Abstract
Background and purpose:
Earlier we reported that 7,8-dihydro-8-oxo-deoxyguanosine (8-oxo-dG), an oxidatively modified guanine nucleoside, exerted anti-inflammatory activity through inactivation of the GTP binding protein, Rac. In the present study, the effects of 8-oxo-dG were investigated on responses to antigen challenge in sensitized mice, as Rac is also involved at several steps of the immune process including antigen-induced release of mediators from mast cells.
Experimental approach:
Mice were sensitized and challenged with ovalbumin without or with oral administration of 8-oxo-dG during the challenge. Effects of 8-oxo-dG were assessed by measuring lung function, cells and cytokines in broncho-alveolar lavage fluid (BALF) and serum levels of antigen-specific IgE. Rac activity in BALF cells was also measured.
Key results:
8-oxo-dG inhibited the increased airway resistance and decreased lung compliance of sensitized and challenged mice to the levels of non-sensitized control mice and lowered the increased leukocytes particularly, eosinophils, in BALF. Furthermore, 8-oxo-dG suppressed allergy-associated immune responses, such as raised anti- ovalbumin IgE antibody in serum, increased expression of CD40 and CD40 ligand in lung, increased interleukin-4, -5, -13, interferon-γ and tumour necrosis factor-α in BALF and mRNA levels of these cytokines in BALF cells, dose-dependently. The corresponding purine, 8-oxo-guanine, showed no effects in the same experiments. Finally, 8-oxo-dG, but not 8-oxo-guanine, inhibited the increased Rac activity in sensitized and challenged mice.
Conclusion and implications:
8-Oxo-dG had anti-allergic actions that might be mediated by Rac inactivation. This compound merits further evaluation of its therapeutic potential in allergic asthma.
Keywords: 8-oxo-deoxyguanosine, 8-oxo-guanine, asthma, allergy, Rac, cytokines
Introduction
Thus far, 8-oxo-GTP and 8-oxo-dGTP, nucleotides whose guanine base is oxidized into 7,8-dihydro-8-oxo-guanine (8-oxo-Gua; 7,8-dihydro-8-hydroxy-guanine) by reactive oxygen species (ROS), were regarded simply as mutagenic wastes (Taddei et al., 1997; Hayakawa et al., 1999). However, we recently found that 8-oxo-GTP inactivates the GTP binding protein, Rac, while GTP activates it (Yoon et al., 2005; Kim et al., 2007b). Interestingly, the corresponding nucleosides, 8-oxo-2′-deoxyguanosine (8-oxo-dG) and 8-oxo-guanosine (8-oxo-G), were also able to inactivate Rac (Kim et al., 2006a; Choi et al., 2007; Lee et al., 2009), and thus inhibit Rac-linked functions of phagocytes (Kim et al., 2006a; Lee et al., 2009), which include ROS production, phagocytosis, chemotaxis, cytokine release and NO production. Consistent with these observations, these nucleosides exerted anti-inflammatory actions when they were given to mice treated with lipopolysaccharide (LPS) (Choi et al., 2007). Simultaneously, suppression of Rac activity was observed in lung tissues of these LPS-treated mice (Choi et al., 2007), suggesting that the anti-inflammatory activity was mediated by inactivation of Rac. In these experiments, 8-oxo-dG exhibited higher potency than 8-oxo-G, while the nucleosides, deoxyguanosine, guanosine and adenosine showed no effect (Kim et al., 2006a; Choi et al., 2007; Lee et al., 2009).
Rac is also known to be involved in allergen-induced secretion of histamine and leukotrienes from mast cells (Hong-Geller et al., 2001) and in other steps of the immune process, for example, antigen-presentation by phagocytosis of antigens (Yamauchi et al., 2004) and antigen-induced B cell activation (Walmsley et al., 2003). It is thus postulated that 8-oxo-dG might modulate immune functions. In the present study, we tested this postulate by investigating the anti-allergic effects of 8-oxo-dG in ovalbumin-sensitized mice.
We have already tested the effects of 8-oxo-G, deoxyguanosine, guanosine and adenosine on Rac activity and Rac-associated functions, and found that 8-oxo-G exhibited very weak activity, while deoxyguanosine, guanosine and adenosine were inactive (Kim et al., 2006a; Choi et al., 2007; Lee et al., 2009). However, 8-oxo-guanine (8-oxo-Gua), the corresponding purine, has never been studied in this context. Thus, the effects of 8-oxo-Gua were also tested in the present study. We observed that 8-oxo-dG inhibited responses to antigen challenge in sensitized mice, including airway hyper-responsiveness and rise of ovalbumin-specific IgE in serum, together with suppression of Rac activation. However, 8-oxo-Gua showed no effects on these responses or on Rac activity. These results indicated that 8-oxo-dG had anti-allergic actions, which could be mediated by Rac inactivation
Methods
Animals
All animal care and experiments were approved by the institutional review board and an ethical committee, and conducted in the Laboratory Animal Research Center of Sungkyunkwan University approved by the Association for the Assessment and Accreditation of Laboratory Animal Care. Female Balb/c mice, 6–8 weeks old, were obtained from ORIENT BIO (Seongnam Co, Korea) and maintained in specific pathogen-free conditions.
Sensitization of mice to ovalbumin and treatment with 8-oxo-dG or 8-oxo-Gua
Female BALB/c mice, 6–8 weeks old, were divided into seven groups, and animal numbers of each group were 8 (n= 8) in all experiments. A (sham), mice injected with phosphate-buffered saline (PBS; pH 7.4) and challenged with PBS; B (ovalbumin/PBS), mice sensitized with ovalbumin and challenged with PBS; C, mice sensitized and challenged with ovalbumin; D, E and F (6, 30 and 60 mg, respectively), mice sensitized and challenged as in group C but treated orally with 6, 30 or 60 mg·kg−1 of 8-oxo-dG, respectively, 6 h before every challenge with ovalbumin; and G (8-oxo-Gua), mice sensitized and challenged as in group C but treated with 60 mg·kg−1 of 8-oxo-Gua orally 6 h before every challenge with ovalbumin. These groups of mice are hereafter denoted by the letters shown previously (A–G).
