Abstract
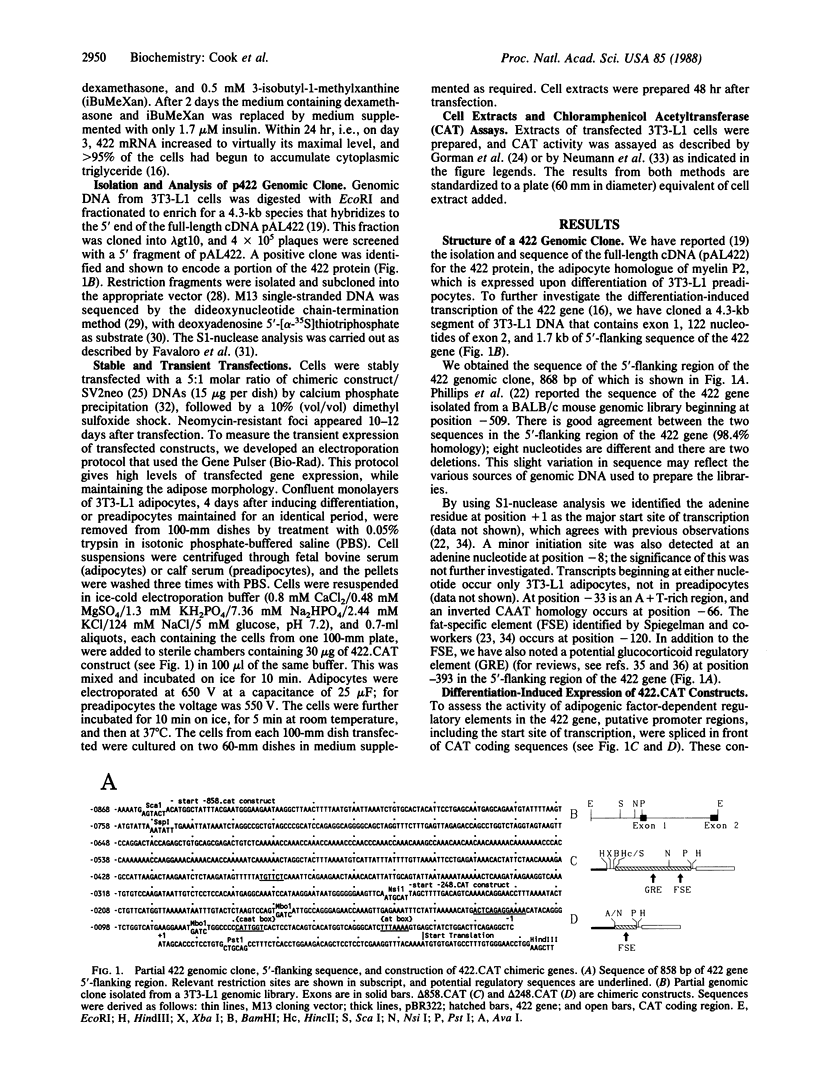
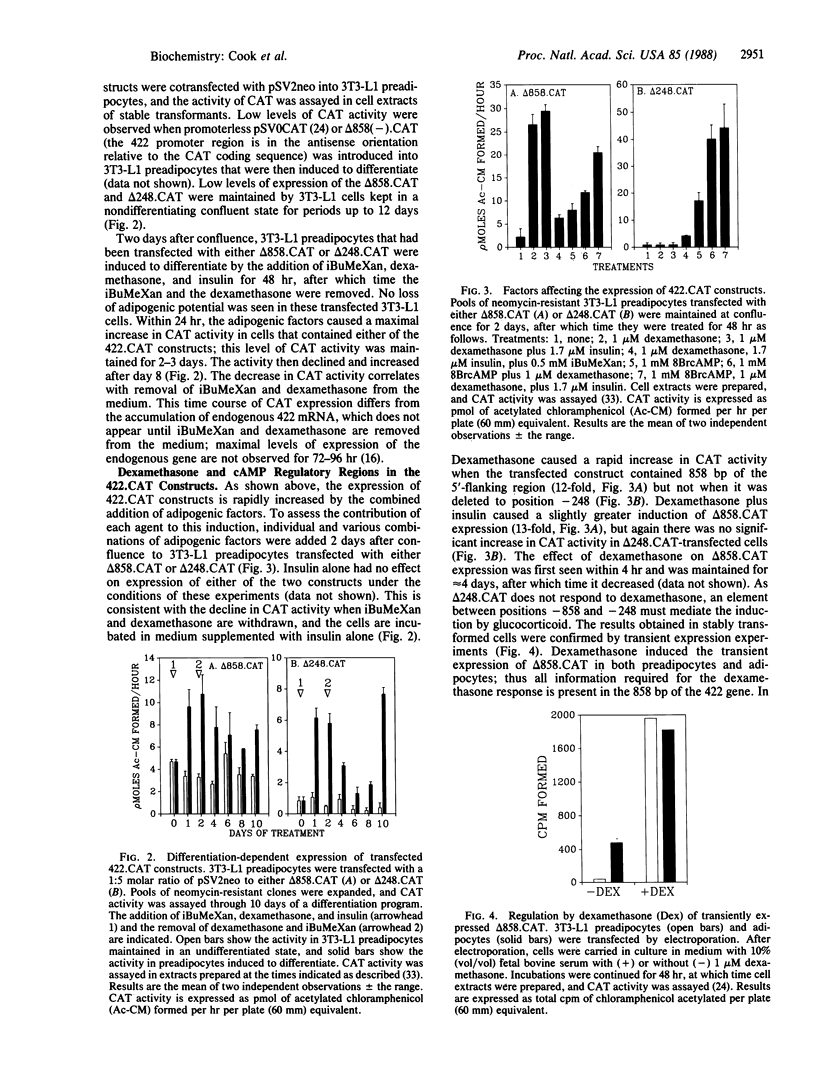
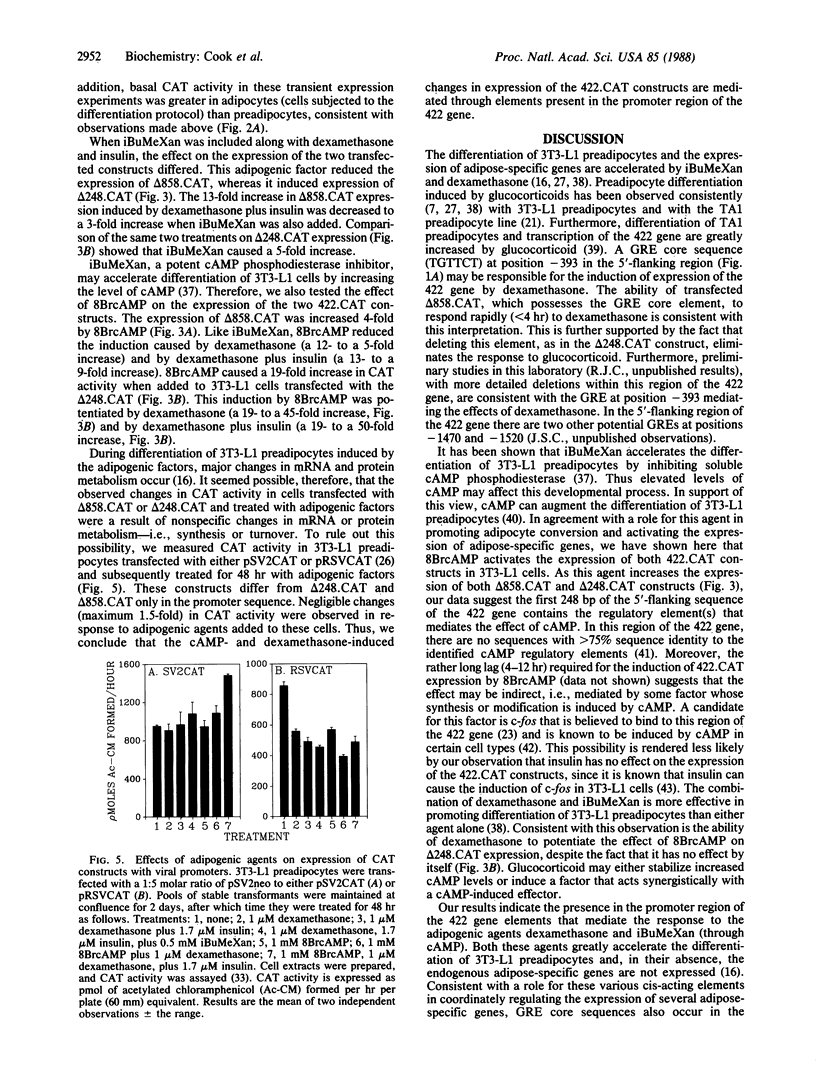
We have isolated and characterized a fragment of the gene encoding adipose fatty acid-binding protein (gene 422) from a 3T3-L1 adipocyte genomic library. The 5'-flanking sequence of the 422 gene contains potential regulatory regions for adipose-specific expression. At position -120 there is a fat-specific element that occurs in several genes expressed as preadipocytes differentiate, and at position -393 there is a glucocorticoid regulatory element core sequence. Chimeric constructs were prepared by ligating 858 base pairs or 248 base pairs of 5'-flanking sequence and 22 nucleotides of 5'-untranslated sequence of the 422 gene to the bacterial gene encoding chloramphenicol acetyltransferase (CAT); these constructs (delta 858.CAT and delta 248.CAT) were transfected into 3T3-L1 preadipocytes. When differentiation was initiated by the adipogenic agents methylisobutylxanthine (a cAMP phosphodiesterase inhibitor), dexamethasone, and insulin, expression of both constructs increased, reaching maximal levels within 24 hr. Both constructs were maximally induced 48 hr before appreciable accumulation of the endogenous 422 mRNA. Expression of delta 858.CAT, but not of delta 248.CAT, was induced by dexamethasone, which correlates with deletion of the potential glucocorticoid regulatory element. Expression of both constructs was induced by 8-bromoadenosine 3',5'-cyclic monophosphate, thus implicating the first 248 base pairs of 5'-flanking sequence of the 422 gene in the response to cAMP. Indirect effects by the adipogenic factors on CAT protein or mRNA synthesis and turnover were ruled out, since replacing the 5'-flanking region of the 422 gene constructs with viral promoters abolished the effects of dexamethasone and 8-bromoadenosine 3',5'-cyclic monophosphate on CAT expression. We conclude that the first 858 base pairs of 5'-flanking sequence of the 422 gene contains elements that mediate activation by dexamethasone and cAMP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus C. W., Lane M. D., Rosenfeld P. J., Kelly T. J. Increase in translatable mRNA for mitochondrial pyruvate carboxylase during differentiation of 3T3 preadipocytes. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1216–1222. doi: 10.1016/0006-291x(81)90252-7. [DOI] [PubMed] [Google Scholar]
- Bernlohr D. A., Angus C. W., Lane M. D., Bolanowski M. A., Kelly T. J., Jr Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5468–5472. doi: 10.1073/pnas.81.17.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr D. A., Bolanowski M. A., Kelly T. J., Jr, Lane M. D. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985 May 10;260(9):5563–5567. [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Neuberg M., Burckhardt J., Almendral J., Wallich R., Müller R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction of cAMP in macrophages. Cell. 1987 Jan 30;48(2):251–260. doi: 10.1016/0092-8674(87)90428-4. [DOI] [PubMed] [Google Scholar]
- Chapman A. B., Knight D. M., Dieckmann B. S., Ringold G. M. Analysis of gene expression during differentiation of adipogenic cells in culture and hormonal control of the developmental program. J Biol Chem. 1984 Dec 25;259(24):15548–15555. [PubMed] [Google Scholar]
- Coleman R. A., Reed B. C., Mackall J. C., Student A. K., Lane M. D., Bell R. M. Selective changes in microsomal enzymes of triacylglycerol phosphatidylcholine, and phosphatidylethanolamine biosynthesis during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1978 Oct 25;253(20):7256–7261. [PubMed] [Google Scholar]
- Cook K. S., Hunt C. R., Spiegelman B. M. Developmentally regulated mRNAs in 3T3-adipocytes: analysis of transcriptional control. J Cell Biol. 1985 Feb;100(2):514–520. doi: 10.1083/jcb.100.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Djian P., Phillips M., Green H. The activation of specific gene transcription in the adipose conversion of 3T3 cells. J Cell Physiol. 1985 Sep;124(3):554–556. doi: 10.1002/jcp.1041240327. [DOI] [PubMed] [Google Scholar]
