Abstract
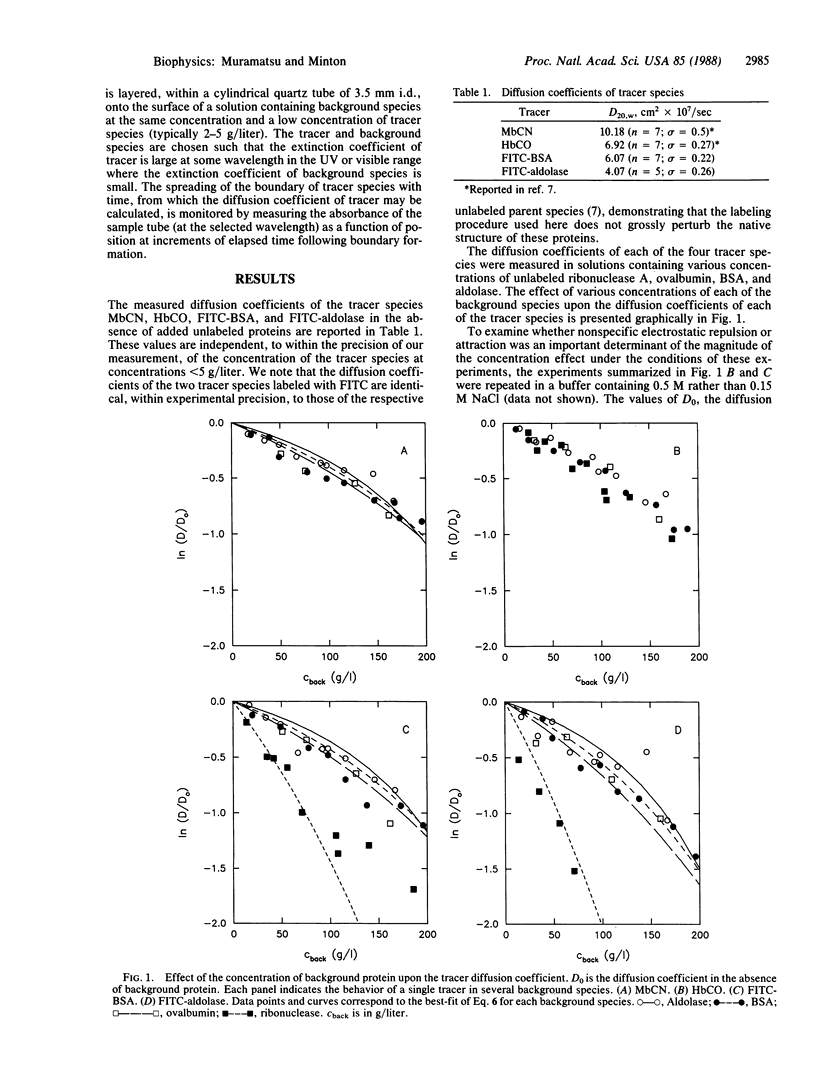
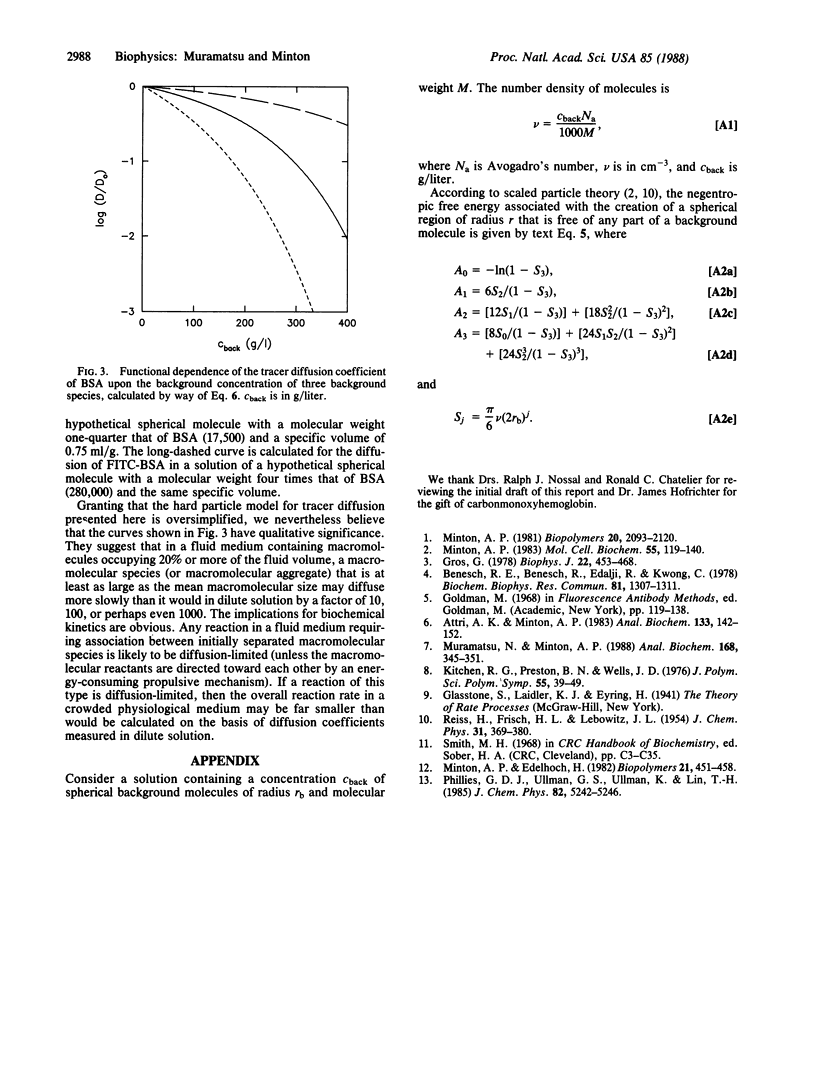
The diffusion of tracer proteins at low concentration was measured in solutions containing "background" proteins at concentrations of up to 200 g/liter. The fractional reduction of the diffusion coefficient of tracer in the presence of a given weight/volume concentration of background species generally increases with increasing size of tracer species and with decreasing size of background species. The dependence of the diffusion constants of three out of four tracer species upon the concentrations of four background species is accounted for semiquantitatively by a simple hard particle model. Extrapolation of model calculations to higher background concentrations suggests that in solutions containing proteins at concentrations comparable to those found in biological fluid media, the diffusive transport of larger proteins and aggregates may be slower than in dilute solution by several orders of magnitude.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attri A. K., Minton A. P. An automated method for determination of the molecular weight of macromolecules via sedimentation equilibrium in a preparative ultracentrifuge. Anal Biochem. 1983 Aug;133(1):142–152. doi: 10.1016/0003-2697(83)90235-x. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Edalji R., Kwong S. Intermolecular effects in the polymerization of hemoglobin S. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1307–1312. doi: 10.1016/0006-291x(78)91278-0. [DOI] [PubMed] [Google Scholar]
- Gros G. Concentration dependence of the self-diffusion of human and Lumbricus terrestris hemoglobin. Biophys J. 1978 Jun;22(3):453–468. doi: 10.1016/S0006-3495(78)85499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton A. P. The effect of volume occupancy upon the thermodynamic activity of proteins: some biochemical consequences. Mol Cell Biochem. 1983;55(2):119–140. doi: 10.1007/BF00673707. [DOI] [PubMed] [Google Scholar]
- Muramatsu N., Minton A. P. An automated method for rapid determination of diffusion coefficients via measurements of boundary spreading. Anal Biochem. 1988 Feb 1;168(2):345–351. doi: 10.1016/0003-2697(88)90328-4. [DOI] [PubMed] [Google Scholar]



