Abstract
Background and purpose:
In previous work, 10 pM BRL37344 and 10 pM clenbuterol stimulated glucose uptake in mouse soleus muscle. Ten nM BRL37344 also stimulated uptake but 100 nM clenbuterol inhibited uptake. Antagonist studies suggested that the opposite effects of 10 nM BRL37344 and 100 nM clenbuterol are mediated by the β2-adrenoceptor. BRL37344 and clenbuterol have been studied in muscles that lack β3-, β2- or all three β-adrenoceptors. Effects of β-adrenoceptor antagonists on responses to the agonists have been studied further using muscles from wild-type mice.
Experimental approach:
Soleus muscles of wild-type or β-adrenoceptor knockout mice were incubated with 2-deoxy[1-14C]-glucose, and β-adrenoceptor ligands. Formation of 2-deoxy[1-14C]-glucose-6-phosphate was measured.
Key results:
Concentration–response relationships were similar for BRL37344 and clenbuterol in normal muscle and muscle lacking β3-adrenoceptors. Ten pM BRL37344 and clenbuterol stimulated glucose uptake in muscle lacking β2-adrenoceptors or all three β-adrenoceptors, but 10 nM BRL37344 did not stimulate uptake in either case, and 100 nM clenbuterol stimulated, rather than inhibited, uptake in muscle lacking β2-adrenoceptors. One hundred nM clenbuterol also stimulated glucose uptake in normal muscle when β2-adrenoceptors were blocked with ICI118551, and this was not prevented by antagonism of β1- or β3-adrenoceptors.
Conclusions and implications:
Ten nM BRL37344 and 100 nM clenbuterol have opposite effects on glucose uptake but both effects are mediated by the β2-adrenoceptor – apparently an example of agonist-directed signalling. Ten pM BRL37344, 10 pM clenbuterol and 100 nM clenbuterol in the presence of ICI118551 stimulate glucose uptake via β-adrenoceptor-independent mechanisms, demonstrating unknown properties for the agonists.
Keywords: β2-adrenoceptor, BRL37344, clenbuterol, soleus muscle, glucose uptake, ligand-directed signalling, β-adrenoceptor knockout
Introduction
β-Adrenoceptor agonists directly and acutely alter the rate of glucose uptake in rat and mouse brown adipocytes (Marette and Bukowiecki, 1990; Shimizu et al., 1996; Chernogubova et al., 2004), rat L6 skeletal muscle cells (Tanishita et al., 1997; Nevzorova et al., 2002) and rat skeletal muscle (Abe et al., 1993; Liu et al., 1996). In brown adipocytes and L6 cells, these effects display the pharmacology of the predominant β-adrenoceptor (normally β3 in adipocytes and β2 in L6 cells); they are solely stimulatory, and they follow a classical concentration–response relationship.
In skeletal muscle, by contrast, very different profiles have been described. In rat skeletal muscle, glucose uptake was stimulated by the selective β3-adrenoceptor agonists (RR+SS)-(±)-4-(2-[(2-(3-chlorophenyl)-2-hydroxyethyl)amino]propyl)phenoxyacetic acid (BRL37344) and disodium 5-[(2R)-2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1,3-benzodioxole-2,2-dicarboxylate (CL-316243) at concentrations well below those reported to stimulate β3-adrenoceptors. Higher concentrations had no effect or elicited an inhibitory response (Abe et al., 1993; Liu et al., 1996). In mouse soleus muscle, we have reported (Ngala et al., 2008) that very low concentrations (10 or 100 pM) of BRL37344 and the selective β2-adrenoceptor agonists clenbuterol and salbutamol stimulated glucose uptake, whereas higher concentrations (100 nM) of clenbuterol and salbutamol that stimulate β2-adrenoceptors inhibited glucose uptake. BRL37344 (10 nM) also displayed a second, more pronounced stimulatory effect, contrasting with its inhibitory effect at similar concentrations in some other studies (Abe et al., 1993; Liu et al., 1996; Board et al., 2000).