- Elks M. L., Manganiello V. C. A role for soluble cAMP phosphodiesterases in differentiation of 3T3-L1 adipocytes. J Cell Physiol. 1985 Aug;124(2):191–198. doi: 10.1002/jcp.1041240204. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Hunt C. R., Ro J. H., Dobson D. E., Min H. Y., Spiegelman B. M. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3786–3790. doi: 10.1073/pnas.83.11.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M., Jensen D. F., Wancewicz E. V., Joy L. L., Khoo J. C., Steinberg D. Hormone-sensitive lipase in differentiated 3T3-L1 cells and its activation by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):732–736. doi: 10.1073/pnas.78.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall J. C., Lane M. D. Role of pyruvate carboxylase in fatty acid synthesis: alterations during preadipocyte differentiation. Biochem Biophys Res Commun. 1977 Dec 7;79(3):720–725. doi: 10.1016/0006-291x(77)91171-8. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- Phillips M., Djian P., Green H. The nucleotide sequence of three genes participating in the adipose differentiation of 3T3 cells. J Biol Chem. 1986 Aug 15;261(23):10821–10827. [PubMed] [Google Scholar]
- Reed B. C., Lane M. D. Insulin receptor synthesis and turnover in differentiating 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):285–289. doi: 10.1073/pnas.77.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B. C., Ronnett G. V., Clements P. R., Lane M. D. Regulation of insulin receptor metabolism. Differentiation-induced alteration of receptor synthesis and degradation. J Biol Chem. 1981 Apr 25;256(8):3917–3925. [PubMed] [Google Scholar]
- Resh M. D. Development of insulin responsiveness of the glucose transporter and the (Na+,K+)-adenosine triphosphatase during in vitro adipocyte differentiation. J Biol Chem. 1982 Jun 25;257(12):6978–6986. [PubMed] [Google Scholar]
- Ringold G. M., Chapman A. B., Knight D. M. Glucocorticoid control of developmentally regulated adipose genes. J Steroid Biochem. 1986 Jan;24(1):69–75. doi: 10.1016/0022-4731(86)90034-8. [DOI] [PubMed] [Google Scholar]
