Abstract
By human sensory analyses, we found that various extracellular calcium-sensing receptor (CaSR) agonists enhance sweet, salty, and umami tastes, although they have no taste themselves. These characteristics are known as “kokumi taste” and often appear in traditional Japanese cuisine. Although GSH is a typical kokumi taste substance (taste enhancer), its mode of action is poorly understood. Here, we demonstrate how the kokumi taste is enhanced by the CaSR, a close relative of the class C G-protein-coupled receptors T1R1, T1R2, and T1R3 (sweet and umami receptors). We identified a large number of CaSR agonist γ-glutamyl peptides, including GSH (γ-Glu-Cys-Gly) and γ-Glu-Val-Gly, and showed that these peptides elicit the kokumi taste. Further analyses revealed that some known CaSR agonists such as Ca2+, protamine, polylysine, l-histidine, and cinacalcet (a calcium-mimetic drug) also elicit the kokumi taste and that the CaSR-specific antagonist, NPS-2143, significantly suppresses the kokumi taste. This is the first report indicating a distinct function of the CaSR in human taste perception.
Keywords: Calcium, G-protein-coupled Receptors (GPCR), Glutathione, Neurobiology/Neuroscience, Peptides, Receptors, Signal Transduction/G-proteins
Introduction
The extracellular calcium-sensing receptor (CaSR)2 (1) is a class C G-protein-coupled receptor consisting (in humans) of 1078 amino acids. It plays a central role in extracellular calcium homeostasis in mammals (2). An increase in the blood calcium level is sensed by the CaSR, which in turn suppresses parathyroid hormone secretion, stimulates the secretion of calcitonin, and induces urinary calcium excretion to reduce blood calcium to normal levels. It has become apparent that the CaSR is expressed not only in the parathyroid glands and kidneys but also in many other tissues such as the liver, heart, lungs, alimentary canal, lymphocytes, pancreas, and the central and peripheral nervous systems, thus suggesting that it is involved in a range of biological functions (3). Several types of substances have been reported to possess CaSR activity, including cations such as Ca2+ and Gd3+, basic peptides such as protamine and polylysine, and polyamines such as spermine (3). In addition, the CaSR is moderately activated by the aromatic amino acids His, Trp, Phe, and Tyr and is weakly activated by other amino acids such as Arg, Lys, Val, and Gly (4, 5). However, the physiological relevance of these CaSR agonists, except calcium, is poorly understood.
Studies investigating the nutritional significance of calcium, mainly in laboratory animals, suggest the involvement of calcium in taste perception. Tordoff and co-workers (6, 7) reported the taste perception of calcium and the physiological mechanisms underlying calcium intake, appetite, and homeostasis and indicated that calcium deprivation increases the palatability of calcium. Although it is presumed that the CaSR (8) or a specific calcium channel is involved, no research provides direct evidence for the involvement of the CaSR in human taste perception. However, it is believed that calcium usually tastes bitter at a high concentration.
Humans can identify five basic tastes, sweet, salty, sour, bitter, and umami, which are believed to be recognized by specific receptors and transduction pathways. In addition to the five basic tastes, taste-enhancing substances are often added to foodstuffs. Substances with kokumi taste (9–12), which is distinct from the five basic tastes, have been used for many years in Japanese cuisine. GSH (γ-glutamylcysteinylglycine), a typical kokumi taste substance, is abundantly present in food-grade yeast extract, which is commercially available and has been used to make foods taste savory and hearty. The kokumi taste was first characterized by Ueda et al. (9, 10), who isolated a kokumi taste substance from water extracts of garlic and onion and identified GSH as the main active ingredient. GSH itself is tasteless; however, in the presence of small amounts of umami taste substances such as monosodium glutamate (MSG) and IMP, GSH synergistically reinforces those tastes.
In this study, we demonstrate that the CaSR is involved in kokumi taste perception in humans and report the discovery of various CaSR agonist peptides, including γ-glutamylvalylglycine (γ-Glu-Val-Gly), a potent kokumi taste substance.
EXPERIMENTAL PROCEDURES
Chemicals
The CaSR agonists used in the human sensory analyses were commercially available food additive products such as calcium lactate (Sky Food), protamine (Asama Chemicals), and polylysine (Nihon Chisso). Cinacalcet (13) and NPS-2143 (14) were chemically synthesized by methods described in the literature, and their activity was determined using HEK-293 cells that were transiently transformed with the human CaSR (hCaSR). All other reagents were a special purity grade purchased from Sigma-Aldrich Japan.
Peptides
The following peptides were used in the study: γ-Glu-Cys-Gly (GSH) and γ-Glu-Cys (Sigma-Aldrich Japan); γ-Glu-Cys(S-nitroso)-Gly (Dojin Chemical Laboratory); γ-Glu-Ala, γ-Glu-Gly, γ-Glu-Met, and γ-Glu-Abu-Gly (Bachem Feinchemikalien AG); and γ-Glu-Thr and γ-Glu-Val (Kokusan Chemical). Other peptides were chemically synthesized and purified by a contract manufacturer using well known techniques. Solutions of the glutamine- and cysteine-containing peptides were prepared immediately before use, whereas those of other peptides were stored at −20 °C after preparation. In the case of acidic and alkaline substances, the solutions were adjusted to pH 7.0–7.4 by the addition of NaOH or HCl. The CaSR agonists and antagonist used in this study are listed in supplemental Table 1.
Preparation of cRNA
cRNA of the hCaSR was prepared from human kidney cDNA (Clontech) using a PCR method. Briefly, primer oligonucleotide DNAs (forward primer, 5′-ACTAATACGACTCACTATAGGGACCATGGCATTTTATAGCTGCTGCTGG-3′, and reverse primer, 5′-TTATGAATTCACTACGTTTTCTGTAACAG-3′; NCBI accession number NM_000388) were synthesized, and PCR was performed using PfuUltra DNA polymerase (Stratagene) under the following conditions. After an initial reaction at 94 °C for 3 min, a cycle of reactions at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 2 min was repeated 35 times, and then a final reaction was performed at 72 °C for 7 min. The plasmid vector pBR322 (Takara) was digested with the restriction enzyme EcoRV. The PCR product was ligated to the EcoRV cleavage site of pBR322 using a ligation kit (Promega). hCaSR cRNA was synthesized using a cRNA preparation kit (Ambion) with this sequence as a template.
