Abstract
Background and purpose:
Monocytes/macrophages are an important population of immune inflammatory cells that have diverse effector functions in which their mobility and adhesion play a very relevant role. Epigallocatechin gallate (EGCG), a major component of green tea, has been reported to have anti-allergic and anti-inflammatory activities, but its effects on monocytes remain to be determined. Here we investigated the effects of EGCG on the migration and adhesion of monocytes.
Experimental approach:
We used a human monocyte cell line (THP-1) to analyse the effects of treatment with EGCG under non-cytotoxic conditions on the expression levels of the monocyte chemotactic protein-1 (MCP-1) and of the MCP-1 receptor (CCR2) and on the activation of β1 integrin. A functional validation was carried out by evaluating the inhibitory effect of EGCG on monocyte adhesiveness and migration in vitro.
Key results:
Treatment of THP-1 cells with EGCG decreased MCP-1 and CCR2 gene expression, together with MCP-1 secretion and CCR2 expression at the cell surface. EGCG also inhibited β1 integrin activation. The effects on these molecular targets were in agreement with the EGCG-induced inhibition of THP-1 migration in response to MCP-1 and adhesion to fibronectin.
Conclusions and implications:
Under our experimental conditions, EGCG treatment inhibited the migration and adhesion of monocytes. These inhibitory effects of EGCG on monocyte function should be considered as a promising new anti-inflammatory response with a potential therapeutic role in the treatment of inflammation-dependent diseases.
Keywords: monocyte, adhesion, migration, α5 integrin, β1 integrin, monocyte chemoattractant protein-1, CCR2
Introduction
Monocytes/macrophages play an important role in the initiation, development and outcome of the immune response and are also involved in inflammation-dependent diseases (Kindt et al., 2007), including the highly prevalent atherosclerosis and inflammation-dependent neoplasia (Coussens and Werb, 2002; Libby, 2002) Migration and adhesion are two key features necessary for monocytes/macrophages to carry out their pathophysiological functions. The migration of circulating monocytes through the arterial wall is largely modulated by the activation of the CC chemokine receptor 2 (CCR2; nomenclature follows Alexander et al., 2008), a dominant monocyte chemotaxis receptor (Han et al., 2005). Integrins are non-covalently associated, heterodimeric, cell surface adhesion molecules that play a well-established and crucial role in cell migration (Rose et al., 2007). These molecules increase intercellular adhesion and act as receptors for extracellular matrix proteins such as fibronectin, collagen and laminin (Hynes, 1992). For example, the α5/β1 integrin complex has been reported to be a specific receptor for fibronectin (Luo et al., 2007).
Green tea, which contains a wide range of catechins, has a variety of modulatory actions on physiological functions, such as antibacterial, radical-scavenging and antioxidant effects. Green tea also has a protective effect on the gastric mucosa and has been implicated in the prevention of atherosclerosis (Cabrera et al., 2006). (–)-[Epigallocatechin-3-gallate] (EGCG), a major component of the tea catechins, has an inhibitory effect on allergic reactions (Gabor, 1986; Suzuki et al., 2000; Katiyar and Mukhtar, 2001). EGCG binds to CD11b, which is expressed on CD8+ T cells, and strongly suppresses the adhesion and migration of lymphocytes (Kawai et al., 2004). It is also a potent anti-inflammatory compound, inhibiting the proinflammatory NF-κB pathway (Bode and Dong, 2004). Another mechanism of the anti-inflammatory effect of EGCG is the induction of apoptosis of monocytes (Kawai et al., 2005). Our group has demonstrated that mast cell histidine decarboxylase (HDC) is another molecular target of EGCG (Rodriguez-Caso et al., 2003). Moreover, EGCG has been shown to have potent anti-tumour effects over a wide range of tumour cell types (Hofmann and Sonenshein, 2003; Sah et al., 2004; Nihal et al., 2005) and inhibits the invasive behaviour of gelatinase-expressing cancer cells (Benelli et al., 2002). Other effects of EGCG include inhibition of endothelial cell differentiation and a reduction in the extracellular matrix remodelling potential (Kondo et al., 2002; Lamy et al., 2002; Singh et al., 2002; Fassina et al., 2004; Lai et al., 2004; Yamakawa et al., 2004). Our group demonstrated very recently that EGCG inhibits aggregation, migration and adhesion to fibronectin of human mast cell line HMC-1 (Melgarejo et al., 2007). In this cell type, EGCG treatment downregulates the expression of several angiogenesis-related genes, such as α5 integrin, β3 integrin and monocyte chemoattractive protein-1 (MCP-1). This chemokine, MCP-1, is a member of the C-C class of the β-chemokine family, has pro-inflammatory properties (Conti and DiGioacchino, 2001) and it was the first chemokine identified as a chemotactic factor responsible for macrophage infiltration into tumours (Bottazzi et al., 1983; Graves et al., 1989; Van Damme et al., 1989; Mantovani et al., 1992). We previously demonstrated that HMC-1 cells treated with EGCG have a reduced capacity to recruit monocytes (Melgarejo et al., 2007), but the effect of EGCG on monocyte mobility has not yet been explored. For this reason, we decided to investigate the effects of EGCG on the migration and adhesion of monocytes.
Our results demonstrated that EGCG treatment downregulated MCP-1 and CCR2 levels in THP-1 human monocyte cells. Moreover, EGCG inhibited monocyte migration in response to MCP-1 and also inhibited adhesion to fibronectin. Because the infiltration of monocytes into tissues is of great importance in the regulation of inflammation, this could be one of the mechanisms underlying the anti-inflammatory effects of EGCG.
