Abstract
Background and purpose:
Although there are many new specific phosphodiesterase inhibitors with anti-inflammatory activity, none have yet reached the market because of their low therapeutic efficacy. Our study was aimed to evaluate the anti-inflammatory and anti-arthritic effect of an established phosphodiesterase inhibitor, theophylline, and to investigate the effect of the nitric oxide (NO) donor, sodium nitroprusside (SNP) or NO synthase inhibitor, L-NG-monomethyl arginine (L-NMMA) on its actions.
Experimental approach:
The effects of theophylline alone and combined with SNP or L-NMMA on the pathogenesis of adjuvant-induced arthritis in rats were evaluated.
Key results:
Prophylactic or therapeutic doses of theophylline significantly ameliorated the pathogenesis of adjuvant arthritis in rats as evidenced by a significant decrease in the arthritis index, hind paws volume, ankle joint diameter, fever, body weight loss and hyperalgesia in a dose-dependent manner. Inflammatory cellular infiltrate in synovium of ankle joint and pannus formation were also markedly inhibited. Interleukin-10 (IL-10) levels were significantly increased in arthritic rats given theophylline alone or in combination with either SNP or L-NMMA. Co-administration of a low dose of SNP or L-NMMA enhanced significantly the anti-inflammatory and anti-arthritic effect of theophylline. In contrast, a high dose of SNP counteracted the anti-inflammatory and anti-arthritic effects of theophylline.
Conclusions and Implication:
These findings confirm the anti-inflammatory and anti-arthritic activities of theophylline and suggest a new approach to enhance the anti-inflammatory and anti-arthritic effects of theophylline would be to administer it in combination with a low dose of a NO donor or a non-specific NO synthase inhibitor.
Keywords: phosphodiesterase inhibitor, adjuvant arthritis, NO synthase inhibitor, nitric oxide donor
Introduction
Rheumatoid arthritis (RA) is an autoimmune disorder of unknown cause and is characterized by chronic inflammation of the synovial joints, which leads to the destruction of articular cartilage and bone (Pincus and Callahan, 1993). Although there are reasonably good drugs used in the symptomatic relief of arthritis (e.g. non-steroidal anti-inflammatory drugs), there are few safe drugs, which modify the fundamental pathological processes responsible for chronic inflammation (Cash and Klippel, 1994)
Phosphodiesterase (PDE) inhibitors have been regarded as promising drugs to be used in asthma therapy because they are known to suppress asthmatic immunopathology caused by chronic inflammatory and immune responses (Giembycz, 2007). At the same time, this approach was also expanded to include PDE inhibitors as possible agents to treat other chronic inflammatory diseases such as RA, because of the elevation of intracellular level of cyclic AMP in leukocytes, which is accompanied by inhibition of the production of tumour necrosis factor–α (TNF-α; Teixeira et al., 1997). It has been reported that both nonselective as well as PDE4-specific inhibitors are effective in ameliorating autoimmune disease in different experimental models of autoimmune encephalomyelitis (Sommer et al., 1995) and collagen-induced arthritis (Nyman et al., 1997). However, the therapeutic utility of PDE4 inhibitors and their new structural classes to suppress inflammation has not been evaluated until now due to lack of tolerance (Giembycz, 2008; Spana, 2008)
A set of conflicting data has been generated over the years regarding the anti-inflammatory effect of theophylline. It has been reported that theophylline fails to reduce the acute inflammatory response in the adjuvant-treated paw of rats and slightly inhibits the chronic inflammation in the contralateral (untreated) hind paws of rats (Bonta et al., 1978). Furthermore, Abdel-Salam et al. (2003) reported that pentoxifylline, not theophylline, inhibited carrageenan-induced oedema in rats. In contrast, Kumar et al. (2000) found that theophylline as well as rolipram exerted dose-dependent analgesic and anti-inflammatory effects against acetic acid-induced writhing in mice and carrageenan-induced paw oedema in rats. More recently, it has been demonstrated that theophylline, not pentoxifylline has a marked anti-inflammatory effect in carrageenan-induced oedema in the rat footpad and that the glucocorticoid-glucocorticoid receptor system is involved in this effect (Watanabe et al., 2008). Additional studies have reported that theophylline possesses anti-inflammatory activities and inhibits the production of free oxygen radicals by human monocytes via inhibition of PDE (Chorostowska-Wynimko et al., 2007; Kanehara et al. (2008)
The mechanism of the anti-inflammatory effect of PDE inhibitors has been studied both in vivo and in vitro. Many authors have reported that PDE inhibitors inhibit nitric oxide (NO) production by macrophages in vivo and in vitro (Beshay et al., 2001; Jae et al., 2004). Indeed, cyclic adenosine monophosphate-elevating PDE inhibitors can influence the activation of inducible NO synthase (iNOS) in different cell types in vitro and their potent anti-inflammatory effects in experimental models of disease and clinical studies have frequently been accompanied by a marked modulation of NO production (Markovic et al., 2003). Yoshikawa et al. (2002) found that all types of PDE inhibitors from I to V (specific and non-specific) suppressed the inducible NO synthase enzyme and the production of NO by mouse microglia and astrocytes stimulated with lipopolysaccharide in a dose-dependent manner. PDE inhibitors such as cilostazol can protect rat chondrocytes against NO-induced apoptosis in vitro and prevent cartilage destruction in osteoarthritis (Lee et al., 2008). The inhibition of NO production by specific and non-specific PDE inhibitors could be beneficial in NO-mediated inflammatory and/or autoimmune disorders (Adrian et al., 1999; Beshay et al., 2001; Tenor et al., 2002).
The rationale of our study is based on the fact that theophylline potentiates the effect of glucocorticoids in bronchial asthma. Both bronchial asthma and RA are immune-mediated diseases involving the release of many inflammatory cytokines and infiltration of inflammatory cells to bronchi and synovial joints respectively. However, it is not clear whether or not theophylline has anti-inflammatory and immunomodulating effects in RA. Also the effect of NO on the actions of theophylline in RA has not been investigated. Therefore, in this study, the effects of theophylline alone and in combination with the NO donor, sodium nitroprusside (SNP) or the NO synthase inhibitor, L-NG-monomethyl arginine (L-NMMA) on the signs, cytokines and histopathology of adjuvant arthritis in rats were carefully evaluated.
Methods
Animals
Adult female albino rats of the Sprague-Dawely strain weighing between 160 and 200 g were used. The animals were acclimatized to a light- and temperature- controlled room with a 12–12 h dark–light cycle. The rats were fed with commercial pelleted rat feed and water was given ad libitum. Food was placed on the floor of the cage to facilitate access, as the pain that accompanies adjuvant-induced arthritis renders the rats immobile and unable to use their hind limbs to obtain food from the cover mesh of the cage. The experimental protocol was approved by the local ethical committee.
Reagents and drugs
Complete Freund's adjuvant (CFA) was purchased from Difco laboratories (Detroit, MI, USA). Squalene was purchased from MP Biomedicals, Inc. Theophylline, L-NMMA and SNP were purchased from Sigma chemical (St. Louis, MO, USA). Hot saline was used as a solvent for theophylline. SNP and L-NMMA were easily dissolved in water.
Experimental induction of arthritis and drug treatment
In this study, adjuvant arthritis was induced in rats according to previously described methods for the evaluation of RA. Based on preliminary experiments, to increase the sensitivity of the rats used to CFA, the method of Trentham et al. (1977) was modified by intradermally injecting 0.1 mL of squalene before the inoculation of CFA into a different site in the sub-plantar surface of the right hind paw. Each animal in all the groups, except those in the control non-adjuvant group, was injected with 0.1 mL squalene and 0.1 mL CFA.
Eighty-eight rats were used in this study. Two groups (I and II) of six animals each served as controls; these non-adjuvant and untreated adjuvant arthritic rats received a daily i.p. injection of saline. Other animals were randomly allocated to two treatment protocols (prophylactic or therapeutic). Each treatment protocol contained six groups of six animals. Drug treatment was started on day 5 until day 14 for the prophylactic protocol and on day 16 until day 25 for the therapeutic protocol. The first three groups (III, IV and V) in each protocol received i.p. theophylline alone at a dose of 45, 30 and 15 mg·kg−1·day−1 respectively. The other three groups (VI, VII and VIII) were treated with 30 mg·kg−1·day−1 theophylline i.p. combined with 1 mg·kg−1·day−1 SNP, 0.01 mg·kg−1·day−1 SNP or 30 mg·kg−1·day−1 L-NMMA respectively.