Determination of CaSR Activity Using Oocytes
CaSR agonist-induced currents were characterized using oocytes microinjected with hCaSR cRNA. Briefly, Xenopus laevis ovarian lobes were surgically removed, defolliculated, and treated with collagenase II. Oocytes were then microinjected with 10–20 ng of hCaSR cRNA and incubated for 36–48 h at 15 °C in Barth's solution. Activation of the CaSR (Gq class G-protein-coupled receptor) expressed in oocytes leads to an increase in intercellular calcium ions. This increase in free calcium activates oocyte endogenous calcium-dependent chloride channels concomitantly with a measurable current. The oocytes were impaled by two electrodes in a voltage-clamp configuration with a GeneClamp 500 (Axon), and responses were recorded using AxoScope 9.0 recording software (Axon) at a membrane potential of −70 mV. The oocytes were challenged with 0.1–1000 μm solutions of CaSR agonists in perfusion buffer containing 96 mm NaCl, 2 mm KCl, 1 mm MgCl2, 1.8 mm CaCl2, and 5 mm Hepes (pH 7.2), and the peak recorded current was deemed the strength of receptor activation.
Determination of CaSR Activity Using HEK-293 Cells
hCaSR cDNA was constructed in the expression vector pcDNA3.1 and transiently transfected into HEK-293 cells. Briefly, the cDNA was diluted with Opti-MEM I medium (Invitrogen), mixed with FuGENE 6 (Roche Applied Science), and poured onto HEK-293 cells grown at a submaximum concentration. After 24 h of culture in a 96-well plate, the cells were incubated with 5 μm Calcium-4 (Calcium-4 assay kit, Molecular Devices) for 45–60 min, and measurements were conducted using an image analyzer (FlexStation, Molecular Devices) and its associated software. Activation of the CaSR expressed in HEK-293 cells leads to an increase in intercellular calcium ions. This increase in free calcium was determined using the calcium dye Calcium-4. The dye binds the free Ca2+, resulting in an increase in dye fluorescence, which is excited at 485 nm and emits at 525 nm. The concentration dependence of the fluorescence intensity was analyzed with various CaSR agonists with or without 0.0002% (4.5 μm) NPS-2143. The assay buffer for calcium imaging contained 0.9 mm CaCl2.
Continuous Kokumi Taste Sensory Analyses of GSH and γ-Glu-Val-Gly
Time course profiles of the kokumi taste of GSH (0.08%) and γ-Glu-Val-Gly (0.01%) were determined by continuous human sensory analyses. Briefly, the test samples contained low concentrations of umami and salty tastes (0.02% MSG, 0.02% IMP, and 0.07% NaCl) with or without kokumi taste substances. The test sample (20 ml) was administered orally, and the taste assessors were instructed to keep the sample in their mouths for at least 30 s, during which time the taste intensity was expressed as a percentage score in comparison with the maximum intensity obtained with a standard solution containing 0.7% MSG and 0.5% NaCl, taken as 100%. Data were recorded every second. The analyses were performed by a panel of 20 well trained assessors.
Kokumi Taste Determination by Human Sensory Analyses with a 5-Point Rating Scale
Samples were dissolved in distilled water and adjusted to pH 6.8–7.2 with NaOH. The human sensory analysis of a sample solution was performed using a previously described method (9). A well trained panel of 20 assessors was asked to evaluate the sample solutions on a 5-point rating scale, from −2 (apparently suppressed) to +2 (apparently strong). The kokumi taste in each basic taste solution was estimated by the thickness, a measure of the taste intensity 5 s after tasting. The results were analyzed by Student's t test. The kokumi taste in the presence of each basic taste, i.e. sweet, salty, or umami, was determined with GSH (0.1%) or γ-Glu-Val-Gly (0.01%) mixed with sucrose (3.3%), NaCl (0.9%), or MSG (0.5%), respectively. The presence of the kokumi taste in foodstuffs was determined as follows. Consommé containing 2% commercial chicken consommé powder (Ajinomoto Co., Inc.) was prepared. GSH (0.02%), γ-Glu-Val-Gly (0.002%), and γ-Glu-Val-Leu (0.02%; a low-activity control peptide) were then dissolved in the consommé. The kokumi taste was determined for three paired scale terms: thickness, continuity, and mouthfulness. Thickness was expressed in terms of increased taste intensity at ∼5 s after tasting; continuity was expressed as the taste intensity at ∼20 s; and mouthfulness was expressed as the reinforcement of the taste sensation throughout the mouth and not just on the tongue. Known CaSR agonists and an antagonist were used at the following final concentrations: calcium lactate (0.35%), protamine (0.02%), polylysine (0.08%), and l-histidine (0.2%). Cinacalcet was dissolved in 99.5% ethanol at a concentration of 1% and then diluted to 0.0015% (38 μm) with the tasting solution for sensory analysis. NPS-2143 was dissolved in 99.5% ethanol at a concentration of 0.1% and then diluted to 0.0002% (4.5 μm) with the tasting solution. The remaining ethanol in the tasting solution did not affect the sensory analysis evaluation. Methods used in the human sensory analyses were approved by the Management Committee of the Food Product Application Center at Ajinomoto Co., Inc., and informed consent was obtained from all assessors.