Methods
Cell culture and treatment
The human monocyte cell line THP-1, kindly supplied by Dr. Daniel Rodríguez-Agudo (Department of Medicine, Veterans Affairs Medical Center and Virginia Commonwealth University, Richmond, VA, USA), was cultured at 37°C in a humidified atmosphere containing 5% CO2. Cells were grown at a starting density of 3 × 105 cells·mL−1 in RPMI medium (Cambrex, Bioscience, Verviers, Belgium) supplemented with 10% fetal calf serum (PAA Laboratories GmbH, Parshing, Austria), 2 mM L-glutamine, penicillin (Cambrex), streptomycin (Cambrex), Fungizone (Gibco, Invitrogen S.A., Part del Llobregat, Spain) and 50 mM 2-mercaptoethanol (Sigma-Aldrich Quimica S.A., Madrid, Spain). The medium was renewed every 6 days. THP-1 cells (7 × 105 cells·mL−1) were treated for 8 h with a fresh solution of EGCG (Sigma, 100 µM final concentration).
Apoptosis assay
The effects of EGCG on the induction of apoptosis in the THP-1 cell line were examined by flow cytometry with the Annexin V-PE apoptosis kit (Pharmingen, BD Biosciences, San Agustin de Guadalix, Spain). Cells were prepared and cultured as described previously in the presence or absence of EGCG. After the incubation time, cells were washed and stained with phycoerythrin (PE)-labelled annexin V (AN) and 7-amino-actinomycin D (7AAD), following the protocol provided with the Annexin V-PE apoptosis detection kit (Pharmingen). Samples of 104 cells were analysed by flow cytometry (FACSort, Becton-Dickinson, San Agustin de Guadalix, Spain), and the AN−/7AAD−, AN+/7AAD−, AN+/7AAD+ and AN−/7AAD+ populations, corresponding to viable, early apoptotic, late apoptotic and necrotic cells, respectively, were enumerated.
Total RNA isolation and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from THP-1 cells (8 × 105 cells per extraction) following the protocol provided with the GenElute Mammalian Total RNA Miniprep Kit (Sigma). RNA yield and purity were assessed spectrophotometrically at 260 and 280 nm. The first-strand cDNA synthesis from the extracted RNA (1 µg) was performed using the iScript cDNA Synthesis Kit (BioRad Laboratories, Alcobendas, Spain) in a final volume of 20 µL according to the recommendations of the manufacturer. Semi-quantitative PCRs were carried out in a final volume of 20 µL containing 1 µL cDNA (synthesized as above), 1x reaction buffer, dNTPs (0.2 mM each), 1 µM forward and reverse primers, and 1 U of Taq polymerase (BioRad). Primers, amplicon size, number of cycles and PCR conditions for each gene are shown in Table 1. The number of cycles for each reaction was chosen to ensure a linear relationship between the quantity of input RNA and quantity of the final product during PCR amplification. Band intensity was quantified by Quantity One Program (BioRad) and β-actin was used as internal control (housekeeping gene). Amplified products were visualized by ethidium bromide fluorescence in 1% agarose gels and were confirmed by sequencing. In every instance, at least three different independent experiments were carried out.
Table 1.
Primers, amplicon size and programme used for PCR amplification
| Gene | Primers | Amplicon size | Programme |
|---|---|---|---|
| MCP-1 | F: 5′-GCCTTAAGTAATGTTAATTCTTAT-3′ | 241 pb | 32 x |
| R: 5′-GGTGTAATAGTTACAAAATATTCA-3′ | (95°C 30″; 51°C 30″; 72°C 40″) | ||
| CCR2 | F: 5′-CCACATCTCGTTCTCGGTTTATCAG-3′ | 582 pb | 31 x |
| R: 5′-CGTGGAAAATAAGGGCCACAG-3′ | (95°C 9″; 64°C 30″; 72°C 25″) | ||
| β-actin | F. 5′-ACCTCATGAAGATCCTGAC-3′ | 524 pb | 24 x |
| R: 5′-ACTCCTGCTTGCCGATCC-3′ | (95°C 30″; 57.4°C 30″; 72°C 60″) |
CCR2, CC chemokine receptor 2; F, forward primer; MCP-1, monocyte chemotactic protein-1; PCR, polymerase chain reaction; R, reverse primer.
Determination of MCP-1 in cell culture supernatants
The level of MCP-1 protein in culture supernatants after the treatment with EGCG was measured using the MCP-1 Human Biotrak Easy ELISA kit (GE Healthcare, Barcelona, Spain) according to the manufacturer's instructions. The limit of detection of MCP-1 by this ELISA kit was determined to be 3.5 pg·mL−1. The absorbance at 450 nm was determined using a microplate reader 680 (BioRad). The measurement of MCP-1 protein levels was carried out after concentration, between 48 and 80 times, of the conditioned medium using Amicon Ultracell 5K centrifugal filter devices (Millipore Iberica, Madrid, Spain), in four independent cultures and the results were expressed as pg·mL−1.