- Ringold G. M. Steroid hormone regulation of gene expression. Annu Rev Pharmacol Toxicol. 1985;25:529–566. doi: 10.1146/annurev.pa.25.040185.002525. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Smith C. J., Hirsch A., Lai E., Rubin C. S. Recent studies of the 3T3-L1 adipocyte-like cell line. Recent Prog Horm Res. 1979;35:477–499. doi: 10.1016/b978-0-12-571135-7.50015-1. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Rubin C. S., Lai E., Rosen O. M. Acquisition of increased hormone sensitivity during in vitro adipocyte development. J Biol Chem. 1977 May 25;252(10):3554–3557. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver B. J., Bokar J. A., Virgin J. B., Vallen E. A., Milsted A., Nilson J. H. Cyclic AMP regulation of the human glycoprotein hormone alpha-subunit gene is mediated by an 18-base-pair element. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2198–2202. doi: 10.1073/pnas.84.8.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980 Sep 25;255(18):8811–8818. [PubMed] [Google Scholar]
- Student A. K., Hsu R. Y., Lane M. D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980 May 25;255(10):4745–4750. [PubMed] [Google Scholar]
- Stumpo D. J., Blackshear P. J. Insulin and growth factor effects on c-fos expression in normal and protein kinase C-deficient 3T3-L1 fibroblasts and adipocytes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9453–9457. doi: 10.1073/pnas.83.24.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I. H., Polakis S. E. Differentiation of 3T3-L1 fibroblasts to adipocytes. The effect of indomethacin, prostaglandin E1 and cyclic AMP on the process of differentiation. Biochem Biophys Res Commun. 1977 Jul 11;77(1):175–186. doi: 10.1016/s0006-291x(77)80180-0. [DOI] [PubMed] [Google Scholar]
- Wise L. S., Sul H. S., Rubin C. S. Coordinate regulation of the biosynthesis of ATP-citrate lyase and malic enzyme during adipocyte differentiation. Studies on 3T3-L1 cells. J Biol Chem. 1984 Apr 25;259(8):4827–4832. [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]