Quantitative Analyses of the Kokumi Taste Compared with the GSH Kokumi Standard
Quantitative analyses were conducted to compare taste thickness. The strength of the kokumi taste was evaluated as a point of subjective equivalence (PSE) as follows. Test samples of γ-Glu-Cys (0.15%), γ-Glu-Val (0.15%), γ-Glu-Ala (0.5%), γ-Glu-Abu-Gly (0.05%), or γ-Glu-Val-Gly (0.01%) were mixed with umami and salty taste solutions (0.05% MSG, 0.05% IMP, and 0.5% NaCl), and their tastes were compared with those of various concentrations of GSH as reference solutions. Eight concentrations of GSH in logarithmically equal steps at 50% intervals were used (0.02, 0.03, 0.044, 0.07, 0.10, 0.15, 0.23, and 0.34%, w/v). Each test sample was paired twice with each reference solution. Assessors judged the PSE between the sample and reference solutions. All judgments were dichotomous, i.e. the assessors were required to rate a test sample as either more or less intense than the reference GSH solution. The PSE was defined as the concentration that was judged to give a sensation that was equivalent to that of the standard solution. The analyses were performed by a panel of 17 well trained assessors.
Reverse Transcription (RT)-PCR Analyses of mRNA Extract of Mouse Taste Buds
C57BL/6N mice (6-week-old males, Charles River) were killed by cervical dislocation. RT-PCR amplification was performed using primers that amplify the mouse CaSR, T1R2, and β-actin. Briefly, tissues containing circumvallate or foliate papillae were injected into the submucosal layer with a mixture of 1 mg/ml collagenase A (Roche Applied Science), 2.5 mg/ml Dispase II (Roche Applied Science), and 1 mg/ml trypsin inhibitor (Sigma-Aldrich Japan) and then incubated for 20 min at room temperature. The papilla-containing epithelium was peeled from the underlying connective tissue. The total RNA was isolated from the epithelial papillae and from the epithelium without taste buds (RNA micro kit, Stratagene). The purified RNA was denatured at 60 °C for 5 min, and first-strand cDNA was synthesized at 50 °C for 60 min using oligo(dT)12–18 primer and reverse transcriptase (SuperScript III, Invitrogen) in a final volume of 20 μl. After synthesizing the cDNA, 1 μl of cDNA was used as a template in 20 μl of PCR mixture with Taq polymerase (Invitrogen). The PCR conditions were as follows: 94 °C for 2 min and 29–35 cycles at 94 °C for 30 s, 58 °C for 20 s, and 72 °C for 45 s. The PCR products were analyzed by gel electrophoresis (2% agarose gel) with GelRed staining (Biotium). The primers used were as follows: mouse CaSR, 5′-TCGAGACCCCTTACATGGAC-3′ (forward) and 5′-AGTAGTTCCCCACCAGGTCA-3′ (reverse); mouse T1R2, 5′-TGGCAGCTACTCAGGGAGAT-3′ (forward) and 5′-GGACAGTCCACACACTCGAA-3′ (reverse); and mouse β-actin, 5-CACCCTGTGCTGCTCACC-3 (forward) and 5′-GCACGATTTCCCTCTCAG-3′ (reverse).
RESULTS
Discovery of CaSR Agonist γ-Glutamyl Peptides
We screened di- and tripeptide libraries for CaSR activity and discovered 46 γ-glutamyl (γ-Glu) peptides, including GSH (γ-Glu-Cys-Gly) (Table 1). Among them, γ-Glu-Val-Gly had the most potent CaSR activity. Amino acids have been reported to bind to the large extracellular Venus flytrap domain of the CaSR, a structure common to all members of class C G-protein-coupled receptors. It was suggested that all amino acids bind at the Venus flytrap domain-binding pocket, through the amino and carboxyl groups (5). This binding motif is common to free amino acids and γ-Glu peptides (γ-glutamic acid (γ-Glu) at the N-terminal end). In contrast, substitution of the γ-Glu residue with β-Asp or β-Ala resulted in loss of activity (data not shown). Furthermore, those γ-Glu tripeptides with a large hydrophobic acid, a basic acid, or an acidic amino acid at the second position were inactive. A thiol group of cysteine at the second position was not essential for CaSR activity. The third-position analogs of γ-Glu-Val-Gly were active, except for Ile, Trp, Tyr, and β-Ala. The presence of the third-position residue, especially one with a free carboxyl terminus and no side chain (Gly), substantially enhanced activity. These results indicate that an exact alignment and the presence of two charged groups in the N-terminal residue are required for CaSR binding. The putative interaction between γ-Glu-Val-Gly and the l-amino acid-binding site of the CaSR is illustrated in supplemental Fig. 1.
TABLE 1.
Determination of CaSR activity using Xenopus oocytes
A two-electrode voltage-clamp assay was performed using oocytes microinjected with hCaSR cRNA at −70 mV. Peptides were added at various concentrations (1000, 500, 300, 100, 50, 30, 10, 3, 1, 0.3, and 0.1 μm), and the minimum effective concentration at which a current was detected was a measure of the strength of CaSR activation. The results show that 46 γ-glutamyl peptides had CaSR activity. No response was observed in oocytes injected with distilled water as a control. In comparison, EC50 values (μm) for six peptides determined by HEK-293 assay are indicated in parentheses. The HEK-293 assay gave a higher sensitivity than the oocyte assay. SNO, S-nitroso; Met(O), Met sulfoxide; Tau, taurine; tLeu, tert-Leu.