The day of inoculation was regarded as day 0, whereas day 16 was the day in which oedema in the contralateral, non-injected, hind paw was observed. This prophylactic schedule of treatment was selected to evaluate the inhibitory effect of theophylline on the development of arthritis in contralateral hind paws. This protocol demonstrates the immunomodulator effect of theophylline. However, a therapeutic protocol was used to assess the anti-inflammatory effect of theophylline on the development of arthritis.
Arthritis index, ankle diameter, volume of oedema in the paws, body weight, rectal temperature and pain threshold to pressure on hind paws, were measured daily from day 0 until day 30 after adjuvant inoculation. At the end of the study, the animals were killed and the blood was collected. Blood samples were immediately centrifuged at 2012 g for 10 min and serum samples were stored at −80°C until assayed for TNF-α and interleukin-10 (IL-10). Specimens of ankle joint tissues were also examined for histopathology.
Arthritis index
Rats were evaluated daily for arthritis. The physical symptoms of arthritis were judged by the following grading system (Wooley et al., 1981) normal paws; 1 = erythema of toes; 2 = erythema and swelling of paws; 3 = swelling of ankles; 4 = complete swelling of the whole leg and inability to bend it. The maximum achievable score is thus 16. Arthritis index for each rat was calculated by adding the four scores of individual paws. A sensitized animal was considered to have arthritis when at least one non-injected paw was inflamed (Philippe et al., 1997).
Measurement of body weight and temperature in arthritic rats
The body weight of each rat was recorded before and daily after adjuvant inoculation to assess food intake and weight gain throughout the period of arthritis. The difference between the body weight on a given day and that on day 0 was calculated to determine the change in body weight in arthritic rats.
Body temperature, as an index of inflammation, was monitored for rats, before and daily after disease induction between 0900 and 1100, using a rectal thermometer.
Measurement of ankle diameter and paw volume changes
Changes in the ankle diameter of both ipsilateral (injected) and contralateral (non-injected) hind paws, from the height on day 0, were assessed daily using a Vernier scale (Marry et al., 1998).
Volumes of hind paws were measured before and daily after adjuvant inoculation by using water displacement plethysmometry (David et al., 2001). The changes in the volumes of the hind paws compared with those on day 0 were calculated.
Analgesimetry
Using a Ugo basile analgesimeter (Ugo Basile Biological Research Apparatus, Comerio–Varese, Italy), a crescent pressure (in grams) was applied separately to the posterior paws until the animal displayed a reaction that consisted of withdrawing the paw and/or vocalizing (Andersen and Tufik, 2000). The slide of the device moved at a speed of 16 mm·s−1. The force on the paw was at rate of 16 g·s−1, so a distance of 11.5 mm is equivalent to 115 g. The pain threshold to pressure on hind paws of rats was measured. The following formula was used to calculate the percentage change in pressure to the hind paws needed to elicit a response on day x for each animal
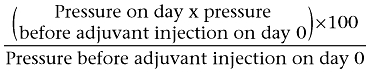 |
TNF-α and IL-10 assays
Animals were killed on day 30 after disease induction and samples of blood were taken to separate sera from all animals. Serum levels of TNF-α and IL-10 were determined using enzyme-linked immunosorbent assay kits (from Bender Medsystems, Vienna, Austria). Antibodies specific for rat TNF-α and IL-10 were coated onto the wells of the microtitre strips and the samples including standards of known rat TNF-α and IL-10 were pipetted into the wells, incubated and washed. Intensity of the colour was determined at 450 nm with a correction wavelength of 630 nm.
Histopathological examination
Ankle joint tissues from control, arthritic and treated rats were excised and fixed in 10% buffered formalin, decalcified in 10% ethylenediaminetetraacetic acid, embedded in paraffin, sectioned and stained with haematoxylin and eosin and then evaluated under a light microscope. The evaluation parameters were mononuclear inflammation, vascular proliferation, oedema, synovial hyperplasia and vasculitis causing fibrinoid necrosis on the vessel wall in periarticular and subcutaneous adipose tissue (McCartney-Francis et al., 2003). The pathological evaluation was performed randomly by a pathologist, blind to the specimens.
Statistical analysis
The results are presented as the mean ± standard error of the mean. Changes in the arthritis index, body weight, temperature, paw volume, pain threshold to paw pressure and serum levels of cytokines measured in different treatment groups were compared with adjuvant untreated control group (group II) and non-adjuvant control group (group I) by one-way analysis of variance and Student's t-test for significance. Also, significance tests were calculated to determine the differences between the effects of different doses of theophylline and theophylline alone compared with theophylline in combination with SNP or L-NMMA.
Results
Arthritis index
Arthritis was successfully induced in the rats by the administration of CFA and squalene. After induction of arthritis in the control untreated group (group II), the injected hind paw (right one) showed, on day 1, obvious swelling of the ankle and small joints of the foot with marked redness of the inflamed joints, whereas the left non-injected hind paw showed swelling and redness on day 16 after adjuvant inoculation. On day 1 after adjuvant inoculation, the arthritis index was 2.5 ± 0.22. The arthritis index peaked on day 18 (6.17 ± 0.17) and slightly decreased on the subsequent days until the end of experiment on day 30 (5.67 ± 0.21) (Figure 1).
Figure 1.
Effect of prophylactic (A) and therapeutic (B) administration of theophylline on the arthritis index of adjuvant arthritic rats. *P < 0.05 versus group II; †P < 0.05 versus group IV.
Prophylactic administration of theophylline significantly decreased the arthritic scores in a dose-dependent manner. The maximum effect of theophylline was recorded on day 30. The arthritic scores of animals treated with 45, 30 and 15 mg·kg−1·day−1 on day 30 were 0.83 ± 0.17, 1.4 ± 0.2 and 1.67 ± 0.2 respectively. The combination of 30 mg·kg−1·day−1 L-NMMA or 0.01 mg·kg−1·day−1 SNP with 30 mg·kg−1·day−1 theophylline significantly enhanced the inhibitory effect of theophylline and decreased the arthritic score from 1.4 ± 0.2 to 0.33 ± 0.2 or 1.0 ± 0.17 respectively. However, SNP at a dose of 1 mg·kg−1·day−1 significantly reduced the inhibitory effect of theophylline and the arthritic score was increased from 1.4 ± 0.2 in the group given a prophylactic dose of 30 mg·kg−1·day−1 theophylline alone to 2.33 ± 0.2 (group VI) (Figure 1).
Similarly, but to a lesser extent, therapeutic administration of theophylline at all doses used (groups III, IV and V) significantly decreased (P < 0.05) the arthritic scores on day 30 (2.83 ± 0.17, 3.33 ± 0.2 and 3.67 ± 0.2, respectively) compared with that of the adjuvant arthritic control (5.67 ± 0.2). Treatment of adjuvant arthritic rats with either 0.01 mg·kg−1·day−1 SNP or 30 mg·kg−1·day−1 L-NMMA (groups VII and VIII) and 30 mg·kg−1·day−1 theophylline caused a further significant reduction of arthritic scores. The arthritic score decreased from 3.33 ± 0.2 with theophylline alone to 1.5 ± 0.2 and 1.33 ± 0.2 with the combined treatment in groups VII and VIII respectively (Figure 1).