Studies with subtype selective β-adrenoceptor antagonists revealed pharmacological profiles for the stimulatory effects of 10 pM clenbuterol and BRL37344 that were not typical of any β-adrenoceptor subtype, whereas the effects of the higher concentrations of both agonists displayed β2-adrenoceptor pharmacology in that they were antagonized by (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol hydrochloride (ICI118551) but not by concentrations of other antagonists that would be expected to block β1- or β3-adrenoceptors. Moreover, glucose uptake in C2C12 cells, in which β2- but not β1- or β3-adrenoceptors could be detected by RT-PCR, responded to clenbuterol and BRL37344 in a similar fashion to soleus muscle. As 100 nM clenbuterol inhibited, whereas 10 nM BRL37344 stimulated glucose uptake, this suggested that these agonists might affect different signalling mechanisms via the β2-adrenoceptor, a phenomenon previously identified with compounds that had originally been described as antagonists of β-adrenoceptors (Summers, 2008).
In order to elucidate further the molecular nature of the receptors that mediate responses to BRL37344 and clenbuterol, we have studied soleus muscle from mice that lack β2-, β3- or all three β-adrenoceptors. Our results confirm and extend our previous conclusions concerning the nature of the receptors.
Methods
Animals and muscle preparation
Breeding, housing and husbandry procedures were conducted in accordance with the UK Government Animals (Scientific Procedures) Act 1986 and approved by The University of Buckingham Ethical Review Board.
Male FVB/N mice aged-matched to β2- and β3-adrenoceptor knockout mice, and male wild-type C57Bl/6 were obtained from Harlan, Bicester, UK. Breeding pairs of FVB/N mice that lacked the β2-adrenoceptor (Chruscinski et al., 1999) were obtained with the permission of Professor Brian Kobilka from Professor Lutz Hein, then at the University of Wuerzburg, Germany. Breeding pairs of FVB/N mice that lacked the β3-adrenoceptor (Susulic et al., 1995) were obtained with the permission of Professor Brad Lowell from Professor Barbara Cannon, Stockholm. Breeding pairs of mice that lacked all three β-adrenoceptors and wild-type mice on the same mixed background (129 Sv/ev, 129 SvJ, FVB/N, C57BL/6J and DBA/2) (Jimenez et al., 2002) were provided by Professor Françoise Rohner-Jeanrenaud and Dr Cedric Asensio, Geneva. Mice were aged 7–14 weeks when used. They were fed ad libitum and killed 3 to 4 h after the onset of the light cycle by a UK Government Animals (Scientific Procedures) Act 1986 schedule 1 method.
The soleus muscle was dissected from each hind leg and held under resting tension by a stainless steel clip as described previously (Wang et al., 2003). Muscles were pre-incubated at 37°C for 45 min in Krebs-Henseleit bicarbonate that contained 10 mM (4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid buffer (HEPES) (pH 7.4) gassed with 95% O2: 5% CO2 and containing 0.14% (w/v) fatty acid-free bovine serum albumin and 5.5 mM glucose, as described previously (Ngala et al., 2008).
2-Deoxyglucose uptake
Muscles were transferred to new flasks and 2-deoxy(1-14C] glucose (0.1 µCi·mL−1) uptake was measured over 45 and 60 min, respectively, in the absence or presence of adrenoceptor agonists and antagonists, insulin (0.1 nM) and glucose (5.5 mM), as described previously (Ngala et al., 2008).
β-Adrenoceptor expression
β-Adrenoceptor expression was measured as described previously (Ngala et al., 2008).
Statistics
Results are expressed as means ± standard error of the mean. Glucose uptake was calculated assuming that 2-deoxyglucose and glucose are not distinguished by the uptake mechanism. Data were analysed by analysis of variance (anova) followed, if the anova was significant by Fisher's least significant difference test. Where a range of concentrations of β-adrenoceptor agonist was used, comparisons were made only with peak effects of the agonists (10 pM and 10 nM BRL37344 in Figure 1). Effects of the β-adrenoceptor agonists were analysed by comparing with corresponding baseline values (asterisks in the figures), and effects of β-adrenoceptor antagonists or absence of β-adrenoceptors by comparing responses at the same concentration of agonist (daggers or double daggers in the figures). Baseline data are reproduced for clarity in the two panels of Figure 3.
Figure 1.
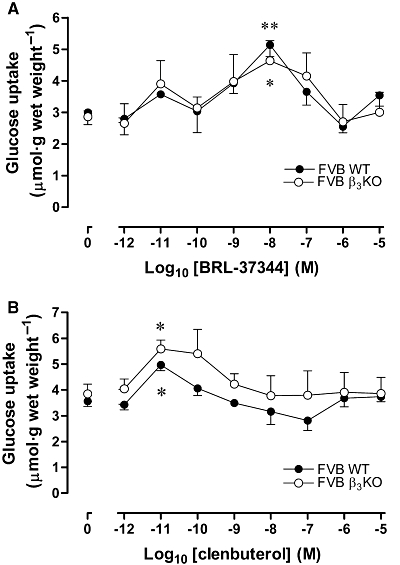
Effects of (A) BRL37344 and (B) clenbuterol on glucose uptake in soleus muscle of FVB wild-type (WT) and β3-adrenoceptor knockout mice (β3KO) (n= 5 to 8). *P < 0.05, **P < 0.01 for the effect of agonist compared with baseline.