| No. | γ-Glu peptide | CaSR activity | No. | γ-Glu peptides | CaSR activity |
|---|---|---|---|---|---|
| μm | μm | ||||
| 1 | γ-Glu-Gly | 1000 | 24 | γ-Glu-Ile-Gly | 100 |
| 2 | γ-Glu-Val-NH2 | 1000 | 25 | γ-Glu-Cys(S-allyl)-Gly | 100 |
| 3 | γ-Glu-Val-ol | 1000 | 26 | γ-Glu-Val-His | 100 |
| 4 | γ-Glu-Met(O) | 1000 | 27 | γ-Glu-Val-Orn | 100 |
| 5 | γ-Glu-Val-Val | 1000 | 28 | γ-Glu-Ala | 50 (3.7) |
| 6 | γ-Glu-Val-Glu | 1000 | 29 | γ-Glu-Thr | 50 |
| 7 | γ-Glu-Val-Lys | 1000 | 30 | γ-Glu-Cys(S-Me) | 30 |
| 8 | γ-Glu-Val-Arg | 1000 | 31 | γ-Glu-Cys-Gly | 30 |
| 9 | γ-Glu-Val-Asp | 1000 | 32 | γ-Glu-Val-Phe | 30 |
| 10 | γ-Glu-Val-Met | 1000 | 33 | γ-Glu-Val-Ser | 30 |
| 11 | γ-Glu-Val-Thr | 1000 | 34 | γ-Glu-Val-Pro | 30 |
| 12 | γ-Glu-Met | 500 | 35 | γ-Glu-Val-Asn | 30 |
| 13 | γ-Glu-Orn | 500 | 36 | γ-Glu-Val | 10 (1.6) |
| 14 | γ-Glu-Ser | 300 | 37 | γ-Glu-Ala-Gly | 10 |
| 15 | γ-Glu-Tau | 300 | 38 | γ-Glu-Ser-Gly | 10 |
| 16 | γ-Glu-Leu-Gly | 300 | 39 | γ-Glu-Val-Gln | 10 |
| 17 | γ-Glu-Thr-Gly | 300 | 40 | γ-Glu-Val-Cys | 10 |
| 18 | γ-Glu-Pro-Gly | 300 | 41 | γ-Glu-Cys | 3 (0.46) |
| 19 | γ-Glu-Val-Ala | 300 | 42 | γ-Glu-Cys-Gly (GSH) | 3 (0.71) |
| 20 | γ-Glu-Cys(S-Me)(O) | 300 | 43 | γ-Glu-Abu-Gly | 3 (0.025) |
| 21 | γ-Glu-Leu | 100 | 44 | γ-Glu-Cys(S-Me)-Gly | 3 |
| 22 | γ-Glu-Ile | 100 | 45 | γ-Glu-Cys(SNO)-Gly | 3 |
| 23 | γ-Glu-tLeu | 100 | 46 | γ-Glu-Val-Gly | 0.1 (0.039) |
Time Course Profiles of Kokumi Taste Intensity of GSH and γ-Glu-Val-Gly
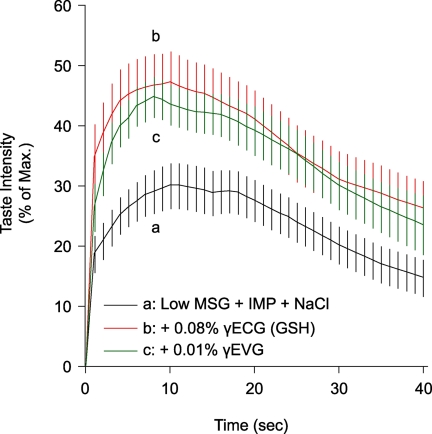
Because GSH is a known authentic kokumi taste substance, we evaluated whether or not the newly identified CaSR agonist peptide γ-Glu-Val-Gly could impart a kokumi taste. Short-term time course profiles of the kokumi taste of GSH and γ-Glu-Val-Gly were determined by human sensory analyses (Fig. 1). In the case of control solutions containing low concentrations of umami and salty substances, the taste intensity increased after oral administration at 0 s, peaked at ∼10 s, and then decreased over a 40-s period. The peak intensity was significantly elevated (i.e. doubled) in the presence of GSH or γ-Glu-Val-Gly, and this elevation remained distinct even after 20 s.
FIGURE 1.
Time course profiles of kokumi taste of GSH and γ-Glu-Val-Gly. GSH (0.1%; a control kokumi taste substance) and γ-Glu-Val-Gly (0.01%; a newly discovered peptide) time course profiles were determined by human sensory analyses performed in a blind test. The taste enhancement intensity was expressed as a percentage score in comparison with the maximum intensity obtained with a standard solution containing 0.07% MSG, 0.07% IMP, and 0.7% NaCl, taken as 100%. Trace a, low concentrations of umami and salty tastes (0.02% MSG, 0.02% IMP, and 0.07% NaCl); trace b, the solution used for trace a plus 0.08% GSH; trace c, the solution used for trace a plus 0.01% γ-Glu-Val-Gly. Data are shown as averages (n = 20) with error bars (S.E.). The solutions used for traces b and c were significantly stronger than that used for trace a at 5 s (p < 0.01) and 20 s (p < 0.05).
Kokumi Taste Intensity of GSH and γ-Glu-Val-Gly
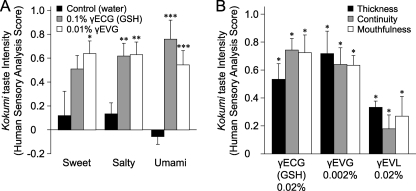
We tested the kokumi taste effects of GSH and γ-Glu-Val-Gly on the basic tastes (sweet, salty, and umami) and found a significant enhancement as shown in Fig. 2A, although they have no taste themselves (data not shown). The intensity of taste enhancement was graded as follows: 0 points for no intense activity, 1 point for fairly intense activity, and 2 points for intense activity. In this study, enhancement of the kokumi taste (so-called “thickness”) was measured. Sensory analysis of γ-Glu-Val-Gly was performed at a concentration of 0.01%, one-eighth that of GSH.
FIGURE 2.