Body weight changes
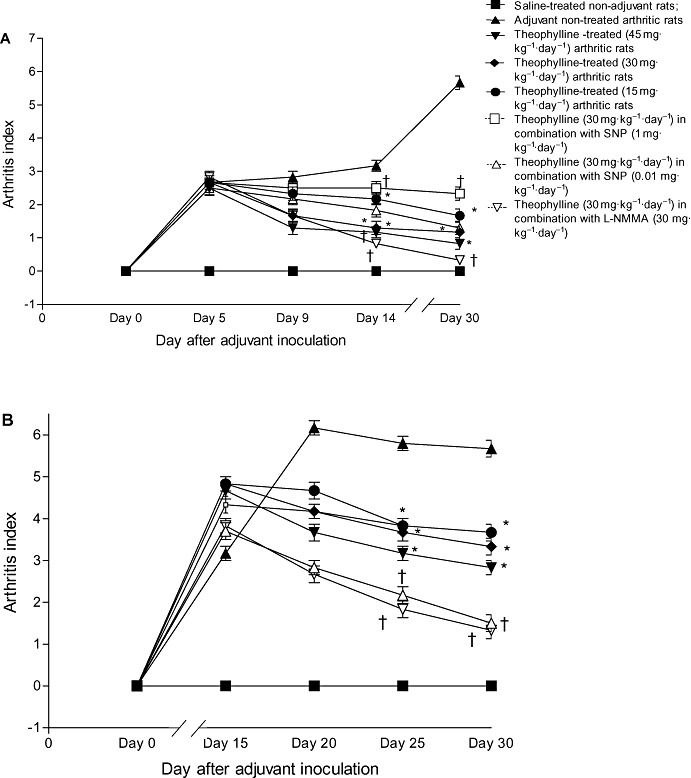
In the present work, the body weight of the non-adjuvant control rats (group I) increased significantly (P < 0.05) over the observation period. However, the body weight of adjuvant untreated control rats (group II) was markedly decreased starting from day 5 and the maximum loss (11.9 ± 0.2 g) was observed on day 30 (Figure 2).
Figure 2.
Effect of prophylactic (A) and therapeutic (B) administration of theophylline on the change in body weight of adjuvant arthritic rats. *P < 0.05 versus group II; †P < 0.05 versus group IV.
Prophylactic and therapeutic treatment of arthritic rats with theophylline alone (groups III, IV and V) and in combination with either 0.01 mg·kg−1·day−1 SNP (group VII) or 30 mg·kg−1·day−1 L-NMMA (group VIII) not only prevented the loss in the body weight of arthritic rats but also increased it. The maximum increase in the body weight of arthritic rats (9 ± 0.1 g) was observed in the group of rats treated with 45 mg·kg−1·day−1 theophylline alone (group III) on day 30. SNP (0.01 mg·kg−1·day−1) or L-NMMA (30 mg·kg−1·day−1) significantly (P < 0.05) enhanced the increase in body weight of arthritic rats caused by administration of 30 mg·kg−1·day−1 theophylline. In contrast, the combination of a therapeutic dose of theophylline with 1 mg·kg−1·day−1 SNP resulted in a loss, rather than an increase, in body weight (group VI) (Figure 2).
Temperature changes
Control rats inoculated with CFA showed a large, transient febrile response with local, acute inflammation, that peaked on day 1 (38.7 ± 0.1°C) followed by a return to the control level on day 3. A second bout of fever that peaked on day 16 (38.1 ± 0.1°C) was observed. Then the body temperature returned to normal levels until the end of the experiments on day 30 (37.5 ± 0.1°C). Treatment of adjuvant arthritic rats with theophylline, alone and in combination with 0.01 mg·kg−1·day−1 SNP or 30 mg·kg−1·day−1 L-NMMA, from day 5 to day 14, abolished the second peak of fever on day 16. In contrast, treating rats with theophylline in combination with 1 mg·kg−1·day−1 SNP did not abolish the febrile peak on day 16.
Changes in ankle diameter
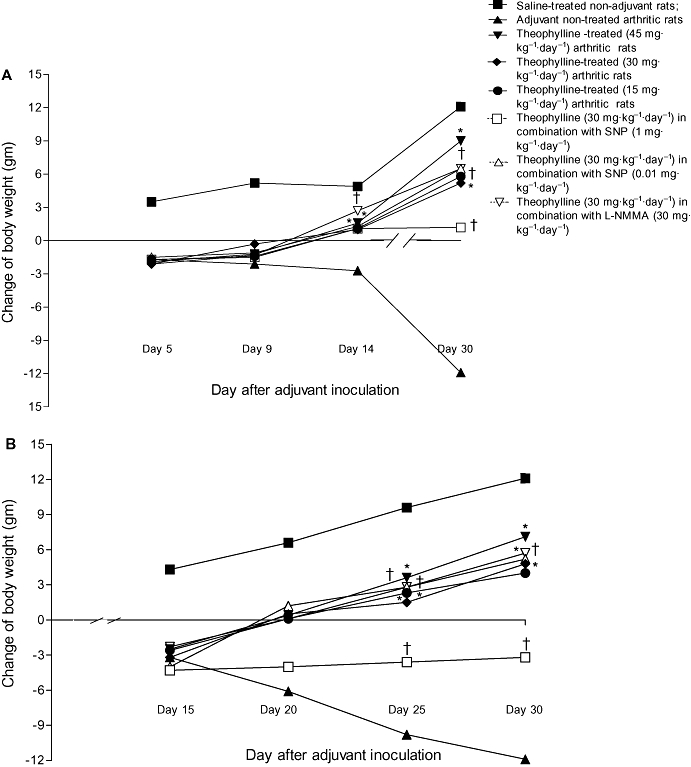
Swelling and erythema with an increase in the diameter of the ankle joint of the injected right hind paw was evident on day 3 in control untreated adjuvant-injected rats (group II). The change in diameter of the right ankle joint significantly increased on day 5 then slightly decreased until day 16. The change of ankle diameter peaked again on day 20 (0.33 ± 0.01 mm) with no marked change on the subsequent days until the end of experiment on day 30 (0.32 ± 0.01 mm). Swelling of the ankle joint of the contralateral non-injected left hind paw was observed on day 16. The change in left ankle diameter was significantly increased on day 20 (0.44 ± 0.01 mm) then decreased on the subsequent days until day 30, when it was 0.34 ± 0.01 mm (Figure 3 and Table 1).
Figure 3.
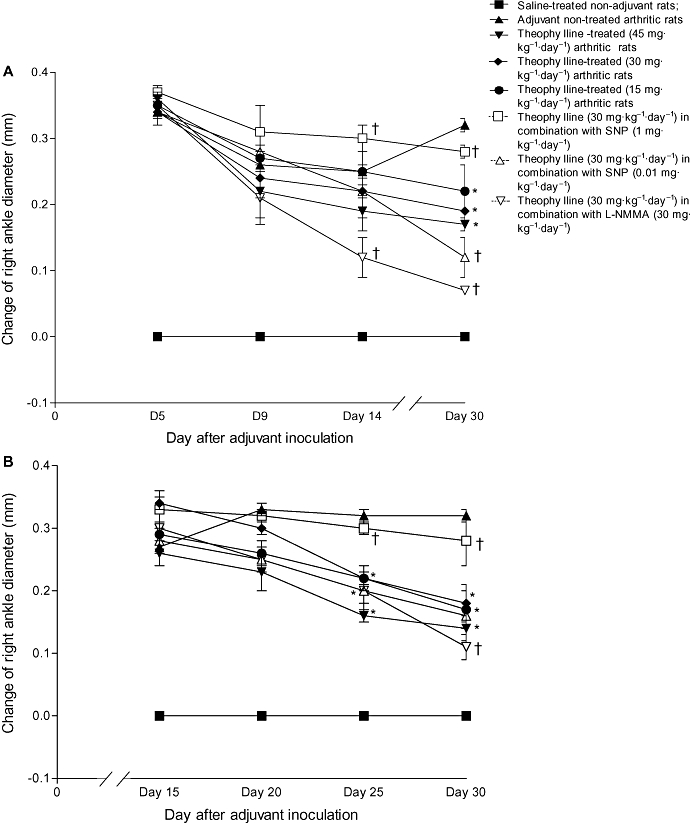
Effect of prophylactic (A) and therapeutic (B) administration of theophylline on the change in right ankle diameter (mm) of adjuvant arthritic rats. *P < 0.05 versus group II; †P < 0.05 versus group IV.
Table 1.