Figure 3.
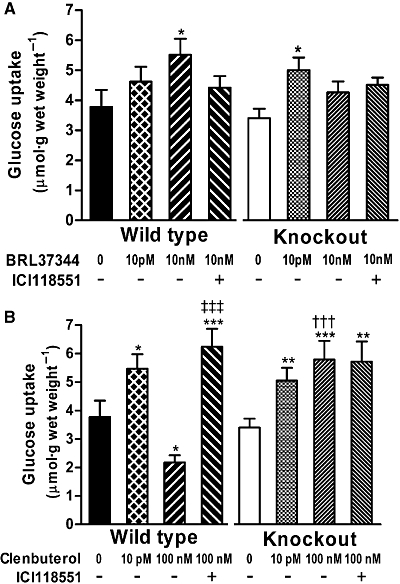
Effects of (A) BRL37344 and (B) clenbuterol on glucose uptake in soleus muscle of FVB wild-type and β2-adrenoceptor knockout mice (n= 6). *P < 0.05, **P < 0.01, ***P < 0.001 for the effect of agonist, or agonist plus 300 nM ICI118551 compared with base line; †††P < 0.001 for the effect of knockout compared with wild type (P= 0.086 for effect of knockout in 10 nM BRL37344-treated mice); ‡‡‡P < 0.001 for the effect of ICI118551 in muscle treated with 100 nM clenbuterol.
Materials
Radiochemicals were obtained from Amersham International (Amersham, Bucks, UK). BRL37344 and clenbuterol were from Tocris Cookson Ltd. (Bristol, UK). Bovine insulin and standard chemicals were from Sigma Aldrich (Gillingham, UK). Nomenclature for receptors and compounds is in accordance with the British Journal of Pharmacology Guide (Alexander et al., 2008).
Results
Glucose uptake in soleus muscle of FVB/N wild-type and knockout mice
Wild-type FVB/N: The concentration–response relationship for the effect of BRL37344 on 2-deoxyglucose uptake (hereafter termed ‘glucose uptake’) in isolated soleus muscle from wild-type FVB/N mice was similar to data reported previously for soleus muscle from C57Bl/6 mice (Ngala et al., 2008). There was a minor peak of stimulation at 10 pM BRL37344 and a major peak at 10 nM BRL37344. The minor peak shown in Figure 1A did not reach statistical significance in that experiment. However, other experiments were conducted on the effect of 10 pM BRL37344 in wild-type FVB/N mice. The data are not included in Figure 1A because the experiments were not conducted in parallel with the study in β3-adrenoceptor knockout mice that is shown in Figure 1A. By using all the data in FVB wild-type mice (including data shown in Figure 3A), a two-way (experiment; concentration of BRL37344) anova conducted on data from three experiments, each involving between 5 and 7 soleus muscles incubated in the absence and presence of 10 pM BRL37344, demonstrated a significant stimulation of glucose uptake by 10 pM BRL37344 (P= 0.028). There was no significant difference between the experiments or interaction between experiment and the effect of BRL37344.
The concentration–response relationship for the effect of clenbuterol on glucose uptake in isolated soleus muscle from wild-type FVB/N mice (Figure 1B) was also broadly similar to that reported previously for soleus muscle from C57Bl/6 mice (Ngala et al., 2008): there was a peak of stimulation at 10 pM clenbuterol and no stimulation at concentrations of 1 nM or higher. In some experiments, including that shown in Figure 1B, there was only a slight trend to suppression of glucose uptake below the baseline value by 100 nM clenbuterol. However, 100 nM clenbuterol suppressed glucose uptake significantly below the baseline value in the experiment shown in Figure 3B, as it had in wild-type C57Bl/6 mice (Ngala et al., 2008).