Human sensory analyses for kokumi taste of GSH and γ-Glu-Val-Gly. A, the kokumi taste intensity of GSH and γ-Glu-Val-Gly for each basic taste was determined. In this experiment, GSH (0.1%) and γ-Glu-Val-Gly (0.01%) were mixed independently with sucrose (3.3%), NaCl (0.9%), or MSG (0.5%). The kokumi taste was rated on a 5-point scale (ranging from −2 (apparently suppressed) to +2 (apparently strong)) in terms of thickness, i.e. taste enhancement ∼5 s after tasting (9, 34). Human sensory analyses were performed in a blind test by a panel of 20 well trained assessors, and the results were analyzed by Student's t test. Data are shown as averages (n = 20) with error bars (S.E.). Asterisks indicate significant differences (Student's t test) from the control: *, p < 0.05; **, p < 0.01; ***, p < 0.001. B, the Kokumi taste profiles of GSH and γ-Glu-Val-Gly for chicken consommé were evaluated as follows. GSH (0.02%), γ-Glu-Val-Gly (0.002%), and γ-Glu-Val-Leu (0.02%; a low-activity control peptide) were dissolved in chicken consommé prepared with 2% of a commercial chicken consommé powder. Human sensory analyses were performed with a focus on kokumi taste characteristics, i.e. thickness, continuity, and mouthfulness (see “Experimental Procedures”). The kokumi taste was evaluated in a blind test on a 5-point rating scale for the three paired scale terms. Data are shown as averages (n = 20) with error bars (S.E.). Asterisks indicate significant differences (Student's t test) from the control: *, p < 0.05.
Further examination was performed to reveal enhancement of the kokumi taste when the CaSR agonist peptides were mixed with a foodstuff. The kokumi taste intensity of γ-Glu-Val-Gly and GSH was much more significant when the peptides were mixed into a solution such as chicken consommé as a result of the coexistence of various flavors that taste sweet, umami, and salty (Fig. 2B). γ-Glu-Val-Gly enhanced all three kokumi taste characteristics (thickness, continuity, and mouthfulness). γ-Glu-Val-Gly produced a potent kokumi taste at a concentration as low as 0.002%, whereas γ-Glu-Val-Leu (a low-activity control peptide) produced a faint kokumi taste (Fig. 2B).
Because GSH has an aroma that is based on the sulfur atom of Cys, it seemed that the smell was related to the kokumi taste (15). It is known that GSH is contained in many kinds of foods, especially meat, and that the hydrogen sulfate derived from cysteine produces a sulfur odor (16). GSH reinforced the aroma, but γ-Glu-Val-Gly did not. However, γ-Glu-Val-Gly produced a kokumi taste, even though its second amino acid is Val instead of Cys. These results suggest that the kokumi taste is integrated into the taste sensory system and is independent of the olfactory sensory system.
Quantitative CaSR Activity of γ-Glu Peptides
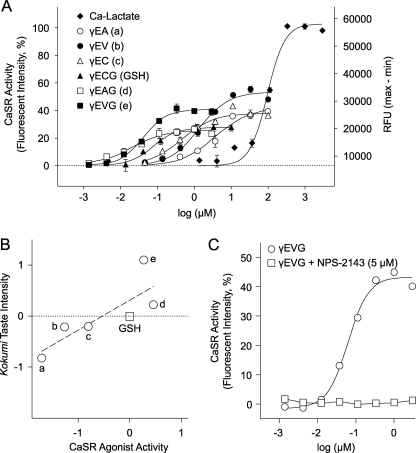
Detailed dose-response curves were determined using HEK-293 cell assays for 6 of the 46 γ-Glu peptides identified as CaSR agonists. Fluorescence intensities observed with these peptides were 26–53% of the maximum intensity observed with Ca2+ or cinacalcet (Fig. 3A and supplemental Fig. 2). The effective concentration (EC50) values for the γ-Glu peptides, which correspond to the concentrations that produced 50% of the maximum intensity for Ca2+, are as follows: γ-Glu-Ala, 3.65 μm/42.6 = Emax; γ-Glu-Val, 1.34 μm/53.3; γ-Glu-Cys, 458 nm/38.0; γ-Glu-Cys-Gly, 76.5 nm/27.6; γ-Glu-Abu-Gly, 18.1 nm/25.6; and γ-Glu-Val-Gly, 41.9 nm/40.8.
FIGURE 3.
Correlation between CaSR activity and kokumi taste intensity. A, HEK-293 cells transiently expressing hCaSRs were used in the calcium imaging method, and the dose dependence was analyzed for six CaSR agonist peptides. The effective concentration (EC50) values are as follows: γ-Glu-Ala (a), 3.65 μm/42.6 = Emax; γ-Glu-Val (b), 1.34 μm/53.3; γ-Glu-Cys (c), 458 nm/38.0; γ-Glu-Cys-Gly (GSH), 76.5 nm/27.6; γ-Glu-Abu-Gly (d), 18.1 nm/25.6; and γ-Glu-Val-Gly (e), 41.9 nm/40.8. γ-Glu peptides showed 26–53% of the maximum intensity (Emax) observed with Ca2+ (calcium = 100%). RFU, relative fluorescence units. B, the relationship between CaSR activity (A, EC50) and kokumi taste intensity (Table 1) of five γ-Glu peptides is plotted on logarithmic scales in comparison with GSH. A Pearson correlation coefficient was used to calculate the relationship between CaSR activity and kokumi taste intensity. A significant positive correlation was found (r2 = 0.660, p = 0.0496). C, shown is the strong inhibitory effect of the CaSR antagonist. The CaSR activity dose response of γ-Glu-Val-Gly was analyzed using HEK-293 cells transiently expressing hCaSR mRNA in the absence and presence of NPS-2143 (5 μm).
Correlations between CaSR Activity and Kokumi Taste Intensity of γ-Glu Peptides
A quantitative sensory evaluation of the kokumi taste was performed with the six γ-Glu peptides, and the results were expressed as PSEs for each γ-Glu peptide test solution in relation to that of the reference solution, GSH (9). The intensity of the kokumi taste was quantified in terms of the GSH concentration required to attain an equal intensity of sensation. γ-Glu-Val-Gly had the most potent kokumi taste; a 0.01% solution produced a kokumi taste equivalent to a GSH concentration of 0.128%. Therefore, we estimated that the kokumi taste of γ-Glu-Val-Gly is 12.8 times stronger than that of GSH. All five CaSR agonist peptides tested against GSH were confirmed to have kokumi taste (Table 2). CaSR activity was investigated in detail using HEK-293 assays to determine EC50 values (Fig. 3A). A significant correlation was observed between CaSR activity and kokumi taste intensity when the intensities were expressed as a value relative to that of GSH and was plotted in two dimensions on a logarithmic scale (Fig. 3B). These peptides were randomly chosen from the CaSR agonist peptides listed in Table 1. These results strongly suggest that the CaSR is involved in the perception of kokumi taste in humans.
