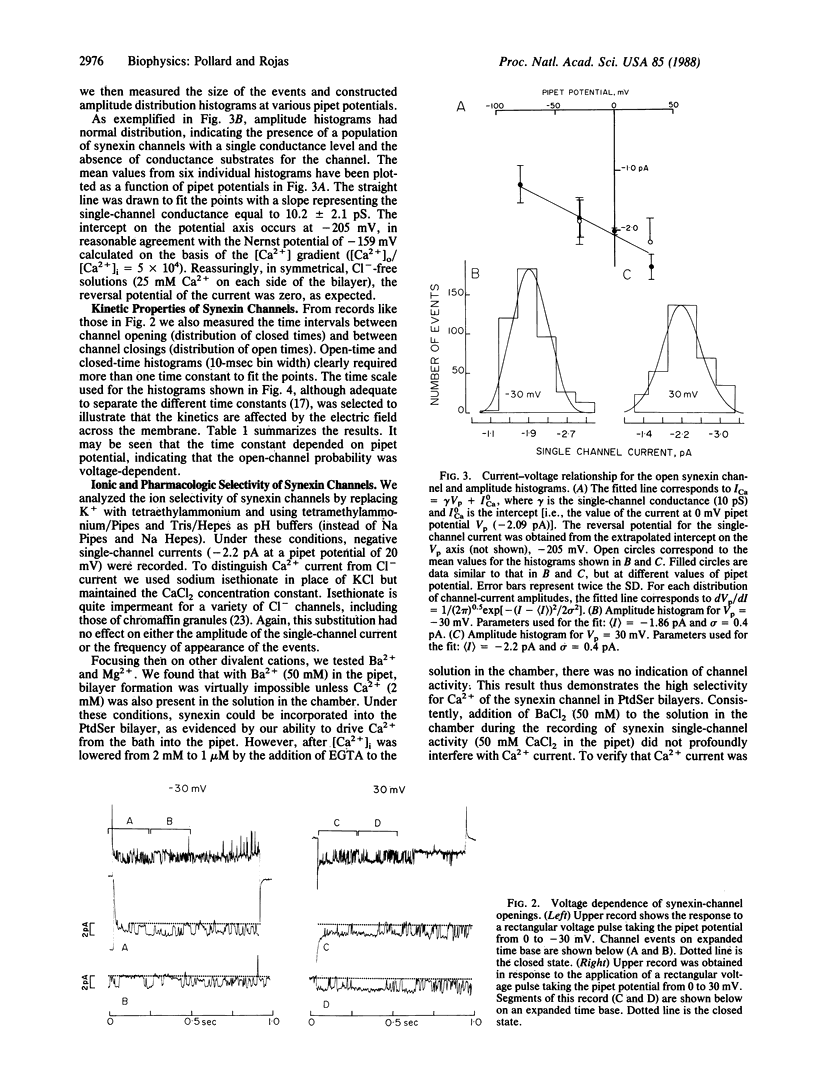
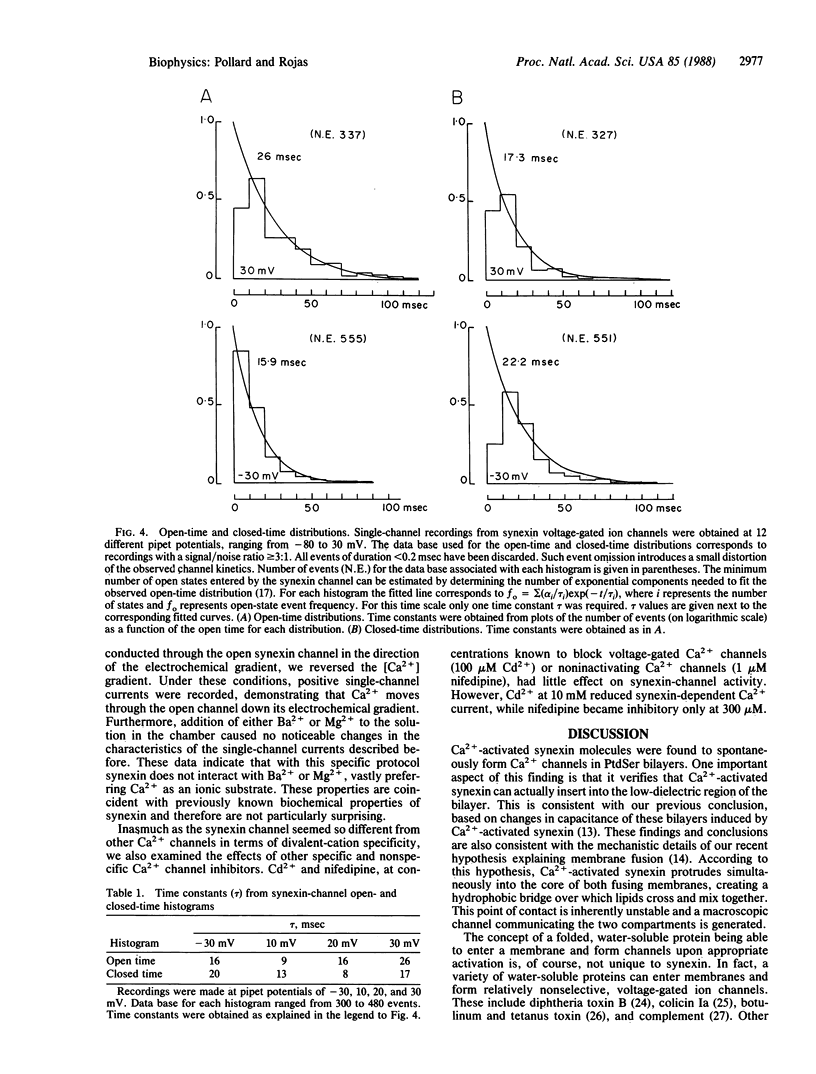
Abstract
Synexin, a cytosolic protein that mediates Ca2+-dependent membrane fusion, was incorporated into acidic phospholipid bilayers, formed at the tip of a patch pipet. The pipet was filled with a high-Ca2+ solution (50 mM) and immersed in a chamber containing a low-Ca2+ solution (1 mM). Brief exposures of the bilayer to synexin increased the capacitance of the bilayer by a factor of 10 and decreased the membrane resistance by a factor of 20. Reduction of Ca2+ in the chamber to 1 microM caused an abrupt increase in the current required to hold the pipet potential at 0 mV. Under certain conditions channel events could be detected, often occurring in bursts. Consistently, open-time histograms were found to be voltage-dependent and to exhibit one time constant in the time range examined here. The slope conductance for the synexin channel was estimated as 10.2 +/- 2.1 pS for the large Ca2+ gradient with low chamber Ca2+. However, for symmetrical, low-Cl- solutions containing 25 mM Ca2+ the conductance was 26.5 +/- 5.2 pS. Ion-replacement studies showed the synexin channel to much prefer Ca2+ over Ba2+ or Mg2+. Cd2+, a potent blocker of other voltage-gated Ca2+ channels at 100 microM, blocked synexin channels only at very high concentrations (greater than or equal to 10 mM). Similarly, nifedipine, an inhibitor of the nonactivating Ca2+ channel, was effective only at extremely high concentrations (greater than 300 microM). The high selectivity for Ca2+ and the lack of response of the channel to various drugs known to block Ca2+ channels thus distinguish the synexin channel from other types of Ca2+ channels hitherto reported.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. Gradual and stepwise changes in the membrane capacitance of rat peritoneal mast cells. J Physiol. 1987 May;386:205–217. doi: 10.1113/jphysiol.1987.sp016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Conti F. Reversible electrical breakdown of squid giant axon membrane. Biochim Biophys Acta. 1981 Jul 6;645(1):115–123. doi: 10.1016/0005-2736(81)90518-6. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Trifluoperazine reduces inward ionic currents and secretion by separate mechanisms in bovine chromaffin cells. J Physiol. 1984 Aug;353:541–564. doi: 10.1113/jphysiol.1984.sp015350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz C. E., Pazoles C. J., Pollard H. B. Identification and purification of an adrenal medullary protein (synexin) that causes calcium-dependent aggregation of isolated chromaffin granules. J Biol Chem. 1978 Apr 25;253(8):2858–2866. [PubMed] [Google Scholar]
- Creutz C. E., Pazoles C. J., Pollard H. B. Self-association of synexin in the presence of calcium. Correlation with synexin-induced membrane fusion and examination of the structure of synexin aggregates. J Biol Chem. 1979 Jan 25;254(2):553–558. [PubMed] [Google Scholar]