β3-adrenoceptor knockout
The concentration–response relationships for both BRL37344 and clenbuterol were similar in soleus muscles from wild-type and β3-adrenoceptor knockout mice (Figure 1), indicating that the stimulatory responses to 10 pM and 10 nM BRL37344, and to 10 pM clenbuterol in FVB/N and C57Bl/6 mice were not mediated by β3-adrenoceptors. We did not investigate whether β1- or β2-adrenoceptors were upregulated in skeletal muscle of these mice. Others have not found increased β1- or β2-adrenoceptor binding in ventricular myocardium of β3-adrenoceptor knockout mice (Ziskoven et al., 2007), but this does not preclude the possibility that β1- or β2-adrenoceptors are up-regulated in skeletal muscle. Nevertheless, these results are consistent with the lack of effect of β3-adrenoceptor blockade on responses to BRL37344 and clenbuterol (Ngala et al., 2008). No conclusion could be drawn from this experiment regarding the suppression of glucose uptake by 100 nM clenbuterol in C57Bl/6 mice because, as explained earlier, a significant suppression below baseline was not seen in the wild-type FVB mice used in this experiment.
β2-adrenoceptor knockout
The expression of β1-adrenoceptor mRNA in soleus muscle was similar in β2-adrenoceptor knockout mice and their wild-type controls, but the mean β3-adrenoceptor mRNA expression in knockout mice was only 45% of that in wild-type mice (Figure 2). In view of other data that preclude a role for the β3-adrenoceptor in responses to BRL37344 and clenbuterol, it is unlikely that ablation of responses to 10 nM BRL37344 and 100 nM clenbuterol described below was due to reduced expression of the β3-adrenoceptor.
Figure 2.
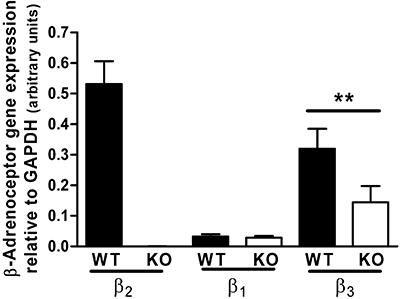
β-Adrenoceptor expression in soleus muscle of FVB wild-type and β2-adrenoceptor knockout mice (n= 6). **P < 0.01 for the effect of knockout of β2-adrenoceptor on expression of β3-adrenoceptors.
For the experiment shown in Figure 3, which was conducted in β2-adrenoceptor knockout and age-matched controls, BRL37344 and clenbuterol were used only at concentrations that elicited peak effects on glucose uptake. For clarity, the effects of BRL37344 and clenbuterol are shown in separate panels with baseline glucose uptake replicated. The β2-adrenoceptor antagonist ICI118551 (300 nM) was added to some incubations with 10 nM BRL37344 and 100 nM clenbuterol because it had blocked the effects of these concentrations in C57Bl/6 mice, transforming the depressant effect of clenbuterol into a stimulation (Ngala et al., 2008).
The stimulatory effect of 10 pM BRL37344 did not reach statistical significance in wild-type FVB/N mice in this experiment, but it did reach statistical significance in the β2-adrenoceptor knockout mice. By contrast, 10 nM BRL37344 stimulated glucose uptake in wild-type but not β2-adrenoceptor knockout mice (Figure 3A). Similarly, 10 nM BRL37344 failed to stimulate glucose uptake in the presence of 300 nM ICI118551 in either wild-type or knockout mice. The presence of ICI118551 did not add to any effect of knocking out the β2-adrenoceptor (Figure 3A). The effect of ICI118551 was not statistically significant, though it was in our previous work in C57Bl/6 mice (Ngala et al., 2008). Thus the β2-adrenoceptor appears to mediate the stimulatory effect of 10 nM BRL37344, but not that of 10 pM BRL37344.
The stimulatory effect of 10 pM clenbuterol was present in both wild-type and β2-adrenoceptor knockout mice. By contrast, 100 nM clenbuterol inhibited glucose uptake in wild-type mice, but stimulated glucose uptake in β2-adrenoceptor knockout mice. ICI118551 similarly changed the inhibitory effect of 100 nM clenbuterol in wild-type mice into a stimulatory effect; it had no effect in β2-adrenoceptor knockout mice (Figure 3B). Thus the inhibitory effect of 100 nM clenbuterol, but neither the stimulatory effect of 10 pM clenbuterol nor that of 100 nM clenbuterol in the presence of ICI118551, was mediated by the β2-adrenoceptor.