Sensitization and challenge were conducted as previously described (Kim et al., 2007a). For purposes of sensitization, 10 µg ovalbumin adsorbed in 250 µg/200 µL aluminium hydroxide gel adjuvant(Superfos Biosector, Vedbaek, Denmark) or 250 µL PBS were injected into mice intraperitoneally (i.p.) on days 0, 5, 14, 21 and 28. For purposes of the challenge, mice sensitized with PBS or ovalbumin were exposed to nebulized PBS or 2% ovalbumin in PBS, for 10 min once a day for 7 days, beginning on day 35 (1 week after the final injection of ovalbumin) until day 41, and on day 49 as the final challenge. All mice were killed on day 50.
For 8-oxo-dG or 8-oxo-Gua treatment, 8-oxo-dG (6, 30 or 60 mg·kg−1) or 8-oxo-Gua (60 mg·kg−1) was dissolved in PBS and administered orally once a day 6 h before every ovalbumin challenge for 7 days (from day 35 to day 41). The data from group A (sham) or group B (ovalbumin/PBS) mice treated with 8-oxo-dG (6, 30, or 60 mg·kg−1) or 8-oxo-Gua (60 mg·kg−1) are not presented because these compounds did not have any significant effects in these groups. We also chose only the high dose (60 mg·mL−1) of 8-oxo-Gua because low concentrations of 8-oxo-Gua did not show any effect on sensitized and challenged mice (group C) in the preliminary experiments.
Measurement of airway hyper-responsiveness (AHR)
AHR was assessed, as described previously (Choi et al., 2006), by measuring changes in the airway resistance (cmH2O·mL−1·s−1) and lung compliance (mL·cmH2O−1) using the Fexivent system (SCIREQ, Montreal, Quebec, Canada) after mice (eight per group) were treated with methacholine (MCh). Anaesthetized (pentobarbital sodium 70–90 mg·kg−1, i.p.) and tracheostomized (18G cannula) mice were mechanically ventilated (160 breaths·min−1, tidal volume of 10 mL·kg−1, positive end-expiratory pressure of 3 cmH2O). Aerosols of increasing concentrations of MCh (3–25 mg·mL−1) were administered by nebulization. Airway resistance (RL) and lung compliance (CL) were measured at 60 and 120 s after administration of MCh. The results of compliance assays were expressed as % change from the baseline value, which was calculated by (baseline value − value at each MCh concentration)/baseline value × 100.
Collection of broncho-alveolar lavage (BAL) fluid and counting of cells in BAL fluid
Mice were anesthetized with pentobarbital sodium 70–90 mg·kg−1, i.p. Blood (0.5 mL) was collected by cardiac puncture immediately. This blood was used for ovalbumin-specific IgE antibody assay. The tracheas were cannulated, and the lungs were lavaged twice with 1 mL and then 0.8 mL of PBS. The PBS lavage was collected as BAL fluid (85–90% of the input PBS was recovered) and cells were harvested from the BAL fluid using a method described previously (Kim et al., 2007a) with slight modifications. Briefly, BAL fluids were centrifuged at 400×g for 5 min at 4°C. The supernatants were used for assaying various cytokines. Cell pellets obtained were resuspended in PBS and total viable cells were counted using Trypan blue exclusion tests. For differential cell counting, the cells were centrifuged with Cytospin III (Shandon, Pittsburgh, PA), and then stained with Diff-Quik (Sysmex Corp., Kobe, Japan). Differential counting was performed using standard morphological criteria. The BAL cells were also used for activity assays and reverse transcriptase chain reaction (RT-PCR) analysis of the mRNA levels of the various cytokines chosen below.
Assay for ovalbumin-specific IgE antibody in serum
Blood collected by cardiac puncture as above was allowed to clot for 30 min and centrifuged at 900×g for 30 min. The sera obtained were used for ovalbumin-specific IgE antibody assays by enzyme-linked immunosorbent assay (ELISA) as described previously (Kim et al., 2007a).
Immunohistochemistry for CD40 and CD40 ligand (CD40L)
After BAL, mouse lungs were perfused with 5 mL PBS via the right ventricle. Left lungs were removed from the chest cavity and fixed in 4% paraformaldehyde. The lobes of the left lungs were isolated, embedded in paraffin and sectioned at 3 µm. Tissue sections were incubated in 3% hydrogen peroxide for 5 min to quench endogenous peroxidase activity, blocked with 1% bovine serum albumin (BSA) in PBS for 1 h, and then incubated with anti-CD40 or anti-CD40L antibody (1:25 dilution, NeoMarkers, Fremont, CA) in blocking solution at 4°C for 24 h. After washing with PBS, the tissue sections were treated with biotinylated secondary antibody for 10 min, streptavidin-horseradish peroxidase (HRP) for 10 min and 3, 3′-diaminobenzidine, a chromogen substrate for 5–10 min; the sections were then counterstained with haematoxylin and examined microscopically under 200× magnifications (Kim et al., 2007a). The intensity of brown colour in peribronchial and perivascular areas in each tissue section was scored into four grades, which were 0, normal (no colour); 1, slight; 2, mild; 3, moderate; and 4, dark. Scoring of sections was carried out without knowledge of the treatments. The grading data obtained were presented as histograms.