- Creutz C. E., Pollard H. B. A Cell-free Model for Protein-Lipid Interactions in Exocytosis: Aggregation and Fusion of Chromaffin Granules in the Presence of Calcium, Synexin, and CIS-Unsaturated Fatty Acids. Biophys J. 1982 Jan;37(1):119–120. doi: 10.1016/S0006-3495(82)84630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz C. E. cis-Unsaturated fatty acids induce the fusion of chromaffin granules aggregated by synexin. J Cell Biol. 1981 Oct;91(1):247–256. doi: 10.1083/jcb.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hidalgo J., Luxoro M., Rojas E. On the role of extracellular calcium in triggering contraction in muscle fibres from barnacle under membrane potential control. J Physiol. 1979 Mar;288:313–330. [PMC free article] [PubMed] [Google Scholar]
- Hoch D. H., Romero-Mira M., Ehrlich B. E., Finkelstein A., DasGupta B. R., Simpson L. L. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Düzgüneş N., Papahadjopoulos D. Modulation of membrane fusion by calcium-binding proteins. Biophys J. 1982 Jan;37(1):297–305. doi: 10.1016/S0006-3495(82)84678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Düzgüneş N., Papahadjopoulos D. Role of synexin in membrane fusion. Enhancement of calcium-dependent fusion of phospholipid vesicles. J Biol Chem. 1981 Apr 25;256(8):3641–3644. [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Morris S. J., Hughes J. M., Whittaker V. P. Purification and mode of action of synexin: a protein enhancing calcium-induced membrane aggregation. J Neurochem. 1982 Aug;39(2):529–536. doi: 10.1111/j.1471-4159.1982.tb03977.x. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Horowitz J. D. Calcium antagonists: a new class of drugs. Pharmacol Ther. 1983;20(2):203–262. doi: 10.1016/0163-7258(83)90040-2. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazoles C. J., Creutz C. E., Ramu A., Pollard H. B. Permeant anion activation of MgATPase activity in chromaffin granules. Evidence for direct coupling of proton and anion transport. J Biol Chem. 1980 Aug 25;255(16):7863–7869. [PubMed] [Google Scholar]
- Pollard H. B., Creutz C. E., Fowler V., Scott J., Pazoles C. J. Calcium-dependent regulation of chromaffin granule movement, membrane contact, and fusion during exocytosis. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):819–834. doi: 10.1101/sqb.1982.046.01.077. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Ornberg R., Levine M., Kelner K., Morita K., Levine R., Forsberg E., Brocklehurst K. W., Duong L., Lelkes P. I. Hormone secretion by exocytosis with emphasis on information from the chromaffin cell system. Vitam Horm. 1985;42:109–196. doi: 10.1016/s0083-6729(08)60062-x. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Rojas E., Burns A. L. Synexin and chromaffin granule membrane fusion. A novel "hydrophobic bridge" hypothesis for the driving and directing of the fusion process. Ann N Y Acad Sci. 1987;493:524–541. doi: 10.1111/j.1749-6632.1987.tb27238.x. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Scott J. H., Creutz C. E. Inhibition of synexin activity and exocytosis from chromaffin cells by phenothiazine drugs. Biochem Biophys Res Commun. 1983 Jun 29;113(3):908–915. doi: 10.1016/0006-291x(83)91085-9. [DOI] [PubMed] [Google Scholar]
- Raymond L., Slatin S. L., Finkelstein A. Channels formed by colicin E1 in planar lipid bilayers are large and exhibit pH-dependent ion selectivity. J Membr Biol. 1985;84(2):173–181. doi: 10.1007/BF01872215. [DOI] [PubMed] [Google Scholar]
- Rojas E., Pollard H. B. Membrane capacity measurements suggest a calcium-dependent insertion of synexin into phosphatidylserine bilayers. FEBS Lett. 1987 Jun 8;217(1):25–31. doi: 10.1016/0014-5793(87)81235-8. [DOI] [PubMed] [Google Scholar]
- Scott J. H., Creutz C. E., Pollard H. B., Ornberg R. Synexin binds in a calcium-dependent fashion to oriented chromaffin cell plasma membranes. FEBS Lett. 1985 Jan 21;180(1):17–23. doi: 10.1016/0014-5793(85)80222-2. [DOI] [PubMed] [Google Scholar]
- Scott J. H., Kelner K. L., Pollard H. B. Purification of synexin by pH step elution from chromatofocusing media in the absence of ampholytes. Anal Biochem. 1985 Aug 15;149(1):163–165. doi: 10.1016/0003-2697(85)90489-0. [DOI] [PubMed] [Google Scholar]
- Stutzin A. A fluorescence assay for monitoring and analyzing fusion biological membrane vesicles in vitro. FEBS Lett. 1986 Mar 3;197(1-2):274–280. doi: 10.1016/0014-5793(86)80341-6. [DOI] [PubMed] [Google Scholar]
- Suarez-Isla B. A., Wan K., Lindstrom J., Montal M. Single-channel recordings from purified acetylcholine receptors reconstituted in bilayers formed at the tip of patch pipets. Biochemistry. 1983 May 10;22(10):2319–2323. doi: 10.1021/bi00279a003. [DOI] [PubMed] [Google Scholar]
- Sussman K. E., Pollard H. B., Leitner J. W., Nesher R., Adler J., Cerasi E. Differential control of insulin secretion and somatostatin-receptor recruitment in isolated pancreatic islets. Biochem J. 1983 Jul 15;214(1):225–230. doi: 10.1042/bj2140225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A., Podack E. R. The ninth component of complement and the pore-forming protein (perforin 1) from cytotoxic T cells: structural, immunological, and functional similarities. Science. 1986 Jul 11;233(4760):184–190. doi: 10.1126/science.2425429. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Curran M., Cohen F. S., Brodwick M. Simultaneous electrical and optical measurements show that membrane fusion precedes secretory granule swelling during exocytosis of beige mouse mast cells. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1585–1589. doi: 10.1073/pnas.84.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]