Flow cytometric analysis of integrins and CCR2 expression
Flow cytometric analysis of the expression of the α5/β1 integrin complex and of β1 integrin activation in THP-1 cells was essentially performed as described elsewhere (Urdiales et al., 1998). Before staining cell surface receptors, THP-1 cells were treated with 100 mM EGCG for 8 h or left untreated. Cells were incubated for 30 min with mouse anti-human VLA-5 (α5/β1) integrin monoclonal antibody (Chemicon International, Temecula, CA, USA) or mouse anti-human CD29 (activated β1 integrin) monoclonal antibody (BD Pharmingen) on ice. Cells were washed twice with phosphate-buffered saline (PBS) and incubated for 30 min with Alexa Fluor 488 goat anti-mouse IgG (Invitrogen S.A., Part del Llobregat, Spain) on ice. For CCR2 expression analysis, an Alexa Fluor 647 mouse anti-human CD192 (CCR2, BD Pharmingen) was used.
Specific fluorescence was detected with a FACSort flow cytometer (Becton-Dickinson) for the integrin expression analysis and a MoFlow (DakoCytomation, Glostrup, Denmark) for the CCR2 expression analysis.
Adhesion assay
Adhesion assays were carried out in 24-well plates. Wells were coated overnight at 4°C with 10 ng·mL−1 fibronectin (Sigma). Plates were then gently washed and any remaining non-specific binding sites were blocked by adding 3% bovine serum albumin (BSA) in PBS for 1 h at 37°C. THP-1 cells were treated with 100 µM EGCG for 8 h or left untreated. The cells were then suspended in the volume necessary to give 3 × 104 cells·mL−1 and 300 µL of the cell suspension was added to each precoated well. Plates were incubated for 1 h at 37°C. Unbound cells were removed by gentle washing with PBS and the remaining cells were counted using a light microscope.
Migration assay
Monocyte chemotaxis was measured by using a 24-well chemotaxis transwell system (Becton-Dickinson) as described previously with minor modifications (Martinez-Poveda et al., 2005). Briefly, control and treated THP-1 cells were labelled in situ with 5 mg·mL−1 Calcein-AM (Molecular Probes, Invitrogen S.A., Part del Llobregat, Spain) in complete culture medium for 1 h at 37°C. After washing, the cells were suspended in fresh medium supplemented with 0.1% BSA. Cells (106) were loaded into the upper chamber of the Micro Chemotaxis chamber (Gibco, Invitrogen). The lower and upper chambers were separated by a polycarbonate membrane (8 µm pore size). Medium with 0.1% BSA and supplemented with 50 ng·mL−1 of recombinant human MCP-1 (Peprotech EC Ltd., London, UK) was used as the chemoattractant in the lower camber. The inserts were incubated in the dark at 37°C and cell migration was determined by taking readings at different times. Fluorescence of cells that had migrated through the inserts was measured on the Fluorescence Microplate Reader (FL600FA, BIO-TEK Instruments, Colmar, France) in the bottom read mode using excitation/emission wavelengths of 485/530 nm. Relative velocities of migration for control and treated cells were compared.
Statistical analysis
Statistical significance for adhesion and migration analysis was determined by the Kolmogorov–Smirnoff and Student's paired sample tests respectively. Values of P < 0.001 (for Kolmogorov–Smirnoff) and P < 0.05 (for Student's paired sample test) were considered to be significant.
Results
Evaluation of the potential toxicity of EGCG treatment on THP-1 cells
EGCG has been shown to induce monocyte apoptosis (Kawai et al., 2005). In this study, we wanted to investigate the effects of EGCG on the migration and adhesion capabilities of monocytes without interference from cytotoxic effects in order to prove that our chosen treatment conditions had no effect on THP-1 cell viability and survival. The effects of EGCG on the induction of apoptosis in the THP-1 cell line after 8 h of treatment with 100 µM EGCG were examined by flow cytometry. Two-colour flow cytometric analysis using Annexin V (AN) and 7-actinomycin D (7AAD) can discriminate four populations: viable (AN−/7AAD−), early apoptotic (AN+/7AAD−), late apoptotic (AN+/7AAD+) and necrotic cells (AN−/7AAD+). Figure 1 shows a representative EGCG toxicity experiment in which cells were treated with EGCG for 8 h. The percentage of early apoptotic cells was relatively low in the untreated cell population and only decreased slightly after EGCG treatment (5.55% control vs. 2.34% treated cells). Similar results were obtained in other experiments. In all cases, the amount of AN+ cells (early and late apoptotic cells) decreased slightly or remained constant after treatment with EGCG.
Figure 1.
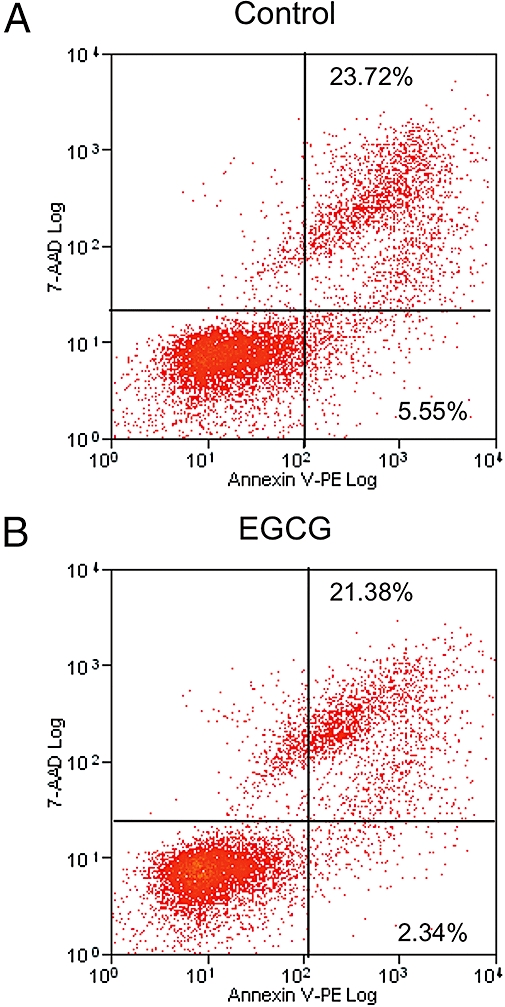
Annexin V apoptosis assay. Human monocyte cell line (THP-1) cells treated with 100 µM epigallocatechin gallate (EGCG) for 8 h (B) or left untreated (A) were stained with phycoerythrin-labelled annexin V (AN) and 7-amino-actinomycin D (7AAD). The percentages of AN+/7AAD− (early apoptotic) and AN+/AAD+ (late apoptotic) cells were calculated. Two independent experiments were carried out, both showing no apoptotic effect of EGCG treatment on THP-1 cells.