Lung tissue preparation and lung histology with haematoxylin and eosin (H & E) staining
After BAL, mouse lungs were perfused with 5 mL PBS via the right ventricle. Left lungs were removed from the chest cavity and fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 3 µm. Tissue sections were stained with H & E (Kim et al., 2007a) to examine inflammatory reaction in lung tissue. Histological appearances of lung tissues were scored into four grades in terms of peribronchial infiltration of inflammatory cells, without knowledge of the treatments. The summary scores of each group (n= 8) are presented as histograms.
Cytokine levels in BAL fluid and lung tissue
The protein levels of interleukin (IL)-4, IL-5, IL-13, tumour necrosis factor (TNF)α and interferon (IFN)γ in BAL fluid (50 µL) were determined using an ELISA kit for each cytokine (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Lung tissues were homogenized in PBS using a Polytron (Kinematica, Littau, Switzerland) and centrifuged (770×g, 10 min). The levels of IL-5 in the lung tissue homogenates (100 mg·100 µl−1) were also determined by ELISA, as previously described. The mRNA levels of these cytokines in BAL cells were determined by RT-PCR. The experimental procedures are presented in the supplementary information.
Rac assay
Rac activity in BAL cells was assayed as previously described (Yoon et al., 2005) using Rac activation kits (Upstate Biotechnology, Lake Placid, NY). BAL cells (1 × 107 cells in 700 µL) were suspended in 0.5 mL of a lysis buffer [25 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1% Nonidet P(NP)-40, 1 mM DL-dithiothreitol(DTT), 5% glycerol, 1 mM phenylmethylsulphonyl fluoride(PMSF), 1 µg·mL−1 aprotinin, 1 µg·mL−1 leupeptin] for 30 min on ice, and supernatants were obtained by centrifugation (13 000×g for 20 min). According to the manufacturer's protocol, the active form of Rac was obtained from the supernatants by affinity precipitation using Pak-1 Rac-binding domain (an effector of Rac), which was fused to GST (glutathione-S-transferase) and visualized by immunoblotting with anti-rabbit Rac1 (1:1000; PIERCE). To measure total Rac protein, supernatants were directly used for immunoblotting. The band densities were quantified using the BAS 2500 imaging analyzer (Fuji Photo Film, Japan). The experiments were repeated four times. In each experiment, seven mice were used, each of which was randomly chosen out of eight mice of each group (A∼G) and a representative result of the four experiments was presented.
Immunoblot analysis of Jun N-terminal kinase (JNK) kinase
Immunoblot analysis of JNK kinase was performed as described earlier (Kim et al., 2007a). BAL cells (1 × 106 cells in 100 µL) were homogenized in a low-salt lysis buffer [10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 2.0 µg·mL−1 aprotinin, 2.0 µg·mL−1 leupeptin] using a Polytron (Kinematica, Littau, Switzerland) and incubated on ice for 10 min. After centrifugation, supernatants obtained as cell lysates (20 µg) were subjected to 10% SDS-PAGE, and the proteins in the gel were transferred to polyvinylidene fluoride membranes (Schleicher & Schuell, Dassel, Germany). Membranes were washed with Tris-buffered saline containing 0.1% Tween 20 (TBST) and then blocked for 1 h in 5% skim milk in TBST. After the membranes were washed with TBST, they were incubated for 60 min at room temperature with antibodies against JNK (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or against their phosphorylated forms, p-JNK (Cell signalling, Beverly, MA) diluted with TBST (1:1000). Membranes were washed with TBST and treated with HRP-conjugated goat anti-mouse or HRP-conjugated rabbit anti-goat IgG (diluted to 1:5000–1:10 000) (Zymed Laboratory Inc., San Francisco, CA) in TBST for 60 min. After washing, the protein bands were visualized by enhanced chemiluminescence (Amersham Biosciences UK Limited, Little Chalfont, Buckinghamshire, UK). The experiments were repeated four times, as in the Rac assay, and a representative result of the four experiments was presented.
Statistical analysis
Unless otherwise mentioned, experimental data are presented as mean ± SE and multiple group comparisons were performed using a one-way analysis of variance (anova) followed by Scheffe's post hoc test using the SPSS statistic program (SPSS Inc., Chicago, IL). P < 0.05 were regarded as significant.
Materials
8-oxo-dG and 8-oxo-Gua were purchased from Berry & Associates, Dexter, MI; ovalbumin (Grade V) from Sigma-Aldrich, St. Louis, MO; DTT, methacholine, leupeptin, aprotinin and PMSF from Sigma (St. Louis, MO), Tween 20 and NP-40 (Amresco, Solon, OH) and aluminium hydroxide gel adjuvant (Superfos Biosector, Vedbaek, Denmark).
Results
Effect of 8-oxo-dG on AHR to MCh
First, we examined the effect of 8-oxo-dG on AHR, a common symptom in allergic asthma. AHR was provoked by nebulization of MCh aerosol and assessed by the changes in RL and CL. The RL of ovalbumin-sensitized and challenged mice (group C) showed a dramatic increase after exposure to nebulized MCh (Figure 1, upper panel) compared to that of PBS-sensitized and challenged mice (group A; Figure 1 upper panel). However, this MCh-induced increase of RL in ovalbumin-sensitized/challenged mice was effectively reduced by 8-oxo-dG treatment. The maximum effect was attained at 30 mg·kg−1 8-oxo-dG. The lower panel of Figure 1 shows the CL, which was dramatically decreased in ovalbumin-sensitized and challenged mice compared to that of PBS-sensitized and challenged mice. This decrease was also effectively prevented by 8-oxo-dG. The data for 6 mg·kg−1 of 8-oxo-dG were approximately in the middle between 30 mg·kg−1 8-oxo-dG and 60 mg·kg−1 8-oxo-Gua (data not shown). However, 8-oxo-Gua (60 mg·kg−1) had no effect on either RL or CL.
Figure 1.