Glucose uptake in soleus muscle of mixed background wild-type and ‘β-less’ mice
Preliminary work using a range of concentrations demonstrated broadly similar effects of BRL37344 and clenbuterol on glucose uptake in isolated soleus muscle from wild-type littermates of the mice that lacked all three β-adrenoceptors (‘β-less’ mice). These results were similar to those reported previously for soleus muscle from C57Bl/6 mice (Ngala et al., 2008). There was stimulation at 10 pM BRL37344, 10 nM BRL37344 and 10 pM clenbuterol and suppression of glucose uptake at 100 nM clenbuterol (Figure 4a)
Figure 4.
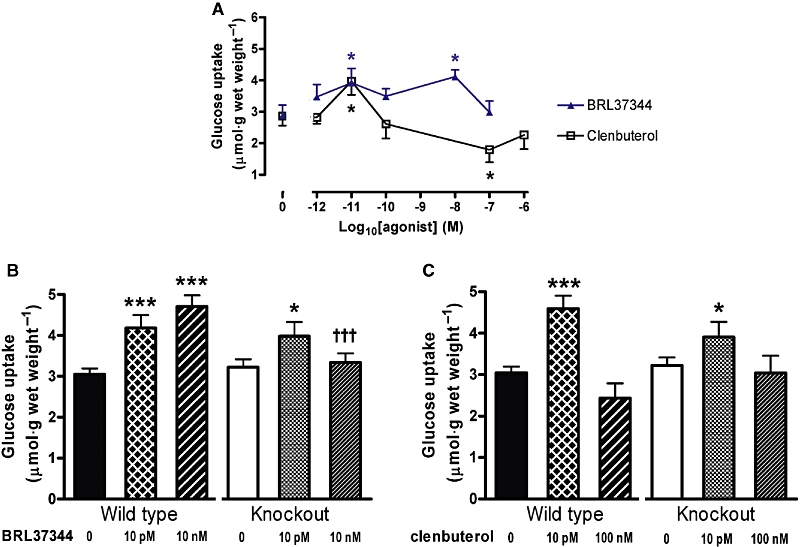
(A) Preliminary study to show concentrations of BRL37344 and clenbuterol that have peak effects on glucose uptake in mixed background wild-type littermates of β-less mice (n= 6). (B and C) Effects of (B) BRL37344 and (C) clenbuterol on glucose uptake in soleus muscle of β-less mice and their mixed background wild-type littermates (n= 18 for wild-type and 12 for β-less mice). *P < 0.05, ***P < 0.001 for the effect of agonist, †††P < 0.001 for the effect of knockout of β-adrenoceptors.
The stimulatory effects of 10 pM BRL37344 and 10 pM clenbuterol were present in both wild-type and β-less mice, indicating that they were not mediated by any β-adrenoceptor, whereas the stimulatory effect of 10 nM BRL37344 was absent (Figure 4B,C), as would be expected since it was absent in FVB/N β2-adrenoceptor knockout mice. One hundred nM clenbuterol did not suppress glucose uptake significantly below the baseline level in this experiment, as happened in the first experiment in wild-type FVB/N mice (Figure 1B), so it is not possible to make any firm statement about the effect of absence of β-adrenoceptors on the response to 100 nM clenbuterol.
Glucose uptake in wild-type C57Bl/6 mice
Further studies were conducted in wild-type C57Bl/6 mice to clarify issues that were not fully resolved using the knockout mice. First, as 100 nM clenbuterol did not inhibit glucose uptake significantly in the β3-adrenoceptor knockout and β-less mice, the possibility that the β3-adrenoceptor contributed to this inhibitory effect was studied in wild-type C57Bl/6 mice using 1 µM SR59230A. A 1 µM SR59230A blocks β3- and possibly also β1-adrenoceptors (Manara et al., 1996; Nisoli et al., 1996; Hutchinson et al., 2001; Hutchinson et al., 2005.) One µM SR59230A has no effect on baseline glucose uptake (Ngala et al., 2008). One hundred nM clenbuterol inhibited glucose uptake to similar degrees in the absence and presence of 1 µM SR59230A (absence: 47% inhibition, P < 0.001; presence 41%, P < 0.001; n= 6 for each treatment).