EGCG downregulates MCP-1 and CCR2 mRNA expression in THP-1 monocytes
In a previous study, we showed that EGCG downregulated MCP-1 expression and secretion in mast cells, inhibiting the ability of these mast cells to recruit monocytes (Melgarejo et al., 2007). However, the direct effect of EGCG on monocytes was not studied. To analyse the effects of EGCG on expression of MCP-1 and CCR2 (a receptor specific for MCP-1) in monocytes, we measured the levels of endogenous mRNA for both genes in THP-1 by RT-PCR. Figure 2 shows the downregulation of MCP-1 and CCR2 after treatment with 100 µM EGCG. Relative expression was assessed as percentage of control after normalization to β-actin expression. EGCG treatment results in a reduction of 39 ± 10% in the level of MCP-1 expression and a reduction of 38 ± 8% for CCR2 expression.
Figure 2.
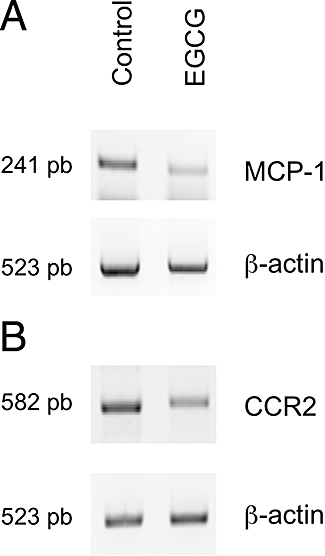
Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis of monocyte chemotactic protein-1 (MCP-1) and CC chemokine receptor 2 (CCR2) expression. RNA extracted from control and EGCG-treated human monocyte cell line (THP-1) cells was reverse transcribed into cDNA and amplified using the conditions listed in Table 1. Amplification was performed under appropriate conditions to ensure a linear relationship between the quantity of input RNA and quantity of the final product during PCR amplification. Relative expression was assessed after normalization to β-actin amplification as described in methods section. Amplifications were carried out in triplicate, at least. (A) MCP-1 expression; (B) CCR2 expression.
EGCG inhibits the secretion of MCP-1 by THP-1 monocytes
As our results showed that EGCG decreased the mRNA levels of MCP-1 in THP-1 cells, we then examined the effect of EGCG on MCP-1 protein secretion. We determined the level of MCP-1 in culture supernatants by ELISA. Treatment of THP-1 cells with 100 µM EGCG for 8 h severely decreased the amount of MCP-1 secreted into the culture medium, with the amount of secreted protein decreasing from 3.4 ± 0.1 pg·mL−1 without treatment, to undetectable levels after EGCG treatment (see Methods).
EGCG decreases the expression levels of CCR2 on the surface of THP-1 monocytes
In order to check if the reduction in mRNA levels for CCR2 in THP-1 cells affected the expression of this receptor at cell surface, we examined the levels of surface expression of CCR2 by flow cytometry. Figure 3 shows that treatment of THP-1 cells with 100 µM EGCG for 8 h was able to reduce the median intensity of fluorescence (MIF) by about 20% with respect to untreated cells.
Figure 3.
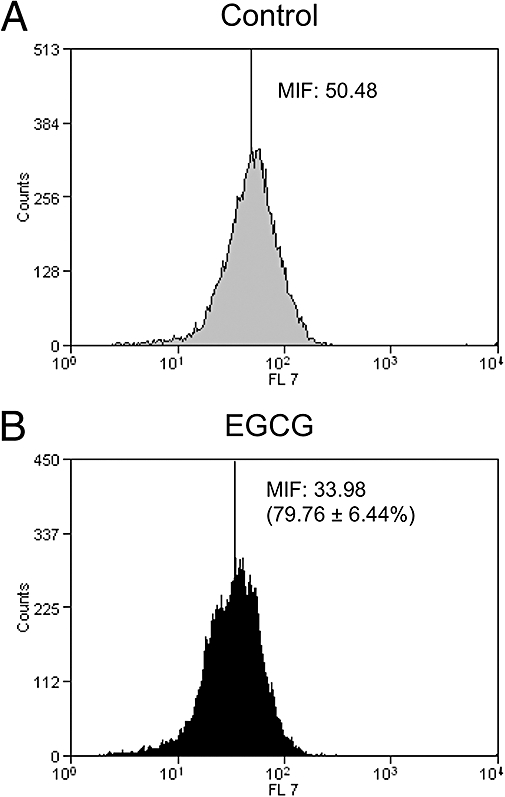
Surface expression of monocyte chemotactic protein-1 receptor on human monocyte cell line cells treated with 100 µM epigallocatechin gallate (EGCG) for 8 h (B) or left untreated (A). Cells were incubated with Alexa Fluor 647 mouse anti-human CD192. The percentages of the median intensities of fluorescence (MIF) were calculated for control and treated cells and are shown in the figure for this experiment. The numbers in parentheses refer to the mean (±SEM) inhibition of % MIF from three independent experiments, after treatment with EGCG.