Effect of prophylactic and therapeutic administration of theophylline on the changes in the left ankle diameter (mm) of adjuvant arthritic rats
| Drug treatment |
Change in left ankle diameter (mm) |
|||
|---|---|---|---|---|
|
Prophylactic |
Therapeutic |
|||
| Day 14 | Day 30 | Day 25 | Day 30 | |
| Saline-treated non-arthritic rats (group I) | 0 | 0* | 0 | 0* |
| Untreated arthritic rats (group II) | 0.1 ± 0.01 | 0.34 ± 0.01 | 0.38 ± 0.02 | 0.34 ± 0.01 |
| Theophylline-treated (45 mg·kg−1·day−1) arthritic rats (groups III) | 0 | 0* | 0.11 ± 0.01* | 0.1 ± 0.001* |
| Theophylline-treated (30 mg·kg−1·day−1) arthritic rats (groups IV) | 0 | 0* | 0.14 ± 0.04* | 0.12 ± 0.02* |
| Theophylline-treated (15 mg·kg−1·day−1) arthritic rats (groups V) | 0 | 0* | 0.16 ± 0.02* | 014 ± 0.01* |
| Theophylline (30 mg·kg−1·day−1) in combination with SNP (1 mg·kg−1·day−1) (groups VI) | 0.1 ± 0.01 | 0.1 ± 0.01* | 0.13 ± 0.01* | 0.15 ± 0.03* |
| Theophylline (30 mg·kg−1·day−1) in combination with SNP (0.01 mg·kg−1·day−1) (groups VII) | 0 | 0* | 0.13 ± 0.01* | 0.1 ± 0.01* |
| Theophylline (30 mg·kg−1·day−1) in combination with L-NMMA (30 mg·kg−1·day−1) (groups VIII) | 0 | 0* | 0.11 ± 0.01* | 0.1 ± 0.01* |
P < 0.05 versus group II.
L-NMMA, L-NG-monomethyl arginine; SNP, sodium nitroprusside.
Prophylactic and therapeutic administration of theophylline alone (groups II, IV and V) significantly (P < 0.05) inhibited the increase in the diameter of the right ankle observed in control arthritic rats (group II). The maximum effect (0.14 ± 0.02 mm) was seen with the therapeutic dose of theophylline, 45 mg·kg−1·day−1. The combination of either 30 mg·kg−1·day−1 L-NMMA (group VIII) or 0.01 mg·kg−1·day−1 SNP (group VII) with 30 mg·kg−1·day−1 theophylline caused a marked enhancement of the inhibitory effect of theophylline. The right ankle diameters of animals in the prophylactic groups, VIII and VII, were 0.07 ± 0.001 mm and 0.12 ± 0.03 mm respectively. However, 1 mg·kg−1·day−1 SNP counteracted the inhibitory effect of theophylline (group VI) (Figure 3).
Prophylactic theophylline administration alone and in combination with either 0.01 mg·kg−1·day−1 SNP or 30 mg·kg−1·day−1 L-NMMA entirely prevented the increased diameter of the left ankle joints diameters observed in control arthritic rats. Although, therapeutic administration of theophylline alone or in combination with either 0.01 mg·kg−1·day−1 SNP or 30 mg·kg−1·day−1 L-NMMA significantly inhibited the increased diameter of the left ankle joint, unlike prophylactic administration of these comounds, it failed to abolish completely the swelling of the left ankle joint (Table 1).
Changes in paw volume
Starting from day 1 after inoculation of CFA, the volumes of the right hind paw of untreated control rats (group II) were significantly increased (P < 0.05) compared with those of non-adjuvant control animals (group I). The change in the volume of the right paw was 1.38 ± 0.15 mL on day 5 and increased on day 30 to 1.57 ± 0.1 mL. On the other hand, little change was noticed in the volume of the left non-injected hind paw before day 16. The increase in volume of the left hind paw peaked on day 20; it was 0.3 ± 0.03 mL then slightly decreased on the subsequent days to 0.26 ± 0.04 mL on day 30 after adjuvant inoculation (Figure 4 and Table 2).
Figure 4.
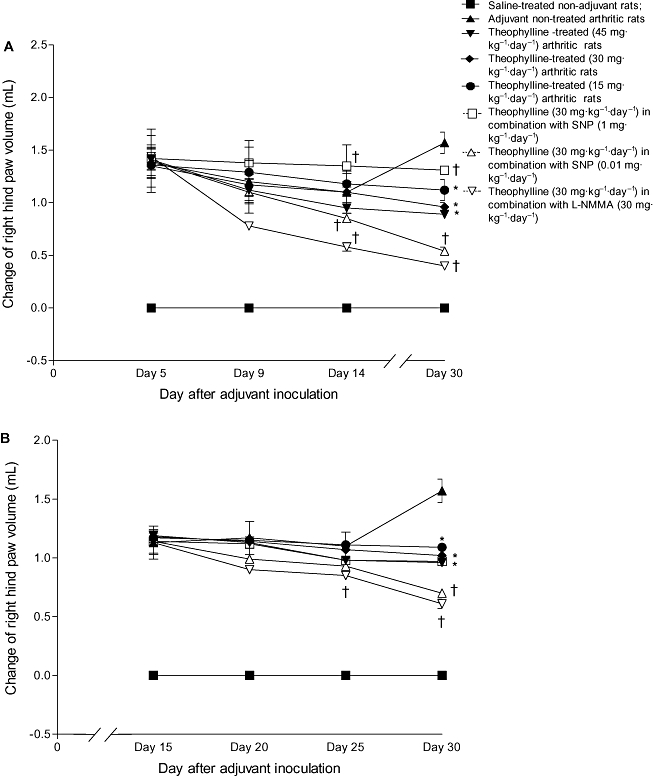
Effect of prophylactic (A) and therapeutic (B) administration of theophylline on the change in right hind paw volume (mL) of adjuvant arthritic rats. *P < 0.05 versus group II; †P < 0.05 versus group IV.
Table 2.
Effect of prophylactic and therapeutic administration of theophylline on the changes in left hind paw volume (mL) of adjuvant arthritic rats
| Drug treatment |
Change of left hind paw volume (mL) |
|||
|---|---|---|---|---|
|
Prophylactic |
Therapeutic |
|||
| Day 30 | Day 25 | Day 30 | Day 14 | |
| Saline-treated non-arthritic rats (group I) | 0 | 0* | 0* | 0* |
| Untreated arthritic rats (group II) | 0.04 ± 0.01 | 0.26 ± 0.04 | 0.26 ± 0.04 | 0.26 ± 0.04 |
| Theophylline-treated (45 mg·kg−1·day−1) arthritic rats (groups III) | 0 | 0* | 0.1 ± 0.01* | 0.1 ± 0.001* |
| Theophylline-treated (30 mg·kg−1·day−1) arthritic rats (groups IV) | 0 | 0* | 0.11 ± 0.01* | 0.1 ± 0.01* |
| Theophylline-treated (15 mg·kg−1·day−1) arthritic rats (groups V) | 0 | 0* | 0.15 ± 0.01* | 0.14 ± 0.01* |
| Theophylline (30 mg·kg−1·day−1) in combination with SNP (1 mg·kg−1·day−1) (groups VI) | 0.07 ± 0.01* | 0.08 ± 0.01* | 0.13 ± 0.01* | 0.14 ± 0.01* |
| Theophylline (30 mg·kg−1·day−1) in combination with SNP (0.01 mg·kg−1·day−1) (groups VII) | 0 | 0* | 0.13 ± 0.01* | 0.07 ± 0.01*† |
| Theophylline (30 mg·kg−1·day−1) in combination with L-NMMA (30 mg·kg−1·day−1) (groups VIII) | 0 | 0* | 0.1 ± 0.01* | 0.05 ± 0.01*† |
P < 0.05 versus group II.
P < 0.05 versus groups IV.
L-NMMA, L-NG-monomethyl arginine; SNP, sodium nitroprusside.