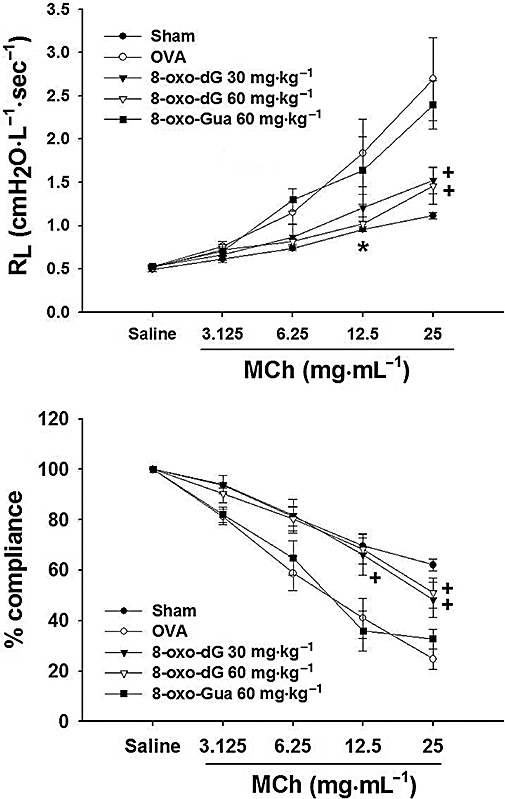
Effects of 8-oxo-dG on AHR of mice sensitized to ovalbumin. Mice were sensitized and then challenged with ovalbumin and AHR was assessed by measuring changes of airway resistance (RL) (upper panel) and lung compliance (lower panel) using the Fexivent system. After each MCh nebulization, lung resistance (cmH2O·L−1·s−1) and compliance (mL·cmH2O−1) were measured and expressed as cmH2O L−1·s−1 and % of the saline-nebulized group respectively. Sham represents mice sensitized and challenged with PBS (group A); OVA represents mice sensitized and challenged with ovalbumin (group C); 8-oxo-dG 30 mg and 8-oxo-dG 60 mg·kg−1, represent mice sensitized and challenged with ovalbumin as in group C but treated orally with 8-oxo-dG at these doses 6 h before every ovalbumin challenge (groups D and E); 8-oxo-Gua 60 mg·kg−1 represents mice as in group C but treated orally with 60 mg·kg−1 8-oxo-Gua 6 h before every ovalbumin challenge (group G). Data are mean ± SE of n= 8. * and +; P < 0.05 and P < 0.01 versus ovalbumin, respectively.
Effect of 8-oxo-dG on recruitment of inflammatory cells into BAL fluid
The results are shown in Figure 2 and Table S1. Leukocyte numbers (total and individual leukocytes) in BAL fluid of ovalbumin-sensitized and challenged mice (group C) were significantly increased compared to those of the PBS-sensitized and challenged control (group A) or ovalbumin-sensitized and PBS challenged mice (group B). The order of increase was eosinophils, neutrophils, lymphocytes and macrophages. However, the increased total and individual cell numbers were reduced by administration of 8-oxo-dG (6, 30 or 60 mg·kg−1; groups D, E and F, respectively) significantly and dose-dependently. Among the individual leukocytes, eosinophils showed the most significant decrease. In contrast, 8-oxo-Gua (60 mg·kg−1; group G) was unable to inhibit the increase of the cell numbers.
Figure 2.
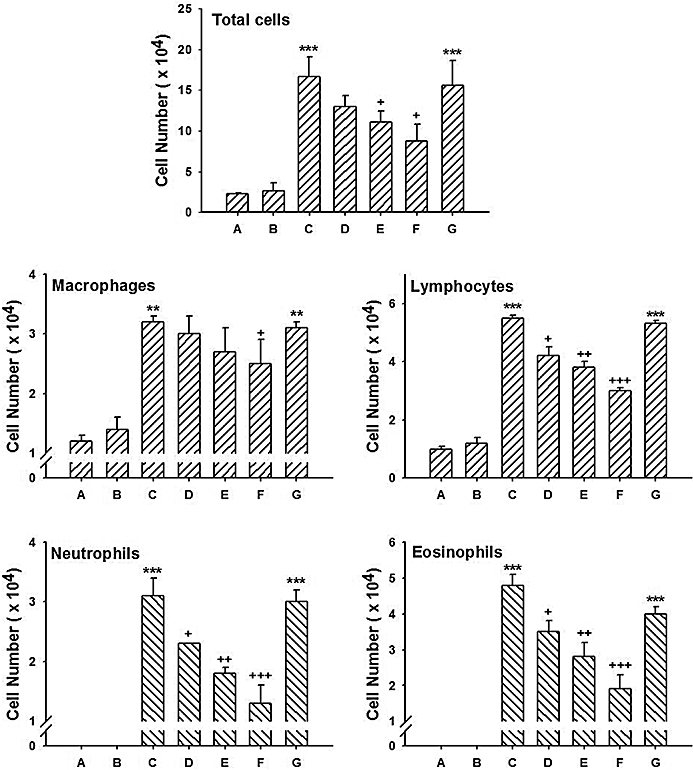
Effects of 8-oxo-dG on recruitment of leukocytes into BAL fluid in mice sensitized to ovalbumin. Sensitization, 8-oxo-dG treatment and cell counts were as described in Methods. The letters below the bars refer to treatment groups. Group A is mice sensitized and challenged with PBS; B, mice sensitized with ovalbumin and challenged with PBS; C, mice sensitized and challenged with ovalbumin; D, E and F, mice as in group C but treated orally with 6, 30 or 60 mg·kg−1 8-oxo-dG, respectively; G, mice as in group C but treated orally with 60 mg·kg−1 8-oxo-Gua. Data expressed are mean ± SE (n= 8). ** and ***; P < 0.01 and P < 0.001, respectively versus group A or group B. +, ++ and +++; P < 0.05, P < 0.01 and P < 0.001, respectively versus group C.