EGCG decreases the levels of both VLA5 and activated β1 integrin in THP-1 monocytes
The effect of EGCG on both VLA5 and activated β1 integrin levels was analysed by flow cytometry. As shown in Figure 4, EGCG treatment decreased the expression of VLA5 (Figure 4A,B) and of activated β1 integrin (Figure 4C,D). From three experiments, the mean inhibition of MIF for VLA5 in EGCG-treated cells (shown in Figure 4B) and for activated β1 integrin (Figure 4D) was essentially identical. We also determined the percentage of cells in an arbitrary region including approximately 95% of VLA5-positive cells in untreated cultures and shown in Figure 4. Treatment with EGCG decreased the proportion of cells expressing VLA5 or that expressing activated β1 integrin on the cell surface.
Figure 4.
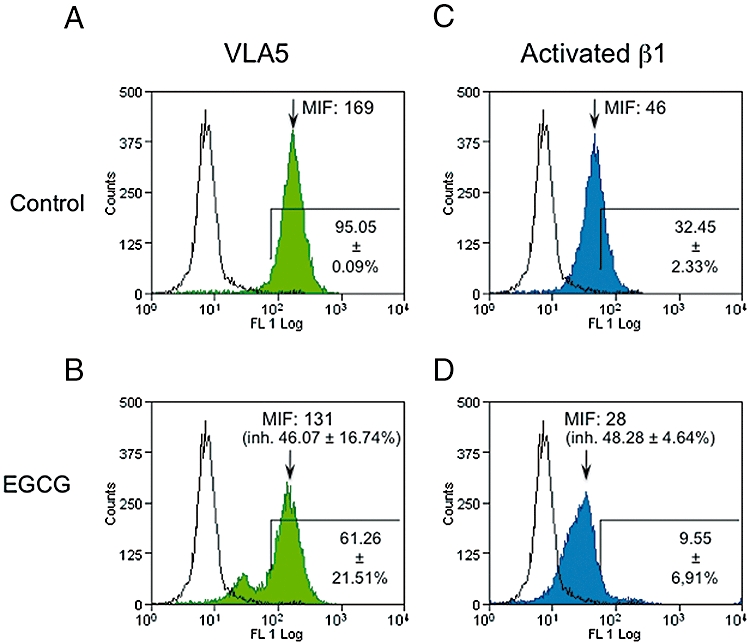
Surface expression of VLA5 and CD29 on human monocyte cell line cells treated with 100 µM epigallocatechin gallate (EGCG) for 8 h or left untreated. The experimental record from one representative experiment is shown. Cells were incubated with anti-human VLA5 (α5/β1 integrin, green histograms) or anti-human CD29 (activated β1 integrin, blue histograms) monoclonal antibodies, followed by incubation with Alexa Fluor 488 goat anti-mouse IgG. Cells incubated with secondary antibody alone are shown as empty histograms in each panel. The percentage of marked cells (mean ± SEM of four different experiments, shown on lower right of each panel) was calculated for (A) control cells stained with anti-VLA5, (B) treated cells stained with anti-VLA5, (C) control cells stained with anti-CD29 and (D) treated cells stained with anti-CD29. In (B) and (D), the percentage (±SEM) reductions of median intensity of fluorescence (MIF) from four independent experiments are shown in parentheses.
EGCG decreases the adhesiveness and migration capability of THP-1 cells
As EGCG treatment produced inhibitory effects on key molecules related to adhesion and migration in THP-1 monocytes, we sought to analyse whether these inhibitory effects affected the adhesion and migration of THP-1 cells. As VLA5 is a mediator of monocyte adhesion to fibronectin in the extracellular matrix, we determined the effects of EGCG treatment on THP-1 cell adhesion to fibronectin. Figure 5 shows that EGCG treatment halved the percentage of THP-1 cells adhering to fibronectin. To test whether EGCG-induced CCR2 downregulation altered THP-1 monocyte chemotactic ability, the response of THP-1 monocytes to recombinant human MCP-1 was measured using a chemotaxis assay. Figure 6 shows that treatment of THP-1 monocytes with EGCG did indeed decrease migration by about 50%.
Figure 5.
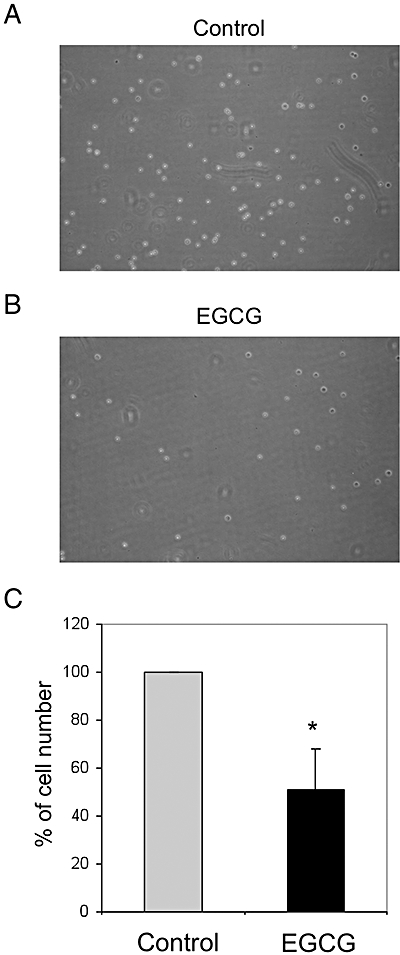
Adhesion of human monocyte cell lineTHP-1 (THP-1) cells to fibronectin. THP-1 cells (3 × 104) were treated with 100 µM EGCG for 8 h (B) or left untreated (A) were seeded on 24-well plates and then incubated for 1 h, after which time the non-adherent cells were removed by gently washing with PBS. The remaining cells were counted. (C) Percentages ± SEM of remaining cells treated with EGCG with respect to the untreated cells calculated from three independent experiments. *Significantly different from control (P < 0.001) according to the Kolmogorov–Smirnoff test.