The increase in the right hind paw volume of arthritic rats was significantly (P < 0.05) inhibited by the prophylactic, as well as the therapeutic, administration of theophylline, in dose-dependent manner; the prophylactic was more effective than the therapeutic treatment. The changes in the right hind paw volume of the groups treated with 45, 30 and 15 mg·kg−1·day−1 theophylline were 0.89 ± 0.03 mL, 0.96 ± 0.04 mL and 1.12 ± 0.1 mL respectively. Simultaneous administration of L-NMMA (30 mg·kg−1·day−1) or SNP (0.01 mg·kg−1·day−1) with theophylline (30 mg·kg−1·day−1) resulted in a further significant (P < 0.05) reduction in the volume of the right hind paw (0.4 ± 0.01 mL or 0.54 ± 0.04 mL respectively). In contrast, a high dose of SNP (1 mg·kg−1·day−1) counteracted the inhibitory effect of theophylline (increase in paw volume = 1.31 ± 0.03 mL) (Figure 4).
Prophylactic theophylline alone and combined with either L-NMMA or a low dose of SNP entirely prevented the change in the left hind paw volume (Table 2). However, the protective effect of the therapeutic administration of theophylline alone or combined with either L-NMMA or a low dose of SNP was less than that of prophylactic theophylline. As with the right hind paw, a high dose of SNP (1 mg·kg−1·day−1) reduced the inhibitory effect of theophylline on the volume of the left hind paw (Table 2)
Analgesimetry
Adjuvant inoculation into control rats (group II) was accompanied by hyperalgesia as evidenced by a reduction in the pain threshold to pressure (grams) in the hind paws; this continued until the end of the experiments on day 30. On day 30 after adjuvant inoculation, the pressure (g) that induced pain in the right and left hind paws was reduced to 54.9 ± 0.3% and 62 ± 0.4%, respectively, of that at day 0 before adjuvant injection. Prophylactic administration of theophylline alone in groups III, IV and V (45, 30 and 15 mg·kg−1·day−1) markedly (P < 0.05) decreased the hyperalgesia of the arthritic rats; the pain threshold to pressure was increased in both hind paws (Table 3). The inhibitory effect of theophylline 30 mg·kg−1·day−1 on the hyperalgesia of arthritic rats was significantly (P < 0.05) enhanced by simultaneous administration of 30 mg·kg−1·day−1 L-NMMA (Table 3). However, the maximum inhibitory effect on hyperalgesia in the right hind paw was induced by a higher dose of theophylline alone (45 mg·kg−1·day−1), which prevented entirely the hyperalgesia. On the other hand, a high dose of SNP (1 mg·kg−1·day−1) counteracted the inhibitory effect of theophylline (prophylactic or therapeutic) on hyperalgesia (Table 3).
Table 3.
Effect of prophylactic and therapeutic administration of theophylline on the pressure needed to induce a response in the right hind paw of adjuvant arthritic rats
| Drug treatment |
% Decrease in pressure on right hind paw |
|||
|---|---|---|---|---|
|
Therapeutic |
Prophylactic |
|||
| Day 30 | Day 25 | Day 30 | Day 14 | |
| Saline-treated non-arthritic rats (group I) | 3.5 ± 0.1 | 2.3 ± 0.1* | 8 ± 0.3* | 2 ± 0.1* |
| Untreated arthritic rats (group II) | 26.7 ± 1.4 | 54.9 ± 3 | 46 ± 2.6 | 54.9 ± 3 |
| Theophylline-treated (45 mg·kg−1·day−1) arthritic rats (groups III) | 2 ± 0.1* | 0 | 14 ± 0.7* | 5 ± 0.2* |
| Theophylline-treated (30 mg·kg−1·day−1) arthritic rats (groups IV) | 14 ± 0.9* | 8.7 ± 0.1* | 18 ± 0.9* | 14 ± 0.5* |
| Theophylline-treated (15 mg·kg−1·day−1) arthritic rats (groups V) | 12.5 ± 0.7* | 10 ± 0.1* | 16 ± 1.1* | 12 ± 0.9* |
| Theophylline (30 mg·kg−1·day−1) in combination with SNP (1 mg·kg−1·day−1) (groups VI) | 35.7 ± 2.1† | 28.6 ± 0.3*† | 28 ± 1.3* | 23 ± 1.4* |
| Theophylline (30 mg·kg−1·day−1) in combination with SNP (0.01 mg·kg−1·day−1) (groups VII) | 23 ± 1.1† | 14.3 ± 0.3* | 13 ± 0.9* | 7 ± 0.8*† |
| Theophylline (30 mg·kg−1·day−1) in combination with L-NMMA (30 mg·kg−1·day−1) (groups VIII) | 6 ± 0.1*† | 4 ± 0.1*† | 14 ± 1.2* | 8 ± 1.1*† |
P < 0.05 versus group II.
P < 0.05 versus groups IV.
L-NMMA, L-NG-monomethyl arginine; SNP, sodium nitroprusside.
Therapeutic and prophylactic administration of theophylline was also effective in reducing the hyperalgesia of the left hind paw induced by adjuvant inoculation. Percentage decrease in pressure was 1.8 ± 0.01, 13 ± 0.1,16 ± 0.1 in the groups injected therapeutically with 45, 30 or 15 mg·kg−1·day−1 theophylline respectively. Simultaneous administration of 30 mg·kg−1·day−1 L-NMMA with 30 mg·kg−1·day−1 theophylline resulted in a further significant reduction of hyperalgesia in the left hind paw (Figure 5).
Figure 5.
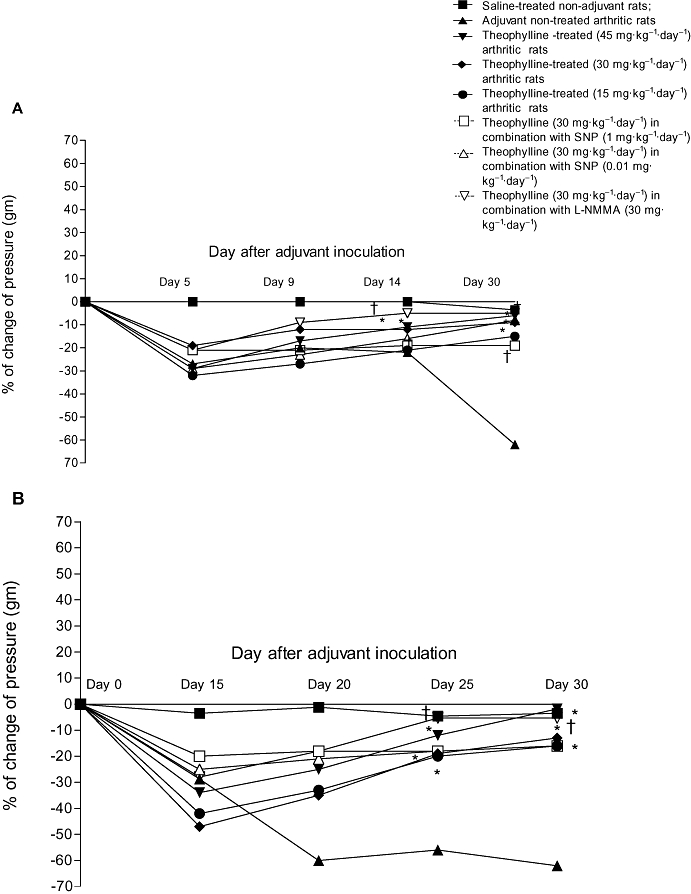
Effect of prophylactic (A) and therapeutic (B) administration of theophylline on the change in pressure (%) of the left hind paw of adjuvant arthritic rats. *P < 0.05 versus group II; †P < 0.05 versus group IV.
Serum TNF-α and IL-10 levels in adjuvant arthritic rats
The serum TNF-α level was only slightly greater in untreated adjuvant arthritic control rats (group II) than that of control non-adjuvant saline-treated animals (group I) on day 30 and this effect was not significant (Table 4). However, the serum IL-10 level was significantly lower (P < 0.05) in adjuvant untreated control arthritic rats compared with that in the control non-arthritic rats. Levels of TNF-αin sera taken from arthritic rats treated with 30 mg·kg−1·day−1 theophylline alone (prophylactically or therapeutically) and in combination with SNP or L-NMMA were also unchanged (P > 0.05) compared with either saline-treated non-adjuvant rats (group I) or untreated adjuvant arthritic control animals (group II) (Table 4). IL-10 levels were significantly higher (P > 0.05) in groups treated with theophylline alone or theophylline with either a low dose of SNP or L-NMMA compared with those in adjuvant arthritic control rats (group II). However, they were not significantly lower than that of saline-treated rats in the non-arthritic control group (group I) (Table 4).