Effect of 8-oxo-dG on serum ovalbumin-specific IgE antibody level
In order to confirm the anti-allergic activity of 8-oxo-dG, the effect of 8-oxo-dG on serum anti-ovalbumin IgE levels was examined. As illustrated in Figure 3 and Table S2, serum IgE levels in the group B mice (sensitized to ovalbumin, challenged with PBS) were higher than those in group A (sensitized and challenged with PBS). However these levels were increased further in group C (sensitized and challenged with ovalbumin). Administration of 8-oxo-dG (6, 30 or 60 mg·kg−1; groups D, E and F, respectively) reduced the elevated IgE level in group C mice, dose-dependently. In contrast to 8-oxo-dG, 8-oxo-Gua (60 mg·kg−1; group G) did not have a significant effect on the IgE levels.
Figure 3.

Effects of 8-oxo-dG on serum ovalbumin-specific IgE levels in mice sensitized to ovalbumin. Experimental details are described in Methods. The letters below the bars refer to treatment groups. Group A is mice sensitized and challenged with PBS; B, mice sensitized with ovalbumin and challenged with PBS; C, mice sensitized and challenged with ovalbumin; D, E and F, mice as in group C but treated orally with 6, 30 or 60 mg·kg−1 8-oxo-dG, respectively; G, mice as in group C but treated orally with 60 mg·kg−1 8-oxo-Gua. Data are expressed as mean ± SE (n= 8). ***; P < 0.001 versus group A +, ++ and +++; P < 0.05, P < 0.01 and P < 0.001, respectively versus group C (OVA).
Effect of 8-oxo-dG on expression of CD40 and CD40L in lung tissues
The observation that 8-oxo-dG suppressed the increase of ovalbumin-specific IgE levels in the ovalbumin-sensitized and challenged mice (Figure 3) strongly suggested that 8-oxo-dG was able to inhibit immune reactions. To confirm this further, we examined its effect on expression of CD40 and CD40L in lung tissues of sensitized mice. CD40L, a cell-surface protein expressed on helper T cells or mast cells, interacts with its receptor CD40 on B cells. Their interaction stimulates B cell functions including IgE production. The results are shown in Figure 4. Expression of CD40 (Figure 4 upper panel) and CD40L (Figure 4 middle panel) were increased in group C (ovalbumin-sensitized and challenged) compared to those in group A and group B. However, 8-oxo-dG (6, 30 or 60 mg·kg−1; groups D, E and F, respectively) reduced staining intensities (dark brown) for both CD40 and CD40L molecules. The staining intensities were quantified and presented in the histograms, which show significant and dose-dependent reductions of staining intensities. However, 8-oxo-Gua (60 mg·kg−1; group G) had no effect on staining for CD40 or CD 40L (shown only as summary data in the histograms).
Figure 4.

Effect of 8-oxo-dG on CD40 or CD40L expression and inflammatory reaction in the lung tissues of mice sensitized to ovalbumin. Lung tissues were removed from mice and immunohistochemistry for CD40 and CD40L and H & E staining were carried out as described in Materials and Methods. Upper panel, CD40; middle panel, CD40L and lower panel, H & E staining. The stained tissues were observed microscopically under 200× magnification; bars in group A indicate 100 µm. The intensity of IHC colour developed in peribronchial and perivascular areas and peribronchial infiltration of inflammatory cells in H &E stained tissues were scored into four grades, which were 0, normal; 1, slight; 2, mild; 3, moderate; and 4, severe. Summary data are presented as histograms to the right of the histological sections. The letters below the bars refer to treatment groups. Group A is mice sensitized and challenged with PBS; B, mice sensitized with ovalbumin and challenged with PBS; C, mice sensitized and challenged with ovalbumin; D, E and F, mice as in group C but treated orally with 6, 30 or 60 mg·kg−1 8-oxo-dG, respectively; G, mice as in group C but treated orally with 60 mg·kg−1 8-oxo-Gua. The data obtained from group G (8-oxo-Gua) is shown only in the histogram. Data in the histograms are mean ± SE of n= 8. ***, P < 0.001 versus group A or group B. ++ and +++; P < 0.01 and P < 0.001, respectively versus group C.
Effect of 8-oxo-dG on histology of lung tissues
The lungs from the group C mice (sensitized and challenged with ovalbumin) model should feature peribronchial inflammation, but this was not demonstrated clearly by the expression of CD40/CD40L (see previous discussion) as the localization of expression appeared to be perivascular. However, peribronchial inflammation was confirmed by H & E staining of lung tissues. As shown in Figure 4 (lowest panel), sections from lungs of group C mice showed marked infiltration of inflammatory cells into peribronchial regions, hypertrophy of bronchial smooth muscle, and epithelial thickening, compared to those from sham (group A) and group B mice (sensitized to ovalbumin, challenged with PBS). Summary data from the scores for H&E staining are presented as histograms (lowest graph, Figure 4). These inflammatory reactions were reduced significantly and dose-dependently by 8-oxo-dG (6, 30 or 60 mg·kg−1, D, E and F respectively).
Effects of 8-oxo-dG on protein and their mRNA levels of cytokines
In order to confirm that 8-oxo-dG exerted anti-allergic activity, we examined the effects of 8-oxo-dG on protein levels of several cytokines (IL-4, IL-5, IL-13, IFN-γ and TNF-α) in BAL fluid (BALF) as well as their mRNA levels in BAL cells. As shown in Figure 5, the protein levels of all cytokines were significantly elevated in BALF of group C mice compared to that of mice in groups A or B. However, 8-oxo-dG (6, 30 or 60 mg·kg−1; groups D, E and F, respectively) reduced the elevated levels in mice sensitized and challenged with ovalbumin, significantly and dose-dependently. The effect of 8-oxo-dG on IL-5 level was also examined in the lung tissues [right-bottom panel (Lung Tissue) in Figure 5]. As in BALF, IL-5 level was also elevated in the lung tissues of group C and 8-oxo-dG (6, 30 or 60 mg·kg−1; groups D, E and F, respectively) reduced the elevated IL-5 level in the lung tissues significantly, confirming that 8-oxo-dG inhibited the inflammatory reaction in lung tissues of mice sensitized and challenged with ovalbumin. 8-oxo-Gua (60 mg·kg−1; group G) had no effect in both BALF and lung tissue. The results of cytokine levels were also presented as a table (Table S3) in the supplementary.