TABLE 2.
Quantitative analyses of the kokumi taste intensity of γ-glutamyl peptides
A quantitative evaluation of the human sensory analyses was performed by a panel of 17 well trained assessors, who judged the PSE between sample and reference GSH solutions. A series of GSH concentrations were chosen in logarithmically equal steps at 50% intervals (0.02, 0.03, 0.044, 0.07, 0.10, 0.15, 0.23, and 0.34%, w/v). Each test sample was paired twice with the reference GSH solution, and the assessors were required to rate the sensation produced by a test sample as either more or less intense than that of the reference solution. The PSE was defined as the concentration that was judged to produce a sensation equal to that of the standard solution. The sample concentration was determined by preliminary tests. Data were analyzed by the probit method.
| Sample concentration | GSH concentration for PSE | Kokumi taste intensity compared with GSH | |
|---|---|---|---|
| % | % | ||
| GSH (γ-Glu-Cys-Gly) | 1 | ||
| γ-Glu-Ala | 0.5 | 0.074 | 0.15 |
| γ-Glu-Val | 0.15 | 0.092 | 0.61 |
| γ-Glu-Cys | 0.15 | 0.094 | 0.63 |
| γ-Glu-Abu-Gly | 0.05 | 0.085 | 1.7 |
| γ-Glu-Val-Gly | 0.01 | 0.128 | 12.8 |
Kokumi Taste Intensity of Known CaSR Agonists and Effect of the Antagonist
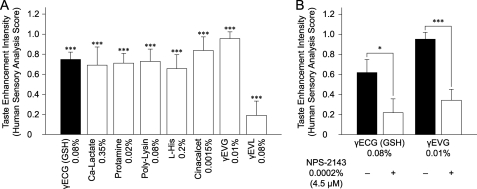
We determined the kokumi taste of known CaSR agonists and the inhibitory effect of the synthetic CaSR antagonist, NPS-2143. Although calcium lactate, protamine, polylysine, and l-histidine are used as food additives, it is not known whether they have their own taste. Cinacalcet, a chemically synthesized allosteric activator of the CaSR, is useful as a drug for the treatment of secondary hyperparathyroidism and hypercalcemia. In our tests, calcium lactate, protamine, polylysine, l-histidine, and cinacalcet showed significant kokumi taste enhancement at concentrations of 0.35, 0.02, 0.08, 0.2, and 0.0015, respectively (Fig. 4A). Some of these substances had bitter tastes at higher concentrations but were suitable for analysis when tested under our conditions. In contrast, the tested CaSR agonist peptides did not possess a bitter taste at any concentration, even though kokumi taste intensity was evaluated by human sensory analyses over a wide range of concentrations.
FIGURE 4.
Kokumi taste profiles of known CaSR agonists and an antagonist. A, human sensory analyses of the CaSR agonists were performed to determine the kokumi taste with known CaSR agonists. CaSR agonists were mixed with a control solution of MSG (0.1%) and NaCl (0.5%) at the following concentrations: GSH, 0.08% (control kokumi taste substance; calcium lactate, 0.35%; protamine, 0.02%; polylysine, 0.08%; l-histidine, 0.2%; cinacalcet, 0.0015%; γ-Glu-Val-Gly, 0.01%; and γ-Glu-Val-Leu, 0.08% (a low-activity control peptide). Human sensory analyses were performed in a blind test focusing on one of the kokumi taste characteristics, i.e. thickness. Data are shown as average scores (n = 20) with error bars (S.E.). Asterisks indicate significant differences (Student's t test) from the control: ***, p < 0.001. B, human sensory analyses of CaSR agonists were performed to determine kokumi taste in the presence or absence of a CaSR antagonist (NPS-2143) using the method described for Fig. 3A. The concentrations used in this experiment were as follows: GSH, 0.08%; γ-Glu-Val-Gly, 0.01%; and NPS-2143, 0.0002% (4.5 μm). Data are shown as average scores (n = 20) with error bars (S.E.). Asterisks indicate significant differences (Student's t test) from NSP-2143: *, p < 0.05; ***, p < 0.001.
Human sensory analyses were performed to determine whether the kokumi tastes of GSH and γ-Glu-Val-Gly were suppressed in the presence of 0.0002% (4.5 μm) NPS-2143 (Fig. 4B). NPS-2143 is a CaSR-specific antagonist that effectively inhibits the activity of both GSH (data not shown) and γ-Glu-Val-Gly (Fig. 3C). These results clearly indicate that the CaSR is involved in kokumi taste perception in humans.
Expression of the CaSR in Peripheral Taste Tissue
RT-PCR analyses in mice have demonstrated that mRNAs of the CaSR and T1R2 (used as the control for taste cells) are expressed in the taste bud-containing epithelium of the circumvallate and foliate papillae. The RT-PCR products for the CaSR were not found in the epithelium surrounding the papillae (supplemental Fig. 2). These findings suggest that the CaSR could be a kokumi taste receptor involved in taste perception. On the other hand, mRNA expression of Gprc6a, which is the closest relative gene of the CaSR, was not detected in circumvallate and foliate papillae in our experiments with four independent sets of primers.