Secondly, clenbuterol stimulated glucose uptake in β2-adrenoceptor knockout mice, and in the presence of ICI118551 in both FVB/N and C57Bl/6 wild-type mice (Figure 3 and Ngala et al., 2008), but it did not stimulate glucose uptake in β-less mice (Figure 4). Three hundred nM ICI118551, like 1 µM SR59230A and 300 nM CGP20712, has no effect on baseline glucose uptake (Ngala et al., 2008). Thus it was possible that the stimulatory effect of 100 nM clenbuterol when β2-adrenoceptors were blocked or absent was mediated by β1-or β3-adrenoceptors. However, the stimulation of glucose uptake by clenbuterol in the presence of ICI118551 was suppressed by neither 300 nM CGP20712, which blocks β1-adrenoceptors (Kaumann, 1986; Zwaveling et al., 1996), nor 1 µM SR59230A, which blocks β3- and possibly also β1-adrenoceptors (Figure 5). Moreover, the combination of ICI118551 and SR59230A also failed to affect the response to 100 nM clenbuterol in the presence of ICI118551 (glucose uptake: baseline, 2.49 ± 0.24; clenbuterol + ICI118551, 3.73 ± 0.44; clenbuterol + all three antagonists, 3.63 ± 0.26 µmol g wet weight−1; n = 8 for all groups; P < 0.001 for both combinations compared with baseline). Thus the stimulation of glucose uptake by clenbuterol in the presence of ICI118551 was not mediated by a β-adrenoceptor.
Figure 5.
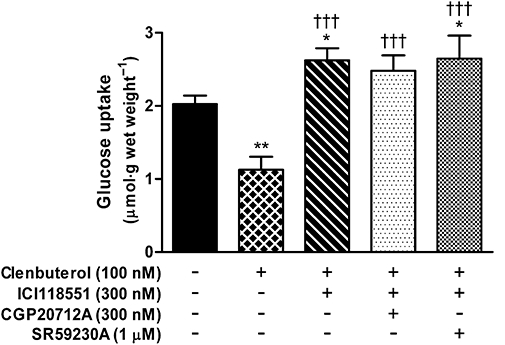
Effects of β1- and β2-adrenoceptor antagonists on the stimulation of glucose uptake by 100 nM clenbuterol in the presence of 300 nM ICI118551 in soleus muscle of wild-type C57BL/6 mice (n= 14). *P < 0.05; **P < 0.01 versus baseline; †††P < 0.001 versus 100 nM clenbuterol.
Discussion
The current study confirms and extends the conclusions of our previous work (Ngala et al., 2008) regarding the nature of the receptors that mediate the effects of 10 pM and 10 nM BRL37344, and 10 pM and 100 nM clenbuterol on glucose uptake in mouse soleus muscle.
An important conclusion of our previous study was that although 100 nM clenbuterol suppressed and 10 nM BRL37344 stimulated glucose uptake, both acted via β2-adrenoceptors. The current study confirms this conclusion because both effects were abolished in β2-adrenoceptor knockout mice. This effect appears to be an example of ligand-directed signalling, previously seen only with β-adrenoceptor ligands that were originally classified as antagonists (Summers, 2008). Clenbuterol and BRL37344 have always been classified as agonists. An alternative interpretation would be that clenbuterol behaves as an inverse agonist at the soleus muscle β2-adrenoceptor. This would mean that constitutively active signalling mechanisms that are further activated by BRL37344 are inhibited by clenbuterol. This possibility is being explored further.
Not only was the inhibitory effect of 100 nM clenbuterol abolished in β2-adrenoceptor knockout mice but it was transformed into a stimulatory effect. This response was also seen when β2-adrenoceptors were blocked with ICI118551 (Ngala et al., 2008). We previously raised the possibility that ICI118551 prevented clenbuterol from signalling to Gαi protein via the β2-adrenoceptor but somehow still allowed signalling via Gαs, so that, like BRL37344, it stimulated glucose uptake. This is clearly not correct because 100 nM clenbuterol stimulated glucose uptake in muscle from mice that lacked β2-adrenoceptors.
It was conceivable that either β1- or β3-adrenoceptors mediated the stimulatory effect of 100 nM clenbuterol in the presence of 300 nM ICI118551 This seemed likely when we found that the stimulatory effect was absent in β-less mice, despite the β2-adrenoceptor being disrupted in the same way in the β-less and β2-adrenoceptor knockout mice (Chruscinski et al., 1999; Jimenez et al., 2002). However, neither 300 nM CGP20712A, nor 1 µM SR59230A, nor both together, blocked the stimulatory effect of 100 nM clenbuterol in the presence of ICI118551 in muscle from wild-type C57Bl/6 mice. It therefore seems that this effect is not mediated by any β-adrenoceptor and that the genetic background of the β-less mice prevented stimulation of glucose uptake by 100 nM clenbuterol plus ICI118551.