Figure 6.
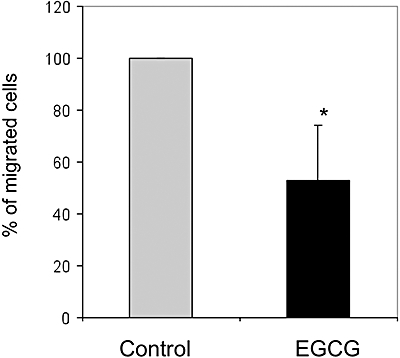
In vitro migration of human monocyte cell line (THP-1) cells. THP-1 cells treated with 100 µM EGCG for 8 h or left untreated were labelled in situ with 5 mg·mL−1 Calcein-AM in complete culture medium for 1 h at 37°C. After washing, cells were re-suspended in medium supplemented with 0.1% bovine serum albumin and placed in a migration chamber. Medium without FBS supplemented with 50 ng·mL−1 MCP-1 was used as a chemoattractant. The inserts were incubated in the dark at 37°C and cell migration was determined by taking readings at different times. The fluorescence of cells that had migrated through the inserts was measured using a Fluorescence Microplate Reader in the bottom read mode using excitation/emission wavelengths of 485/530 nm and a gain setting of 75. The number of migrated cells after 3 h was calculated for both treated and untreated cells. Data shown are the mean ± SEM of three independent experiments. *Significantly different from control (P < 0.05); Student's t-test for paired samples (two tailed).
Discussion
Transmigration of monocytes through endothelial monolayers is induced by chemotaxis, which is an essential step in the recruitment of monocytes to the vascular wall. MCP-1, a member of the C-C class of the β-chemokine family and one of the key factors responsible for initiating the inflammatory process, triggers chemotaxis and transendothelial migration of monocytes to inflammatory lesions by interacting with the CCR2 on monocytes (Mackay, 1996). Although there are different, MCP-1-independent, ways of monocyte recruitment (Janardhan et al., 2006), upregulation of MCP-1 is related to macrophage recruitment, angiogenesis and survival in human breast cancer (Ueno et al., 2000), and MCP-1 transfection has been shown to induce angiogenesis and tumourigenesis in gastric carcinoma (Kuroda et al., 2005). Moreover, MCP-1 appears to be the most potent monocyte chemoattractant and it is thought to play a major role in the obligatory recruitment of monocytes into arterial lesions during the advancement of atherosclerosis. We and other groups previously demonstrated that EGCG inhibits MCP-1 expression and secretion in HMC-1 (Melgarejo et al., 2007) and vascular endothelial cells (Ahn et al., 2008), and that it has an inhibitory role on monocyte recruitment (Yamakawa et al., 2004), but the effect of this compound on monocyte CCR2 was unknown. Moreover, monocytes are able to secrete MCP-1 too.
In this work we analysed the effects of EGCG on CCR2 in cultured, non-activated monocytes simulating their physiological condition as circulating blood cells, before they are recruited to sites of inflammation, where they are differentiated into macrophages and become activated within the tissue. We used the human monocyte cell line THP-1, and because monocytes also express MCP-1, we analysed the effect of EGCG on this marker. In the literature, EGCG is used in a wide range of concentrations. We decided to use 100 µM EGCG, on the basis of our earlier work on mast cells (Melgarejo et al., 2007) and some other recent references (Hussain et al., 2005; Choi et al., 2009; Magyar et al., 2009). We wanted to study the effect of EGCG on THP-1 cells after a single dose/treatment, not trying to imitate the effect of a ‘chronic’ treatment as the regular drinking of green tea should be considered. At this concentration and after 8 h exposure, EGCG did not induce apoptosis on THP-1 cells (Figure 1). Kawai et al. (2005) have shown that EGCG induces apoptosis on peripheral blood monocytes when treated at 50 µM for 24 h. The different cell model, longer treatment and/or lower concentration, all could explain this apparent discrepancy. We demonstrated that EGCG modulated MCP-1 mediated monocyte chemotactic activity in THP-1 cells under treatment conditions that did not induce monocyte apoptosis. We found that EGCG downregulated both CCR2 and MCP-1 gene expression (Figure 2) in this cell type. Furthermore, the amount of the CCR2 receptor at THP-1 cell surface was decreased (Figure 3) and a marked inhibition of MCP-1 protein secretion in treated cells was shown, by ELISA.