DISCUSSION
Taste plays a crucial role in the response to sweet, bitter, sour, salty, and umami stimuli and in the detection of nutritional status. These stimuli are believed to be recognized by specific receptors and transduction pathways. It has been previously shown that the sweet receptor is a heterodimer of T1R2 and T1R3 (17, 18), that the umami receptor is a heterodimer of T1R1 and T1R3 (17, 19), and that the bitter receptors are a family of T2R receptors (20, 21). The epithelial sodium channel (22) and transient receptor potential family members PKD1L3 and PKD2L1 (23) are plausible candidates as receptors for the salty and sour (acid) tastes, respectively. A common characteristic of kokumi taste substances such as GSH is that they are tasteless by themselves but enhance basic tastes. Because GSH appears on the list of identified CaSR agonists (Table 1), we hypothesized that CaSR agonist peptides such as γ-Glu-Val-Gly might impart a kokumi taste.
In this study, we have shown that CaSR agonists, including calcium, are possible appetite stimulants that enhance basic tastes by providing a kokumi taste and have demonstrated that the CaSR is involved in human kokumi taste perception. CaSR agonists may directly activate the CaSR expressed on the surface of taste cells and subsequently be integrated in the brain through the central nervous system. Until now, the physiological roles of calcium appetite were less well understood than those of other appetites. Some behavioral studies have focused on characterizing the specificity of the calcium appetite, and abundant behavioral experimental data on rats and mice are available, although the data are scarce for humans.
Attempts have been made to develop kokumi taste ingredients for commercial use from natural products such as autolyzed yeast extracts and hydrolyzed vegetable proteins (24); however, there are few examples of the isolation of pure kokumi taste substances. Recently, new kokumi taste substances have been isolated from an extract of edible beans, and these have been characterized by human sensory analysis (10). The active substances were identified as the γ-Glu dipeptides γ-Glu-Val and γ-Glu-Leu, which appear in the list of CaSR agonist peptides that we identified (Table 1). These γ-Glu peptides are also found in beech fruit (25) and in bovine brain (26); γ-Glu-Cys is present in yeast extracts (27). The identification of the kokumi taste receptor allowed us to screen for new kokumi taste substances using in vitro high-throughput assay methods. These kokumi taste substances are useful as taste enhancers in many kinds of food.
Wang et al. (28) reported that GSH does not directly activate the CaSR but potentiates calcium-induced responses. They showed that GSH does not activate the CaSR in an assay buffer containing 0.5 mm calcium, but activation occurred under similar conditions when the assay buffer was supplemented with an additional 0.3 mm calcium administered simultaneously. In our study, GSH and γ-Glu-Val-Gly activated the CaSR in an assay buffer containing ≥0.75 mm calcium (supplemental Fig. 2), thus suggesting that a certain basal level of calcium, corresponding to a physiological concentration, is necessary for activation of the CaSR by γ-Glu peptides. γ-Glu peptides such as γ-Glu-Val-Gly may act as partial allosteric agonists in a physiological environment, including plasma and saliva, in which the calcium concentration is ∼0.75 mm or higher.
CaSR gene expression in taste buds was investigated using mouse tissue specimens. RT-PCR analyses showed that the CaSR mRNA was expressed in taste bud-containing circumvallate and foliate papilla epithelium (supplemental Fig. 3). However, RT-PCR products for the CaSR were not found in the epithelium surrounding the papillae. The same results were shown by immunohistological observation of rat and mouse taste buds (29). Among the class C G-protein-coupled receptors, the CaSR has the highest homology with GPRC6A. It has been reported that a chimeric receptor consisting of the goldfish 5.24 (mammalian GPRC6A homolog) outer membrane domain and the mouse GPRC6A trans- and intramembrane domains is activated by GSH but that neither a chimeric receptor consisting of the GPRC6A outer membrane domain and 5.24 trans- and intramembrane domains nor full-length GPRC6A itself is functionally active (28). Wellendorph et al. (30) reported that GPRC6A is expressed in rat taste bud-containing epithelia. However, in our experiment, GPRC6A mRNA expression was not detected in circumvallate and foliate epithelia from adult mice. These findings suggest that the kokumi taste is mediated by CaSR activation, and it is unlikely that GPRC6A is involved in human taste perception.
Overall, our results show that CaSR agonists are recognized by the CaSR in taste cells and produce a desirable kokumi taste sensation in humans. Various kinds of CaSR agonists, including calcium, exist widely in nature and are found in plants, animals, and microbes. The involvement of calcium in taste perception has been previously suggested by some behavioral studies on mice that focused on calcium appetite, from which the involvement of T1R3 in calcium and magnesium recognition was proposed (31). No previous research has established direct evidence for the involvement of the CaSR in human taste perception. In addition to being expressed in taste buds, the CaSR is also expressed in the gastrointestinal tract (32, 33), thus suggesting that CaSR agonists present in foodstuffs such as cations, amino acids, and peptides could be determinants of physiological processes such as motility, digestion, absorption, and secretion. Taken together, our findings indicate that there is an appetite for CaSR agonists in humans and highlight the importance of further detailed studies to elucidate the physiological significance of the palatability of kokumi taste substances in humans.
Supplementary Material
Acknowledgments
We sincerely thank Yasuhito Uezono and Seiji Fukumoto for valuable comments on electrophysiology and the CaSR, respectively. We thank Kiyoshi Miwa, Tohru Kouda, and Hiroaki Takino for encouragement and continued support of this work. We are grateful to Hisashi Uneyama, Chiori Ijichi, Mitsuo Takahashi, Sayaka Asari, Kaoru Takenaka, Reiko Yasuda, Seiichi Sato, Megumi Kaneko, Takako Hirose, Orie Yokoi, and Yasuhisa Manabe for helpful discussions and assistance. We are also grateful to members of the taste panel at the Food Products Global R&D Center in Ajinomoto Co., Inc.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3 and Table 1.
- CaSR
- calcium-sensing receptor
- hCaSR
- human CaSR
- MSG
- monosodium glutamate
- Abu
- α-aminobutyric acid
- PSE
- point of subjective equivalence
- RT
- reverse transcription.