Figure 5.

Effects of 8-oxo-dG on cytokine proteins in BAL fluid and lung tissues of mice sensitized to ovalbumin. Cytokine levels in BAL fluids (BALF) and lung tissues (Lung Tissue) were determined by ELISA as described in Methods. The letters below the bars refer to treatment groups. Group A is mice sensitized and challenged with PBS; B, mice sensitized with ovalbumin and challenged with PBS; C, mice sensitized and challenged with ovalbumin; D, E and F, mice as in group C but treated orally with 6, 30 or 60 mg·kg−1 8-oxo-dG, respectively; G, mice as in group C but treated orally with 60 mg·kg−1 8-oxo-Gua. Data are expressed as mean ± SE (n= 8). ***; P < 0.001 versus group A (sham) or group B (OVA/PBS). +, ++ and +++; P < 0.05, P < 0.01 and P < 0.001, respectively versus group C (OVA).
Effects of 8-oxo-dG on activation of Rac and Rac-linked kinase in BAL cells
Finally, we tested the effect of 8-oxo-dG on Rac activity in mice sensitized and challenged with ovalbumin. As shown in Figure 6, Rac was markedly activated in group C mice, compared to that in group A and group B mice. But this activation was inhibited dose-dependently by 8-oxo-dG (6, 30 or 60 mg·kg−1; groups D, E and F respectively). As expected, 8-oxo-Gua had no effect on Rac activation. Figure 6 also shows the effect of 8-oxo-dG on the activity of JNK (assessed by formation of its phosphorylated form), which is a downstream effector protein of Rac. The results were similar to those of Rac activity and thus, provide strong evidence that 8-oxo-dG suppressed the activation of Rac in the ovalbumin-sensitized and challenged mice.
Figure 6.

Effects of 8-oxo-dG on activation of Rac and Rac-linked kinases in BAL cells of mice sensitized to ovalbumin. Collection of cells from BALF and activity assays of Rac1 and JNK were performed as described in Methods. The letters above the record refer to treatment groups. Group A is mice sensitized and challenged with PBS; B, mice sensitized with ovalbumin and challenged with PBS; C, mice sensitized and challenged with ovalbumin; D, E and F, mice as in group C but treated orally with 6, 30 or 60 mg·kg−1 8-oxo-dG, respectively; G, mice as in group C but treated orally with 60 mg·kg−1 8-oxo-Gua. The experiments were repeated four times. In each experiment, eight mice were used, each of which was randomly chosen out of each group of A∼G (n= 8) and a representative result of the four experiments was presented. Numerical values below the record with * are the ratio of band density of each group versus that of group A (sham), normalized to the band density of total Rac1.
Discussion and conclusions
In the present study, we demonstrated the anti-allergic activity of 8-oxo-dG in the lungs of ovalbumin-sensitized and challenged mice by showing effective inhibition of the AHR shown to MCh (Figure 1), along with a dramatic reduction of increased eosinophil count in the BALF (Figure 2) and of increased inflammatory cells in the lung tissues (Figure 4 lower panel). The anti-allergic activity of 8-oxo-dG was further supported by the immunological data, showing a inhibition of increased expression of CD40 and CD40L upon treatment with 8-oxo-dG (Figure 4 upper and lower panels) and a subsequent suppression of increased ovalbumin-specific IgE levels (Figure 3). CD40L is a surface protein of helper T cells and mast cells, and CD40 is a surface protein of dendritic cells, macrophages and B cells. Thus, CD40L-CD40 interaction results in activation of T cells and mast cells by antigen-presenting dendritic cells (Grewal and Flavell, 1998; Gonzalez-Carmona et al., 2008), activation of macrophages to destroy engulfed microbes (Qin et al., 2005) and stimulation of B cells to produce antibodies (Lipsky et al., 1997). Therefore, inhibition of increased expression of CD40 and CD40L by 8-oxo-dG in ovalbumin-sensitized and challenged mice suggests inhibition of these effector functions of helper T cells, leading to suppression of immune function during this allergic condition. Inhibition of the rise of IgE level in serum (Figure 3) was one of the consequences of this suppression of immune function.
8-Oxo-dG also inhibited the increased protein levels of inflammatory cytokines (IL-4, IL-5, IL-13, IFN-γ and TNF-α) in BAL fluid and lung tissue and their mRNA levels in BAL cells of these ovalbumin-sensitized and challenged animals (Figure 5 and Figure S1). Th2 cells play a central role in allergic immune processes by secreting IL-4, IL-5 and IL-13. IL-4 is a major cytokine for IgE production by stimulating B cell Ig heavy chain class switching to the IgE isotype (Lebman and Coffman, 1988). IL-5 is a major stimulator of growth and differentiation of eosinophils and a major activator of mature eosinophils (Takatsu and Nakajima, 2008). IL-13 stimulates mucus production by lung epithelial cells and may play a role in asthma (Fulkerson et al., 2006; Kasaian and Miller, 2008). Knockout mice lacking IL-13 receptor showed decreased IgE production and allergic reaction (Munitz et al., 2008). Therefore, the effects of 8-oxo-dG in suppressing the increases of protein and mRNA levels of IL-4, IL-5, IL-13 (Figure 5 and Figure S1) are more evidence of the anti-allergic action of 8-oxo-dG. IFN-γ (Smart and Kemp, 2002) and TNF-α (Howarth et al., 2005) are also involved in asthma and atopic diseases. Figure 1 shows that in addition to suppressing eosinophil counts, 8-oxo-dG suppressed the increased counts of neutrophils and macrophages in BAL fluid of the allergic mice. This seems to be related to inhibition of TNF-α increase since the principal function of TNF-α is to stimulate recruitment of neutrophils and monocytes to sites of inflammation (Lukacs et al., 1995).