Intravasation and extravasation of normal and neoplastic monocytes involves close interactions between these cells and the extracellular matrix glycoproteins (Albelda, 1993; Brown, 1997; Kinashi, 2005). Adhesion molecules are commonly divided into the superfamily of immunoglobulins and the families of integrins, selectins and cadherins (Carlos and Harlan, 1990). Integrins are integral glycoproteins of the cell membrane that are composed of an α and a β chain (Hynes, 1992). EGCG reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells (Ludwig et al., 2004). We chose to analyse α5/ β1 integrins because they are the primary integrins that can assemble monocytes on the extracellular matrix under normal conditions. EGCG did not produce any decrease in the mRNA expression of these integrins (data not shown). However, we did find an inhibitory effect on the surface expression of α5/β1 integrin and on β1 integrin activation (Figure 4). This effect resulted in an inhibition of the adhesion of THP-1 cells to fibronectin (Figure 5) and would support the role of EGCG in reducing adhesion of monocytes to the arterial wall, as an early step in atherosclerosis.
After showing the inhibitory effects of EGCG on MCP-1 expression and secretion, on CCR2 expression and on THP-1 adhesion to fibronectin, a decrease in THP-1 migration in response to MCP-1 could be expected. In fact, this was the case, as demonstrated in the present study by showing that EGCG treatment produced potent inhibitory effects on the migration of THP-1 cells in assays in which MCP-1 was used as a chemoattractant (Figure 6).
Under our experimental conditions, EGCG treatment inhibited the expression of both MCP-1 and its receptor CCR2, which are key mediators. Furthermore, EGCG also had an inhibitory effect on the dimerization of α5 and β1 integrins and on β1 integrin activation. As a consequence, the migration and adhesion of monocytes were compromised. The inhibitory effects of EGCG on monocyte functions should be considered as a promising new anti-inflammatory response with a potential therapeutic role in the treatment of inflammation-dependent diseases such as atherosclerosis, Crohn's disease and asthma, among others.
Acknowledgments
This study was supported by Grants SAF2008-02522 (Spanish Ministry of Science and Innovation), Fundación Ramón Areces, P07-CVI-02999 and funds given to group BIO-267 (Andalusian Government). The ‘CIBER de Enfermedades Raras’ is an initiative of the ISCIII.
Glossary
Abbreviations:
- CCR2
CC chemokine receptor 2
- EGCG
epigallocatechin gallate
- HDC
histidine decarboxylase
- MCP-1
monocyte chemotactic protein-1
- MIF
mean intensity of fluorescence
- NF-κB
nuclear factor kappa B
- PBS
phosphate-buffered saline
- SEM
standard error of the mean
References
- Ahn HY, Xu Y, Davidge ST. Epigallocatechin-3-O-gallate inhibits TNFalpha-induced monocyte chemotactic protein-1 production from vascular endothelial cells. Life Sci. 2008;82(17–18):964–968. doi: 10.1016/j.lfs.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Albelda SM. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993;68(1):4–17. [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli R, Vene R, Bisacchi D, Garbisa S, Albini A. Anti-invasive effects of green tea polyphenol epigallocatechin-3-gallate (EGCG), a natural inhibitor of metallo and serine proteases. Biol Chem. 2002;383(1):101–105. doi: 10.1515/BC.2002.010. [DOI] [PubMed] [Google Scholar]
- Bode AM, Dong Z. Targeting signal transduction pathways by chemopreventive agents. Mutat Res. 2004;555(1–2):33–51. doi: 10.1016/j.mrfmmm.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Bottazzi B, Polentarutti N, Balsari A, Boraschi D, Ghezzi P, Salmona M, et al. Chemotactic activity for mononuclear phagocytes of culture supernatants from murine and human tumor cells: evidence for a role in the regulation of the macrophage content of neoplastic tissues. Int J Cancer. 1983;31(1):55–63. doi: 10.1002/ijc.2910310110. [DOI] [PubMed] [Google Scholar]
- Brown EJ. Adhesive interactions in the immune system. Trends Cell Biol. 1997;7(7):289–295. doi: 10.1016/S0962-8924(97)01076-3. [DOI] [PubMed] [Google Scholar]
- Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea – a review. J Am Coll Nutr. 2006;25(2):79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- Carlos TM, Harlan JM. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990;114:5–28. doi: 10.1111/j.1600-065x.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Choi KC, Jung MG, Lee YH, Yoon JC, Kwon SH, Kang HB, et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009;69(2):583–592. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- Conti P, DiGioacchino M. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc. 2001;22(3):133–137. doi: 10.2500/108854101778148737. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassina G, Vene R, Morini M, Minghelli S, Benelli R, Noonan DM, et al. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin Cancer Res. 2004;10(14):4865–4873. doi: 10.1158/1078-0432.CCR-03-0672. [DOI] [PubMed] [Google Scholar]
- Gabor M. Anti-inflammatory and anti-allergic properties of flavonoids. Prog Clin Biol Res. 1986;213:471–480. [PubMed] [Google Scholar]
- Graves DT, Jiang YL, Williamson MJ, Valente AJ. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989;245(4925):1490–1493. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- Han KH, Ryu J, Hong KH, Ko J, Pak YK, Kim JB, et al. HMG-CoA reductase inhibition reduces monocyte CC chemokine receptor 2 expression and monocyte chemoattractant protein-1-mediated monocyte recruitment in vivo. Circulation. 2005;111(11):1439–1447. doi: 10.1161/01.CIR.0000158484.18024.1F. [DOI] [PubMed] [Google Scholar]