REFERENCES
- 1.Brown E. M., Gamba G., Riccardi D., Lombardi M., Butters R., Kifor O., Sun A., Hediger M. A., Lytton J., Hebert S. C. (1993) Nature 366, 575–580 [DOI] [PubMed] [Google Scholar]
- 2.Chattopadhyay N., Vassilev P. M., Brown E. M. (1997) Biol. Chem. 378, 759–768 [PubMed] [Google Scholar]
- 3.Brown E. M., MacLeod R. J. (2001) Physiol. Rev. 81, 239–297 [DOI] [PubMed] [Google Scholar]
- 4.Conigrave A. D., Quinn S. J., Brown E. M. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 4814–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conigrave A. D., Hampson D. R. (2006) Trends Endocrinol. Metab. 17, 398–407 [DOI] [PubMed] [Google Scholar]
- 6.McCaughey S. A., Forestell C. A., Tordoff M. G. (2005) Physiol. Behav. 84, 335–342 [DOI] [PubMed] [Google Scholar]
- 7.Reed D. R., Li X., McDaniel A. H., Lu K., Li S., Tordoff M. G., Price R. A., Bachmanov A. A. (2003) Mamm. Genome 14, 302–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tordoff M. G., Reed D. R., Shao H. (2008) Genes Brain Behav. 7, 618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda Y., Sakaguchi M., Hirayama K., Miyajima R., Kimizuka A. (1990) Agric. Biol. Chem. 54, 163–169 [Google Scholar]
- 10.Ueda Y., Yonemitsu M., Tsubuku T., Sakaguchi M., Miyajima R. (1997) Biosci. Biotechnol. Biochem. 61, 1977–1980 [DOI] [PubMed] [Google Scholar]
- 11.Dunkel A., Köster J., Hofmann T. (2007) J. Agric. Food Chem. 55, 6712–6719 [DOI] [PubMed] [Google Scholar]
- 12.Toelstede S., Dunkel A., Hofmann T. (2009) J. Agric. Food Chem. 57, 1440–1448 [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez M., Nemeth E., Martin D. (2005) Am. J. Physiol. Renal. Physiol. 288, F253–F264 [DOI] [PubMed] [Google Scholar]
- 14.Rybczynska A., Lehmann A., Jurska-Jasko A., Boblewski K., Orlewska C., Foks H., Drewnowska K. (2006) J. Endocrinol. 191, 189–195 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Chen M., Ho C. (1988) J. Agric. Food Chem. 36, 992–996 [Google Scholar]
- 16.Mecchi E. P., Pippen E. L., Lineweaver H. (1964) J. Food Sci. 29, 393–399 [Google Scholar]
- 17.Hoon M. A., Adler E., Lindemeier J., Battey J. F., Ryba N. J., Zuker C. S. (1999) Cell 96, 541–551 [DOI] [PubMed] [Google Scholar]
- 18.Nelson G., Hoon M. A., Chandrashekar J., Zhang Y., Ryba N. J., Zuker C. S. (2001) Cell 106, 381–390 [DOI] [PubMed] [Google Scholar]
- 19.Nelson G., Chandrashekar J., Hoon M. A., Feng L., Zhao G., Ryba N. J., Zuker C. S. (2002) Nature 416, 199–202 [DOI] [PubMed] [Google Scholar]
- 20.Chandrashekar J., Mueller K. L., Hoon M. A., Adler E., Feng L., Guo W., Zuker C. S., Ryba N. J. (2000) Cell 100, 703–711 [DOI] [PubMed] [Google Scholar]
- 21.Adler E., Hoon M. A., Mueller K. L., Chandrashekar J., Ryba N. J., Zuker C. S. (2000) Cell 100, 693–702 [DOI] [PubMed] [Google Scholar]
- 22.Rotin D., Bar-Sagi D., O'Brodovich H., Merilainen J., Lehto V. P., Canessa C. M., Rossier B. C., Downey G. P. (1994) EMBO J. 13, 4440–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishimaru Y., Inada H., Kubota M., Zhuang H., Tominaga M., Matsunami H. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aaslyng M. D., Martens M., Poll L., Nielsen P. M., Flyge H., Larsen L. M. (1998) J. Agric. Food Chem. 46, 481–489 [DOI] [PubMed] [Google Scholar]
- 25.Kristensen I., Larsen P., Sørensen H. (1974) Phytochemistry 13, 2803–2811 [Google Scholar]
- 26.Kanazawa A., Kakimoto Y., Nakajima T., Sano I. (1965) Biochim. Biophys. Acta 111, 90–95 [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Wei G., Chen J. (2004) Appl. Microbiol. Biotechnol. 66, 233–242 [DOI] [PubMed] [Google Scholar]
- 28.Wang M., Yao Y., Kuang D., Hampson D. R. (2006) J. Biol. Chem. 281, 8864–8870 [DOI] [PubMed] [Google Scholar]
- 29.San Gabriel A., Uneyama H., Maekawa T., Torii K. (2009) Biochem. Biophys. Res. Commun. 378, 414–418 [DOI] [PubMed] [Google Scholar]
- 30.Wellendorph P., Burhenne N., Christiansen B., Walter B., Schmale H., Bräuner-Osborne H. (2007) Gene 396, 257–267 [DOI] [PubMed] [Google Scholar]
- 31.Tordoff M. G., Shao H., Alarcon L. K., Margolskee R. F., Mosinger B., Bachmanov A. A., Reed D. R., McCaughey S. A. (2008) Physiol. Genomics 34, 338–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hebert S. C., Cheng S., Geibel J. (2004) Cell Calcium 35, 239–247 [DOI] [PubMed] [Google Scholar]
- 33.Conigrave A. D., Brown E. M. (2006) Am. J. Physiol. Gastrointest. Liver Physiol. 291, G753–G761 [DOI] [PubMed] [Google Scholar]
- 34.Scheffer H. (1952) J. Am. Stat. Assoc. 47, 381–400 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.