Figure 6 shows that 8-oxo-dG inhibited the allergy-induced activation of Rac effectively and dose-dependently (Figure 6). p-JNK, a downstream effector of Rac, also showed a behaviour similar to Rac, further supporting the inhibition of Rac by 8-oxo-dG. We have already reported that 8-oxo-dG showed anti-inflammatory activity by inactivating Rac in LPS-treated mice (Choi et al., 2007) and we assumed that Rac inactivation was also the principal factor involved in the anti-allergic activity of 8-oxo-dG observed in this study. As mentioned earlier, Rac may be involved in at least three steps during the progression of immune reactions in allergy. At present, we can neither provide direct evidence for the involvement of Rac at each step in the anti-allergic action of 8-oxo-dG, nor can we quantify the contribution of Rac at each step. However, we have provided indirect evidence, showing that 8-oxo-dG inhibited Rac, and exerted anti-allergic and immune suppressive effects, while 8-oxo-Gua did not inhibit Rac and showed very weak or negligible effects on all the symptoms observed in the ovalbumin-sensitized and challenged mice.
8-oxo-dG appeared to block virtually all the variables measured, raising the question of which was the key mediator or pathway inhibited. However, this would be difficult to identify, particularly in whole animal experiments because Rac is linked to many proteins and thus, is involved in many signalling pathways. Production of ROS from phagocytes (Bromberg et al., 1994) and secretion of the allergic mediators from mast cells (Hong-Geller et al., 2001) are typical Rac-linked functions and thus, might be primary targets of 8-oxo-dG. ROS and the allergic mediators affect a variety of proteins or signalling pathways. Therefore, inhibition of the secretion of ROS and allergic mediators by 8-oxo-dG are likely to induce a number of changes, as observed in this study.
Incidences of various allergic disorders are increasing but therapeutic measures for them are not still satisfactory. The results obtained from the present study suggest that 8-oxo-dG could be a potential anti-allergic candidate for further confirmatory studies. The possibility of 8-oxo-dG acting as a mutagen was excluded, as 8-oxo-dG was not phosphorylated to the corresponding nucleotide, 8-oxo-deoxyguanosine monophosphate (8-oxo-dGMP) and thus was not incorporated into DNA (Kim and Chung, 2006; Kim et al., 2006b).
Acknowledgments
This work was funded by grants from the Korean Ministry of Science & Technology (Grant 2009-0065519) and from Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine (Grant SBRI S-M 200106).
Glossary
Abbreviations:
- 8-oxo-dG
7,8-dihydro-8-oxo-deoxyguanosine (7,8-dihydro-8-hydroxy-deoxyguanosine)
- 8-oxo-Gua
7,8-dihydro-8-oxo-guanine (7,8-dihydro-8-hydroxy-guanine)
- AHR
airway hyper-responsiveness
- BAL
broncho-alveolar lavage
- CD40L
CD40 ligand
- CL
lung compliance
- DTT
DL-dithiotreitol
- GST
glutathione-S-transferase
- IFNγ
interferon γ
- IL
interleukin
- MCh
methacholine
- NP-40
Nonidet P-40
- PMSF
phenylmethanesulphonyl fluoride
- RL
airway resistance
- ROS
reactive oxygen species
- TNFα
tumor necrosis factor α
Statement of conflicts of interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Effects of 8-oxo-dG on expression of various cytokine mRNAs in BAL cells of ovalbumin (OVA) sensitized mice. Experimental details were described in Methods. A, sham; B OVA/PBS; C, OVA; D, E and F, OVA mice (group C) orally administered 6, 30, and 60 mg·kg−1 8-oxo-dG, respectively; G (8-oxo-Gua), OVA mice orally administered 60 mg·kg−1 8-oxo-Gua. The entire experiments were repeated four times by choosing a mouse randomly out of each group of A∼G (n = 8, each) and a representative result of the four experiments was presented. Numbers are the ratio of density of the band of each group versus that of sham (A), normalized to the band density of GAPDH mRNA.
Table S1 Effects of 8-oxo-dG on recruitment of leukocytes into BAL fluid in ovalbumin (OVA)-induced allergic mice.
Table S2 Effects of 8-oxo-dG on serum ovalbumin (OVA)-specific IgE levels in OVA-induced allergic mice.
Table S3 Effects of 8-oxo-dG on cytokine protein levels in BAL fluid and lung tissues.