Table 4.
Effect of prophylactic theophylline alone or in combination with either SNP or L-NMMA on serum levels of TNF-α and IL-10 in adjuvant arthritic rats
| Group | Drug treatment |
Serum levels (pg) |
|
|---|---|---|---|
| TNF-α | IL-10 | ||
| I | Saline-treated (non-adjuvant) | 30.7 ± 2.3 | 345.6 ± 44.4† |
| II | Adjuvant arthritis (untreated) | 31.04 ± 1.4 | 171.6 ± 24* |
| III | Theophylline-treated (45 mg·kg−1·day−1) | 27.2 ± 1.8 | 340 ± 38.6† |
| IV | Theophylline-treated (30 mg·kg−1·day−1) | 27.8 ± 1.22 | 286.5 ± 31.7† |
| V | Theophylline-treated (15 mg·kg−1·day−1) | 28.3 ± 1.8 | 275 ± 32.4† |
| IV | Theophylline-treated (30 mg·kg−1·day−1) in combination with SNP (1 mg·kg−1·day−1) | 28.8 ± 0.7 | 340.9 ± 60.6† |
| VII | Theophylline (30 mg·kg−1·day−1) in combination with SNP (0.01 mg·kg−1·day−1) | 30.2 ± 2.4 | 312.2 ± 43.4† |
| VIIII | Theophylline (30 mg·kg−1·day−1) in combination with L-NMMA (30 mg·kg−1·day−1) | 29.6 ± 3.7 | 293.6 ± 28.8† |
Samples were taken from rats on day 30 after adjuvant inoculation.
P < 0.05 versus saline-treated non-arthritic rats (group I).
P < 0.05 versus saline-treated arthritic rats (group II).
IL-10, interleukin-10; L-NMMA, L-NG-monomethyl arginine; SNP, sodium nitroprusside; TNF-α, tumour necrosis factor–α.
Histopathological examination
Histopathological examination of the ankle joint tissue of saline-treated, non-arthritic rats (group I) showed that the joint space, synovial lining, articular cartilage and subchondral bone were normal (Figure 6A). In contrast, the synovium of adjuvant-arthritic control rats was oedematous and thickened with a dense perivascular inflammatory infiltrate composed of lymphocytes, plasma cells and macrophages, which filled the synovial stroma. The vascularity was increased and the inflamed and hyperaemic synovium crept over the articular cartilage forming a pannus and caused erosion of the underlying cartilage (Figure 6B). In adjuvant-induced arthritic rats given 30 mg·kg−1·day−1 theophylline, the proliferation and infiltration of the inflammatory cells in the synovial tissue were inhibited and the erosion of the articular cartilage was alleviated when compared with the untreated adjuvant arthritic rats (Figure 6C). Arthritic rats given 45 mg·kg−1·day−1 theophylline have minimal infiltration of inflammatory cells into the synovium; the pannus was partly inhibited and the destruction of the articular cartilage was alleviated when compared with control adjuvant arthritic rats (Figure 6D). The maximum inhibitory effect was seen in the group of rats given therapeutic 30 mg·kg−1·day−1 theophylline in combination with 0.01 mg·kg−1·day−1 SNP; the inflammatory cellular infiltrate, in the synovium of this group of arthritic rats was inhibited and the articular cartilage erosion attenuated. It is clear that this dose of SNP markedly enhanced the inhibitory effect of theophylline (Figure 6E). In arthritic rats given 30 mg·kg−1·day−1 theophylline in combination with L-NMMA, there was minimal inflammatory cellular infiltrate in synovial tissue and the pannus formation was partly inhibited; there was some focal superficial cartilage erosion when compared with untreated adjuvant arthritic rats (Figure 6F).
Figure 6.
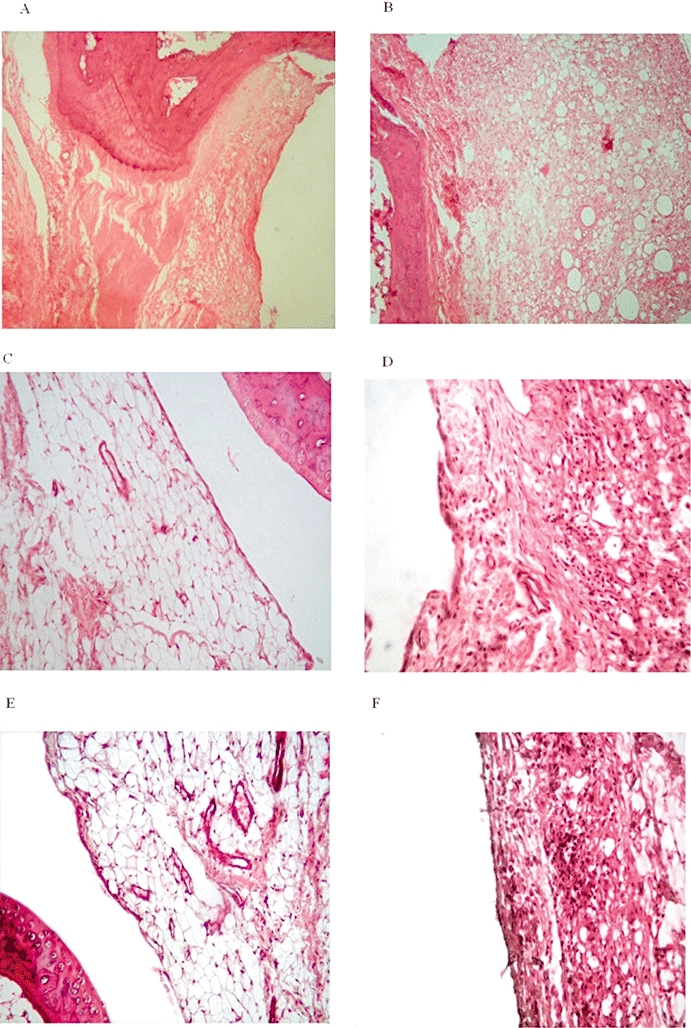
Representative histopathology of left ankle joints of (A) normal control rats (normal synovial lining, articular cartilage and subchondral bone), (B) untreated adjuvant arthritic rats, (C) adjuvant arthritic rats treated with 30 mg·kg−1·day−1 theophylline from day 16 to day 25, (D) adjuvant arthritic rats treated with 45 mg·kg−1·day−1 theophylline from day 16 to day 25, (E) adjuvant arthritic rats treated with 30 mg·kg−1·day−1 theophylline in combination with 0.01 mg·kg−1·day−1 sodium nitroprusside (SNP) from day 16 to day 25, (F) adjuvant arthritic rats treated with 30 mg·kg−1·day−1 theophylline in combination with 30 mg·kg−1·day−1 L-NG-monomethyl arginine from day 16 to day 25. Note the dramatic inhibition of inflammatory cellular infiltrate and alleviation of articular cartilage erosion by therapeutic administration of theophylline in combination with a low dose of SNP.
Discussion
In the present study, we carefully evaluated the prophylactic and therapeutic potential of the non-specific PDE inhibitor, theophylline, for the treatment of adjuvant-induced arthritis in rats. Our findings show that theophylline has anti-inflammatory and anti-arthritic effects. It was observed that theophylline, after either prophylactic or therapeutic administration, markedly suppressed the adjuvant arthritis syndrome in rats. It reduced significantly the arthritis index, loss in body weight, second peak of fever, increase in ankle joint diameter, increase in hind paw volume, inflammatory cellular infiltrate in the synovium of the ankle joints and pannus formation. These protective and inhibitory effects of theophylline were dose-dependent. The maximum effect was seen with the largest dose of theophylline used (45 mg·kg−1·day−1). Prophylactic administration (from day 5 to day 14) was more effective against arthritis than therapeutic administration (from day 16 to day 25) of theophylline. However, histopathological findings revealed that the anti-inflammatory effect of therapeutic theophylline was more effective than prophylactic.