The current study also supports our previous conclusions that the stimulatory effects of 10 pM BRL37344 and 10 pM clenbuterol are not mediated by any β-adrenoceptor. For 10 pM clenbuterol, the conclusion was less secure because, although 10 pM is 250-fold below the EC50 value of clenbuterol for relaxation of rat uterus and guinea pig trachea (Ngala et al., 2008), its effect was blocked by 300 nM ICI118551 and not by 1 µM atenolol, which might suggest the involvement of the β2-adrenoceptor. However, any β2-adrenoceptor involvement now seems very unlikely because both 10 pM BRL37344 and 10 pM clenbuterol stimulated glucose uptake in soleus muscle from mice that lack β2-, β3- or all three β-adrenoceptors. A remaining possibility is that these effects were mediated by a disrupted β2-adrenoceptor in the knockout mice. In view of the major insertion made in the β2-adrenoceptor (Chruscinski et al., 1999), in our following comments we do not classify such a receptor as a β-adrenoceptor. It is most unlikely that a disrupted β3-adrenoceptor could be involved because the β3-adrenoceptor was disrupted in different ways in the β3-adrenoceptor and β-less mice, and the disrupted β3-adrenoceptor is not expressed in the β-less mice (Susulic et al., 1995; Jimenez et al., 2002). It is also most unlikely that a disrupted β1-adrenoceptor could be involved because it is largely ablated in the β-less mice (Rohrer et al., 1996).
We do not know the molecular nature of the receptors that mediate responses to 10 pM BRL37344, 10 pM clenbuterol or 100 nM clenbuterol in the presence of ICI118551. Our previous paper (Ngala et al., 2008) showed that the effect of 10 pM BRL37344 was blocked by both ICI118551 and atenolol, and that of 10 pM clenbuterol by ICI118551 (both antagonists at 1 µM). As discussed in that paper, other workers have reported that BRL37344 interacts with other receptors, but only at higher concentrations than those used here. Similarly, clenbuterol blocks sodium channels, but only in the 1–100 µM range (Fischer et al., 2001). Ongoing studies are investigating the signalling mechanisms involved in both the β2-adrenoceptor- and non-β-adrenoceptor-mediated responses.
In conclusion, 10 nM BRL37344 and 100 nM clenbuterol had opposite effects on glucose uptake in mouse soleus muscle but both effects were mediated by the β2-adrenoceptor. Ten pM BRL37344 and 10 pM clenbuterol stimulated glucose uptake via β-adrenoceptor-independent mechanisms. In the presence of ICI118551, 100 nM clenbuterol stimulated glucose uptake via a β-adrenoceptor-independent mechanism.
Acknowledgments
RAN was supported by a bursary from the Ghanaian government.
Glossary
Abbreviations:
- RT
reverse transcriptase
Conflict of interest
J Arch and M Cawthorne consult for various pharmaceutical companies but have no dealings with these companies that present any conflict with the results in this paper.
References
- Abe H, Minokoshi Y, Shimazu T. Effect of a β3-adrenergic agonist, BRL 35135A, on glucose uptake in rat skeletal muscle in vivo and in vitro. J Endocrinol. 1993;139:479–486. doi: 10.1677/joe.0.1390479. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn.) 2008;153(Suppl. 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board M, Doyle P, Cawthorne MA. BRL37344, but not CGP12177, stimulates fuel oxidation by soleus muscle in vitro. Eur J Pharmacol. 2000;406:33–40. doi: 10.1016/s0014-2999(00)00671-3. [DOI] [PubMed] [Google Scholar]
- Chernogubova E, Cannon B, Bengtsson T. Norepinephrine increases glucose transport in brown adipocytes via β3-adrenoceptors through a cAMP, PKA, and PI3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology. 2004;145:269–280. doi: 10.1210/en.2003-0857. [DOI] [PubMed] [Google Scholar]
- Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the β2-adrenergic receptor gene. J Biol Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- Fischer W, Kittner H, Regenthal R, Malinowska B, Schlicker E. Anticonvulsant and sodium channel blocking activity of higher doses of clenbuterol. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:182–192. doi: 10.1007/s002100000341. [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Evans BA, Summers RJ. β1-Adrenoceptors compensate for b3-adrenoceptors in ileum from β3-adrenoceptor knock-out mice. Br J Pharmacol. 2001;132:433–442. doi: 10.1038/sj.bjp.0703828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson DS, Sato M, Evans BA, Christopoulos A, Summers RJ. Evidence for pleiotropic signaling at the mouse β3-adrenoceptor revealed by SR59230A [3-(2-Ethylphenoxy)-1-[(1,S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propa nol oxalate. J Pharmacol Exp Ther. 2005;312:1064–1074. doi: 10.1124/jpet.104.076901. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Leger B, Canola K, Lehr L, Arboit P, Seydoux J, et al. β1/β2/β3-adrenoceptor knockout mice are obese and cold-sensitive but have normal lipolytic responses to fasting. FEBS Lett. 2002;530:37–40. doi: 10.1016/s0014-5793(02)03387-2. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ. The β2-adrenoceptor antagonist CGP 20712 A unmasks β2-adrenoceptors activated by (-)-adrenaline in rat sinoatrial node. Naunyn Schmiedebergs Arch Pharmacol. 1986;332:406–409. doi: 10.1007/BF00500096. [DOI] [PubMed] [Google Scholar]
- Liu YL, Cawthorne MA, Stock MJ. Biphasic effects of the β-adrenoceptor agonist, BRL 37344, on glucose utilization in rat isolated skeletal muscle. Br J Pharmacol. 1996;117:1355–1361. doi: 10.1111/j.1476-5381.1996.tb16736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manara L, Badone D, Baroni M, Boccardi G, Cecchi R, Croci T, et al. Functional identification of rat atypical β-adrenoceptors by the first β3-selective antagonists, aryloxypropanolaminotetralins. Br J Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marette A, Bukowiecki LJ. Mechanism of norepinephrine stimulation of glucose transport in isolated rat brown adipocytes. Int J Obes. 1990;14:857–867. [PubMed] [Google Scholar]
- Nevzorova J, Bengtsson T, Evans BA, Summers RJ. Characterization of the β-adrenoceptor subtype involved in mediation of glucose transport in L6 cells. Br J Pharmacol. 2002;137:9–18. doi: 10.1038/sj.bjp.0704845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngala RA, O'Dowd J, Wang SJ, Agarwal A, Stocker C, Cawthorne MA, et al. Metabolic responses to BRL37344 and clenbuterol in soleus muscle and C2C12 cells via different atypical pharmacologies and β2-adrenoceptor mechanisms. Br J Pharmacol. 2008;155:395–406. doi: 10.1038/bjp.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Landi M, Carruba MO. Functional studies of the first selective β3-adrenergic receptor antagonist SR 59230A in rat brown adipocytes. Mol Pharmacol. 1996;49:7–14. [PubMed] [Google Scholar]
- Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP, Jr, Barsh GS, et al. Targeted disruption of the mouse β1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci USA. 1996;93:7375–7380. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Kielar D, Minokoshi Y, Shimazu T. Noradrenaline increases glucose transport into brown adipocytes in culture by a mechanism different from that of insulin. Biochem J. 1996;314:485–490. doi: 10.1042/bj3140485. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers RJ. Atypical pharmacologies at β-adrenoceptors. Br J Pharmacol. 2008;155:285–287. doi: 10.1038/bjp.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, et al. Targeted disruption of the β3-adrenergic receptor gene. J Biol Chem. 1995;270:29483–29492. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- Tanishita T, Shimizu Y, Minokoshi Y, Shimazu T. The β3-adrenergic agonist BRL37344 increases glucose transport into L6 myocytes through a mechanism different from that of insulin. J Biochem (Tokyo) 1997;122:90–95. doi: 10.1093/oxfordjournals.jbchem.a021744. [DOI] [PubMed] [Google Scholar]
- Wang S, Subramaniam A, Cawthorne MA, Clapham JC. Increased fatty acid oxidation in transgenic mice overexpressing UCP3 in skeletal muscle. Diabetes Obes Metab. 2003;5:295–301. doi: 10.1046/j.1463-1326.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- Ziskoven C, Grafweg S, Bolck B, Wiesner RJ, Jimenez M, Giacobino JP, et al. Increased Ca2+ sensitivity and protein expression of SERCA 2a in situations of chronic β3-adrenoceptor deficiency. Pflugers Arch. 2007;453:443–453. doi: 10.1007/s00424-006-0137-7. [DOI] [PubMed] [Google Scholar]
- Zwaveling J, Winkler Prins EA, Pfaffendorf M, van Zwieten PA. The influence of hyperthyroidism on β-adrenoceptor-mediated relaxation of isolated small mesenteric arteries. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:438–444. doi: 10.1007/BF00261441. [DOI] [PubMed] [Google Scholar]