Supplementary Text Methods. Reverse-transcriptase polymerase chain reaction (RT-PCR) analysis of cytokines.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Bromberg Y, Shani E, Joseph G, Gorzalczany Y, Sperling O, Pick E. The GDP-bound form of the small G protein Rac1 p21 is a potent activator of the superoxide-forming NADPH oxidase of macrophage. J Biol Chem. 1994;269:7055–7058. [PubMed] [Google Scholar]
- Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, et al. Intranasal delivery of cytoplasmic domain of CTLA-4 using a novel protein transduction. Nat Med. 2006;12:574–579. doi: 10.1038/nm1385. [DOI] [PubMed] [Google Scholar]
- Choi S, Choi HH, Lee SH, Ko SH, You HJ, Ye SK, et al. Anti-inflammatory effects of 8-hydroxy-2′-deoxyguanosine on lipopolysaccharide-induced inflammation via Rac suppression in Balb/c mice. Free Rad Biol Med. 2007;43:1594–1603. doi: 10.1016/j.freeradbiomed.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Fulkerson PC, Fischetti CA, Hassman LM, Nikolaidis NM, Rothenberg ME. Persistent effects induced by IL-13 in the lung. Am J Respir Cell Mol Biol. 2006;35:337–346. doi: 10.1165/rcmb.2005-0474OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Carmona MA, Lukacs-Kornek V, Timmerman A, Shabani S, Kornek M, Vogt A, et al. CD40ligand-expressing dendritic cells induce regression of hepatocellular carcinoma by activating innate and acquired immunity in vivo. Hepatology. 2008;48:157–168. doi: 10.1002/hep.22296. [DOI] [PubMed] [Google Scholar]
- Grewal IS, Flavell RA. CD40, CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Hofer A, Thelander L, Kitajima S, Cai Y, Oshiro S, et al. Metabolic fate of oxidized guanine ribonucleotides in mammalian cells. Biochemistry. 1999;38:3610–3614. doi: 10.1021/bi982361l. [DOI] [PubMed] [Google Scholar]
- Hong-Geller E, Holowka D, Siraganian RP, Baird B, Cerione RA. Activated Cdc42/Rac reconstitutes Fcepsilon RI-mediated Ca2+ mobilization and degranulation in mutant RBL mast cells. Proc Natl Acad Sci USA. 2001;98:1154–1159. doi: 10.1073/pnas.98.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60:1012–1018. doi: 10.1136/thx.2005.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaian MT, Miller DK. IL-13 as a therapeutic target for respiratory disease. Biochem Pharmacol. 2008;76:147–155. doi: 10.1016/j.bcp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Kim DY, Ryu SY, Lim JE, Lee YS, Ro JY. Anti-inflammatory mechanism of simvastatin in mouse allergic asthma model. Eur J Pharmacol. 2007a;557:76–86. doi: 10.1016/j.ejphar.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Yoon SH, Ryu HO, Yoon BH, Choi S, Ye SK, et al. 8-Oxo-7, 8-dihydroguanosine triphosphate (8-oxoGTP) down-regulates respiratory burst of neutrophils by antagonizing GTP toward Rac, a small GTP binding protein. Free Radic Res. 2007b;41:655–662. doi: 10.1080/10715760701250270. [DOI] [PubMed] [Google Scholar]
- Kim HS, Ye SK, Cho IH, Jung JE, Kim DH, Choi S, et al. 8-Hydroxydeoxyguanosine suppresses NO production and COX2 activity via Rac1/STATs signaling in LPS-induced brain microglia. Free Radic Biol Med. 2006a;41:1392–1403. doi: 10.1016/j.freeradbiomed.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Kim JE, Chung MH. 8-Oxo-7,8-dihydro-2′-deoxyguanosine is not salvaged for DNA synthesis in human leukemic U937 cells. Free Radic Res. 2006;40:461–466. doi: 10.1080/10715760600570539. [DOI] [PubMed] [Google Scholar]
- Kim JE, Hyun JW, Hayakawa H, Choi S, Choi J, Chung MH. Exogenous 8-oxo-dG is not utilized for nucleotide synthesis but enhances the accumulation of 8-oxo-Gua in DNA through error-prone DNA synthesis. Mutat Res. 2006b;596(1–2):128–136. doi: 10.1016/j.mrfmmm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Lebman DA, Coffman RL. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988;168:853–862. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Han ST, Choi SW, Sung SY, You HJ, Ye SK, et al. Inhibition of Rac and Rac-linked functions by 8-oxo-2′-deoxyguanosine in murine macrophages. Free Radic Res. 2009;43:78–84. doi: 10.1080/10715760802609432. [DOI] [PubMed] [Google Scholar]
- Lipsky PE, Attrep JF, Grammer AC, McIlraith MJ, Nishioka Y. Analysis of CD40-CD40 ligand interactions in the regulation of human B cell function. Ann N Y Acad Sci. 1997;815:372–383. doi: 10.1111/j.1749-6632.1997.tb52088.x. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Chensue SW, Widmer M, Kunkel SL. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J Immunol. 1995;154:5411–5417. [PubMed] [Google Scholar]
- Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci USA. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Wilson CA, Lee SJ, Zhao X, Benveniste EN. LPS induces CD40 gene expression through the activation of NF-kappaB and STAT-1alpha in macrophages and microglia. Blood. 2005;106:3114–3122. doi: 10.1182/blood-2005-02-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart JM, Kemp AS. Increased Th1 and Th2 allergen-induced cytokine responses in children with atopic disease. Clin Exp Allergy. 2002;32:796–802. doi: 10.1046/j.1365-2222.2002.01391.x. [DOI] [PubMed] [Google Scholar]
- Taddei F, Hayakawa H, Bouton M, Cirinesi A, Matic I, Sekiguchi M, et al. Counteraction by MutT protein of transcriptional errors caused by oxidative damage. Science. 1997;278:128–130. doi: 10.1126/science.278.5335.128. [DOI] [PubMed] [Google Scholar]
- Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288–294. doi: 10.1016/j.coi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Walmsley MJ, Ooi SK, Reynolds LF, Smith SH, Ruf S, Mathiot A, et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- Yamauchi A, Kim C, Li S, Marchal CC, Towe J, Atkinson SJ, et al. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol. 2004;173:5971–5979. doi: 10.4049/jimmunol.173.10.5971. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Hyun JW, Choi J, Choi EY, Kim HJ, Lee SJ, et al. In vitro evidence for the recognition of 8-oxoGTP by Ras, a small GTP-binding protein. Biochem Biophys Res Commun. 2005;327:342–348. doi: 10.1016/j.bbrc.2004.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