The anti-inflammatory activity of theophylline has been mainly studied at the cellular or clinical level in the treatment of airway inflammation (Sansone et al., 1998; Page, 1999; Yamaguchi, 2003; Kanehara et al., 2008). However, a few studies have evaluated the effect of theophylline in animal models of inflammation and arthritis. The most recent one of these reported that subcutaneous injection of theophylline dose-dependently inhibited carrageenan-induced oedema in the rat foot (Watanabe et al., 2008). These authors suggested that the glucocorticoid receptor system is involved in the anti-inflammatory activity of theophylline. Previous studies had shown that theophylline exerted a dose-dependent analgesic effect against acetic acid-induced writhing in mice and an anti-inflammatory effect against carrageenan-induced paw oedema in rats (Kumar et al., 2000). In our study we also observed that theophylline protected rats against hyperalgesia induced by adjuvant inoculation in a dose-dependent manner; the maximum protective effect was observed after therapeutic administration of 45 mg·kg−1·day−1 theophylline.
In the present study, it was observed that there was a significant loss in body weight of untreated arthritic rats. This finding is in agreement with those of others who reported that body weight loss is an indication of an abnormal condition (Dardick et al., 1989; Koufany et al., 2008). Weight loss in rats with adjuvant-induced arthritis can be explained by the decrease in food intake observed throughout the period of the study due to the immobility accompanying the hyperalgesia. Our results demonstrate that theophylline inhibits the loss in body weight of arthritic rats and increases the food intake. This effect may be attributed to an improvement in the condition of these rats as a result of the amelioration of the hyperalgesia and inflammation associated with arthritis.
In the development of arthritis, a marked proliferation of synovial fibroblasts in the joint leads to pannus formation, which is the critical step in the destruction of cartilage and bones. In the present study, we observed that theophylline inhibited the infiltration of inflammatory cells into the synovium of arthritic rats and alleviated the destruction of the articular cartilage. It also partially inhibited the pannus formation. However, the pannus formation was inhibited most in the group of rats injected with theophylline and a low dose of SNP. Similar results have been reported in the literature with the administration of cyclic AMP elevating drugs. Kobayashi et al. (2007) observed that administration of PDE4 inhibitors as rolipram, KF 66490 and SB207499 suppressed the pannus-like inflammation by inhibiting cytokine production by macrophages and the proliferation of fibroblasts into the synovium .
The exact mechanism of the anti-inflammatory and anti-arthritic action of theophylline cannot be determined from the results of this study but several hypotheses can be put forward. It is well known that the elevation of the intracellular level of cyclic AMP in leukocytes is accompanied by a significant inhibition of the production of cytokines and release of other inflammatory mediators (Teixeira et al., 1997). Yamaguchi (2003) and others (Banner and Page, 1995) have suggested that theophylline induces an anti-inflammatory effect by suppressing the production of cytokines and the resulting proliferative response.
The results from the present study demonstrated that serum levels of IL-10 are significantly higher in the groups injected with theophylline compared with those in untreated arthritic rats. Keeping in mind the fact that IL-10 levels are significantly lower in untreated arthritic rats compared with non-arthritic control rats, we can cautiously suggest that theophylline normalizes the IL-10 levels in arthritic rats. Our observations are consistent with those of Mascali et al. (1996); they found that theophylline slightly inhibited TNF-α levels but its administration was associated with a 2.8-fold increase in the spontaneous production of the anti-inflammatory cytokine, IL-10.
However, in our study neither the prophylactic nor the therapeutic administration of theophylline significantly altered the serum level of TNF-α. With regard to the contribution of TNF-α to the severity of adjuvant arthritis, our study showed that serum TNF-α levels in untreated arthritic rats on day 30 after inoculation of the adjuvant were not significantly higher than those in normal rats. This observation is consistent with those of Philippe et al. (1997) who reported that the systemic TNF-α concentration had significantly increased 6 h after adjuvant injection, peaked at 12 h, returned to near control concentration on day 2, and was only slightly increased on day 20 and thereafter.
Cyclic AMP elevating PDE inhibitors can influence NO synthase (iNOS) activation in different cell types in vitro, and their potent anti-inflammatory effects in experimental disease models and clinical studies are frequently accompanied by a profound modulation of NO production (Markovic et al., 2003). Yoshikawa et al. (2002) suggested that all types of phosphodiestrase inhibitors from types I to V, specific and non-specific, suppress the production of NO by mouse microglia and astrocytes stimulated with lipopolysaccharide in a dose-dependent manner. In addition, the non-specific PDE inhibitor, pentoxifylline, and the specific PDE4 inhibitor, rolipram, have been found to suppress iNOS at the mRNA level and NO production in vitro and in vivo; an effect that could be beneficial in NO-mediated inflammatory and/or autoimmune disorders (Beshay et al., 2001)
Our present results are in remarkably good agreement with those of the aforementioned studies and provide evidence that inhibition of NO production is involved in the anti-inflammatory and anti-arthritic effects of PDE inhibitors. In our experiments, the anti-inflammatory and anti-arthritic actions of theophylline were potentiated markedly by L-NMMA, a non-specific NOS inhibitor. In contrast, a high dose of SNP, a NO donor, counteracted the anti-inflammatory and anti-arthritic effect of theophylline.
Another interesting finding in our study was the enhancement of the anti-inflammatory and anti-arthritic effect of theophylline by a low dose of SNP, a NO donor. Therefore, we propose that a low dose of the NO donor, SNP, may exert a negative feedback regulation of iNOS activities and have a reverse effect of the high dose of SNP (Gomaa et al., 2009).
In summary, we present data showing that theophylline, a non-specific PDE inhibitor, has an anti-inflammatory, anti-arthritic and anti-hyperalgesia effects adjuvant arthritic rats that are dose-dependent. These effects were markedly enhanced by the non-specific NOS inhibitor, L-NMMA, and a low dose of the NO donor, SNP. We suggest that a low dose of the NO donor exerts negative feedback regulation of iNOS, an enzyme that catalyses the synthesis of NO, and possesses a similar action to the NOS inhibitor, L-NMMA. These findings suggest that theophylline in combination with a non-specific NOS inhibitor or a low dose of a NO donor may have therapeutic value for various chronic inflammatory diseases such as RA.
Glossary
Abbreviations:
- IL-10
interleukin-10
- iNOS
inducible nitric oxide synthase
- L-NMMA
L-NGmonomethyl-L- arginine
- NO
nitric oxide
- PDE
phosphodiesterase
- SNP
sodium nitroprusside
- RA
rheumatoid arthritis
- TNF-α
tumour necrosis factor–α
Conflicts of Interest
None.