- Hofmann CS, Sonenshein GE. Green tea polyphenol epigallocatechin-3 gallate induces apoptosis of proliferating vascular smooth muscle cells via activation of p53. FASEB J. 2003;17(6):702–704. doi: 10.1096/fj.02-0665fje. [DOI] [PubMed] [Google Scholar]
- Hussain T, Gupta S, Adhami VM, Mukhtar H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int J Cancer. 2005;113(4):660–669. doi: 10.1002/ijc.20629. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Janardhan KS, Sandhu SK, Singh B. Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front Biosci. 2006;11:1569–1576. doi: 10.2741/1904. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Mukhtar H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J Leukoc Biol. 2001;69(5):719–726. [PubMed] [Google Scholar]
- Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, et al. Epigallocatechin gallate attenuates adhesion and migration of CD8+ T cells by binding to CD11b. J Allergy Clin Immunol. 2004;113(6):1211–1217. doi: 10.1016/j.jaci.2004.02.044. [DOI] [PubMed] [Google Scholar]
- Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, et al. Epigallocatechin gallate induces apoptosis of monocytes. J Allergy Clin Immunol. 2005;115(1):186–191. doi: 10.1016/j.jaci.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5(7):546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- Kindt TJ, Osborne BA, Goldsby RA. Kuby Immunology. 6th edn. San Francisco, CA: W.H. Freeman; 2007. [Google Scholar]
- Kondo T, Ohta T, Igura K, Hara Y, Kaji K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002;180(2):139–144. doi: 10.1016/s0304-3835(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Kuroda T, Kitadai Y, Tanaka S, Yang X, Mukaida N, Yoshihara M, et al. Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin Cancer Res. 2005;11(21):7629–7636. doi: 10.1158/1078-0432.CCR-05-0798. [DOI] [PubMed] [Google Scholar]
- Lai HC, Chao WT, Chen YT, Yang VC. Effect of EGCG, a major component of green tea, on the expression of Ets-1, c-Fos, and c-Jun during angiogenesis in vitro. Cancer Lett. 2004;213(2):181–188. doi: 10.1016/j.canlet.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Lamy S, Gingras D, Beliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62(2):381–385. [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Lorenz M, Grimbo N, Steinle F, Meiners S, Bartsch C, et al. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem Biophys Res Commun. 2004;316(3):659–665. doi: 10.1016/j.bbrc.2004.02.099. [DOI] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay CR. Chemokine receptors and T cell chemotaxis. J Exp Med. 1996;184(3):799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar JE, Gamberucci A, Konta L, Margittai E, Mandl J, Banhegyi G, et al. Endoplasmic reticulum stress underlying the pro-apoptotic effect of epigallocatechin gallate in mouse hepatoma cells. Int J Biochem Cell Biol. 2009;41(3):694–700. doi: 10.1016/j.biocel.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13(7):265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- Martinez-Poveda B, Quesada AR, Medina MA. Hypericin in the dark inhibits key steps of angiogenesis in vitro. Eur J Pharmacol. 2005;516(2):97–103. doi: 10.1016/j.ejphar.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Melgarejo E, Medina MA, Sanchez-Jimenez F, Botana LM, Dominguez M, Escribano L, et al. Epigallocatechin-3-gallate interferes with mast cell adhesiveness, migration and its potential to recruit monocytes. Cell Mol Life Sci. 2007;64(19–20):2690–2701. doi: 10.1007/s00018-007-7331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114(4):513–521. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Caso C, Rodriguez-Agudo D, Sanchez-Jimenez F, Medina MA. Green tea epigallocatechin-3-gallate is an inhibitor of mammalian histidine decarboxylase. Cell Mol Life Sci. 2003;60(8):1760–1763. doi: 10.1007/s00018-003-3135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Sah JF, Balasubramanian S, Eckert RL, Rorke EA. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of ERK1/2 and AKT kinases. J Biol Chem. 2004;279(13):12755–12762. doi: 10.1074/jbc.M312333200. [DOI] [PubMed] [Google Scholar]
- Singh AK, Seth P, Anthony P, Husain MM, Madhavan S, Mukhtar H, et al. Green tea constituent epigallocatechin-3-gallate inhibits angiogenic differentiation of human endothelial cells. Arch Biochem Biophys. 2002;401(1):29–37. doi: 10.1016/S0003-9861(02)00013-9. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yoshino K, Maeda-Yamamoto M, Miyase T, Sano M. Inhibitory effects of tea catechins and O-methylated derivatives of (-)-epigallocatechin-3-O-gallate on mouse type IV allergy. J Agric Food Chem. 2000;48(11):5649–5653. doi: 10.1021/jf000313d. [DOI] [PubMed] [Google Scholar]
- Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6(8):3282–3289. [PubMed] [Google Scholar]
- Urdiales JL, Becker E, Andrieu M, Thomas A, Jullien J, van Grunsven LA, et al. Cell cycle phase-specific surface expression of nerve growth factor receptors TrkA and p75(NTR) J Neurosci. 1998;18(17):6767–6775. doi: 10.1523/JNEUROSCI.18-17-06767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J, Decock B, Lenaerts JP, Conings R, Bertini R, Mantovani A, et al. Identification by sequence analysis of chemotactic factors for monocytes produced by normal and transformed cells stimulated with virus, double-stranded RNA or cytokine. Eur J Immunol. 1989;19(12):2367–2373. doi: 10.1002/eji.1830191228. [DOI] [PubMed] [Google Scholar]
- Yamakawa S, Asai T, Uchida T, Matsukawa M, Akizawa T, Oku N. Epigallocatechin gallate inhibits membrane-type 1 matrix metalloproteinase, MT1-MMP, and tumor angiogenesis. Cancer Lett. 2004;210(1):47–55. doi: 10.1016/j.canlet.2004.03.008. [DOI] [PubMed] [Google Scholar]