References
- Abdel-Salam OM, Baiuomy AR, El-Shenawy SM, Arbid MS. The anti-inflammatory effects of the phosphodiesterase inhibitor pentoxifylline in the rat. Pharmacol Res. 2003;37:331–340. doi: 10.1016/s1043-6618(03)00002-1. [DOI] [PubMed] [Google Scholar]
- Adrian JH, Annie H, Salvador M. Inhibition of nitric oxide synthase as potential therapeutic target. Annu Rev Pharmacol Toxicol. 1999;39:191–220. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Tufik S. Altered sleep and behavioral patterns of arthritic rats. Sleep Res Online. 2000;3:161–167. [PubMed] [Google Scholar]
- Banner KH, Page CP. Theophylline and selective phosphodiesterase inhibitors as anti-inflammatory drugs in treatment of bronchial athma. Eur Respir J. 1995;8:996–1000. [PubMed] [Google Scholar]
- Beshay E, Croze F, Prud'homme GJ. The phosphodiesterase inhibitors pentoxifylline and rolipram suppress macrophage activation and nitric oxide production in vitro and in vivo. Clin Immunol. 2001;98:272–279. doi: 10.1006/clim.2000.4964. [DOI] [PubMed] [Google Scholar]
- Bonta IL, Parnham MJ, Vliet LV. Combination of theophylline and prostaglandine E1 as inhibitors of the adjuvant-induced arthritis syndrome of rats. Ann Rheum Dis. 1978;37:212–217. doi: 10.1136/ard.37.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash JM, Klippel JH. Second-line drug therapy for rheumatoid arthritis. N Engl J Med. 1994;330:1368–1375. doi: 10.1056/NEJM199405123301908. [DOI] [PubMed] [Google Scholar]
- Chorostowska-Wynimko J, Kus J, Skopinska-Rozewska E. Theophylline inhibits free oxygen radicals production by human monocytes via phosphodiesterase inhibition. J Physiol Pharmacol. 2007:95–103. [PubMed] [Google Scholar]
- Dardick SJ, Basbaum AL, Levine JD. The contribution of pain to disability in experimentally induced arthritis. Arthritis Rheum. 1989;29:1017–1022. doi: 10.1002/art.1780290811. [DOI] [PubMed] [Google Scholar]
- David IB, Elizabeth AK, Michael FJ, Chih-Hung L, Shripad SB, Michael W, et al. Anti-inflammatory effects of ABT-702, a novel non-nucleoside adenosine kinase inhibitor in rat adjuvant arthritis. J Pharmacol Exp Ther. 2001;296:495–500. [PubMed] [Google Scholar]
- Giembycz MA. Re-inventing the wheel: non-selective phosphodiesterase inhibitors for chronic inflammatory diseases. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. Boca Raton: CRC Press; 2007. pp. 649–665. [Google Scholar]
- Giembycz MA. Can the inti-inflammatory potential of PDE4 inhibitors be realized: guarded optimism or wishful thinking ? Br J Pharmacol. 2008;155:288–290. doi: 10.1038/bjp.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa A, Elshenawy M, Afifi N, Mohammed E, Thabit R. Dual effect of nitric oxide donor on adjuvant arthritis. Int Immunopharmacol. 2009;9:439–447. doi: 10.1016/j.intimp.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Jae YC, Joon SP, Kyong UB, June GL, Hyun PK, Eun SY, et al. Defferential effect of phosphodiesterase IV inhibitor RP 73401 on various inflammatory and immune responses relevant to rheumatoid arthritis. Pharmacol Res. 2004;49:423–431. doi: 10.1016/j.phrs.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Suda T, Manabe H, Miki I. Administration of PDE4 inhibitors suppressed the pannus-like inflammation by inhibition of cytokine production by macrophages and synovial fibroblast proliferation. Mediators Inflamm. 2007;2007:1–9. doi: 10.1155/2007/58901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehara M, Yokoyama A, Tomoda Y, Shiota N, Iwamoto H, Ishikawa N, et al. Anti-inflammatory effects and clinical efficacy of theophylline and tulobuterol in mild-to-moderate chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2008;21:874–878. doi: 10.1016/j.pupt.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Koufany M, Moulin D, Bianchi A, Muresan M, Sebillaud S, Netter P, et al. Anti-inflammatory effect of antidiabetic thiazolidinediones prevents bone resorption rather than cartilage change in experimental polyarthritis. Arthritis Res Ther. 2008;10:R6, 15. doi: 10.1186/ar2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Jain NK, Kulkarni SK. Analgesic and anti-inflammatory effect of phosphodiesterase inhibitors. Indian J Exp Biol. 2000;28:26–30. [PubMed] [Google Scholar]
- Lee SW, Song YS, Shin SH, Kim KT, Park YC, Park BS, et al. Cilostazol protects rat chondrocytes against nitric oxide-induced apoptosis in vitro and prevents cartilage destruction in a rat model of osteoarthritis. Arthritis Rheum. 2008;58:790–800. doi: 10.1002/art.23220. [DOI] [PubMed] [Google Scholar]
- Markovic M, Miljkovic D, Trajkovic V. Regulation of inducible nitric oxide synthase by cAMP-elevating phosphodiesterase inhibitors. Curr Drug Targets Inflamm Allergy. 2003;2:63–79. 17. doi: 10.2174/1568010033344471. [DOI] [PubMed] [Google Scholar]
- Marry RL, Geetha T, Varalakshmi P. Effect of Vernonia Cinerea less flower extract in adjuvant-induced arthritis. Gen Pharmac. 1998;31:601–606. doi: 10.1016/s0306-3623(98)00049-4. [DOI] [PubMed] [Google Scholar]
- Mascali J, Cvielusa P, Negri J, Borish L. Anti-inflammatory effects of theophylline: modulation of cytokine production. Ann Allergy Asthma Immunol. 1996;77:34–38. doi: 10.1016/S1081-1206(10)63476-X. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis NL, Chan J, Wahl SM. Inflammatory joint disease: clinical, histological, and molecular parameters of acute and chronic inflammation and tissue destruction. In: Winyard PG, Willoughby DA, editors. Inflammation Protocols. Totowa, New Jersey: Humana Press; 2003. pp. 147–159. [DOI] [PubMed] [Google Scholar]
- Nyman U, Mussener A, Larsson E, Lorentzen J, Klareskog L. Amelioration of collagen II-induced arthritis in rats by the type IV phosphodiesterase inhibitor Rolipram. Clin Exp Immunol. 1997;108:415–419. doi: 10.1046/j.1365-2249.1997.3931291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page CP. Recent advances in our standing of the use of theophylline in treatment of asthma. J Clin Pharmacol. 1999;39:237–240. [PubMed] [Google Scholar]
- Philippe L, Gegout-Pottie P, Guingamp C, Bord JIK, Terlain B, Netter P, et al. Relations between functional, inflammatory, and degenerative parameters during adjuvant arthritis in rats. Am J Physiol. 1997;273:R1550, R1556. doi: 10.1152/ajpregu.1997.273.4.R1550. [DOI] [PubMed] [Google Scholar]
- Pincus T, Callahan LF. What is the natural history of rheumatoid arthritis? Rheum Dis Clin North Am. 1993;19:123–151. [PubMed] [Google Scholar]
- Sansone GR, Matin A, Wang SF, Bouboulis D, Frieri M. Theophylline inhibits the production of nitric oxide by peripheral blood mononuclear cells from patients with asthma. Ann Allergy Asthma Immunol. 1998;81:90–95. doi: 10.1016/S1081-1206(10)63114-6. [DOI] [PubMed] [Google Scholar]
- Sommer N, Loschmann P, Northoff G, Weller M, Steinbrecher A, Steinbach J, et al. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nature Med. 1995;1:244–248. doi: 10.1038/nm0395-244. [DOI] [PubMed] [Google Scholar]
- Spana D. Phosphodiesterase 4 inhibitors: current status. Br J Pharmacol. 2008;155:308–315. doi: 10.1038/bjp.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MM, Gristwood RW, Cooper N, Hellewell PG. Phosphodiesterase (PDE)4 inhibitors: anti-inflammatory drugs of the future? Tends Pharmacol Sci. 1997;18:164–167. doi: 10.1016/s0165-6147(97)01049-3. [DOI] [PubMed] [Google Scholar]
- Tenor H, Hedbom E, Häuselmann HJ, Schudt C, Hatzelmann A. Phosphodiesterase isoenzyme families in human osteoarthritis chondrocytes – functional importance of phosphodiesterase 4. Br J Pharmacol. 2002;135:609–618. doi: 10.1038/sj.bjp.0704480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen: an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Yamakami J, Tsuchiya M, Terajima T, Kizu J, Hori S. Anti-inflammatory effect of theophylline in rats and its involvement of the glucocorticoid-glucocorticoid receptor system. J Pharmacol Sci. 2008;106:566–570. doi: 10.1254/jphs.fp0071816. [DOI] [PubMed] [Google Scholar]
- Wooley PH, Luthra HS, Stuart JM, David CS. Type II collagen-induced arthritis inmice. Major histocompatibility complex (1 region) linkage and antibody response. J Exp Med. 1981;154:688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K. Antiinflammatory effect of theophylline. Allergy Pract. 2003;305:514–517. [Google Scholar]
- Yoshikawa M, Suzumura A, IT OA, Tumaru T, Takayanagi T. Effect of phosphodiesterase inhibitors on nitric oxide production by glial cells. Tohoku J Exp Med. 2002;196:167–177. doi: 10.1620/tjem.196.167. [DOI] [PubMed] [Google Scholar]


