Abstract
The accessory protein polymerase (pol) γB of the human mitochondrial DNA polymerase stimulates the synthetic activity of the catalytic subunit. pol γB functions by both accelerating the polymerization rate and enhancing polymerase-DNA interaction, thereby distinguishing itself from the accessory subunits of other DNA polymerases. The molecular basis for the unique functions of human pol γB lies in its dimeric structure, where the pol γB monomer proximal to pol γA in the holoenzyme strengthens the interaction with DNA, and the distal pol γB monomer accelerates the reaction rate. We further show that human pol γB exhibits a catalytic subunit- and substrate DNA-dependent dimerization. By duplicating the monomeric pol γB of lower eukaryotes, the dimeric mammalian proteins confer additional processivity to the holoenzyme polymerase.
Introduction
Most DNA polymerases dissociate from their template DNA too rapidly for efficient replication; as a consequence, only a short DNA product is synthesized per binding event. To perform processive DNA synthesis, the catalytic subunit of a DNA polymerase associates with an accessory subunit, forming a holoenzyme that displays a markedly increased affinity for DNA. Consequently, the number of nucleotides incorporated at each binding event is significantly increased.
DNA replicases use a variety of accessory subunits for processivity enhancement. Whereas their catalytic subunits of all DNA replicases present a recognizable polymerase (pol)2 core structure, the accessory subunits differ considerably in shape and size. The processivity factor β-sliding clamp for bacterial DNA polymerases II and III, gp45 for T4 DNA polymerase, UL44 for cytomegalovirus DNA polymerase, UL30 for herpes simplex virus I DNA polymerase, and proliferating cell nuclear antigen for eukaryotic DNA polymerases δ and ϵ, are all toroidal oligomeric complexes that encircle the duplex DNA (1–4). The rate of dissociation of the holoenzyme from DNA is thereby dramatically decreased. The processivity factor for bacteriophage T7 gene 5 product protein is the small monomeric Escherichia coli protein thioredoxin, which increases polymerase processivity by direct DNA contact and by enhancing interactions of the catalytic subunit with the template DNA (5, 6).
The processivity factor for mitochondrial DNA polymerase, pol γB, bears no resemblance to these processivity factors. It is structurally homologous to Class II aminoacyl tRNA synthetases (7) and has a unique mode of action for processivity enhancement. In the holoenzyme, pol γB simultaneously increases the polymerization rate and suppresses the exonuclease activity of the catalytic subunit (8, 9). By increasing polymerization rate, more nucleotides are incorporated into the product DNA per unit time (thus per binding event).
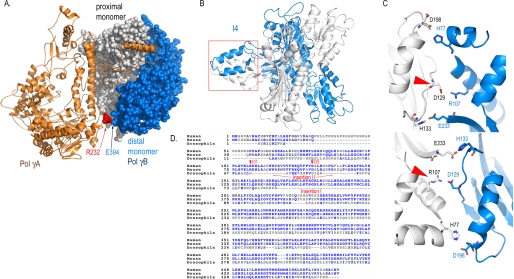
A crystal structure of human mitochondrial DNA polymerase has shed some light on how processivity is achieved by the holoenzyme (10). pol γ is a heterotrimer where one molecule of pol γA binds to a dimeric pol γB (Fig. 1A). pol γB positions the positively charged AID subdomain of pol γA to bind DNA and preferentially directs the primer terminus into the polymerase active site. The structure reveals that pol γA interacts primarily with one monomer of pol γB and makes only limited contacts with the distal monomer. Interestingly, whereas the mammalian pol γBs are dimeric, that in Drosophila melanogaster is a monomer (11). The structure of human holoenzyme suggests that a monomeric pol γB could be completely functional; the question then arises as to the necessity and function of a dimeric pol γB in mammals.
FIGURE 1.
Structural and bioinformatic basis for construction of pol γB variants. A, structure of a trimeric human pol γ holoenzyme shows pol γA forms extensive interactions with the proximal pol γB monomer but limited contacts with the distal monomer. Alignment of human, mouse, and Drosophila pol γB reveals two inserted regions (D). One forms a four-helical bundle I4 (B); the second forms interdimer H-bonds (C). Both regions are important for dimerization of mammalian pol γB.
Mutations affecting pol γA are common causes of human mitochondrial diseases. The human pol γ structure has rationalized several disease-implicated pol γA mutations that interact with the proximal pol γB monomer (10). However, interpreting some specific clinical observations with the help of the holoenzyme structure suggests that both pol γB monomers may be important to humans. A patient who died at 6 months of age was found to carry the R232G substitution in pol γA, and other patients were found to carry R232H in trans with other mutations (12, 13). Arg232 provides the most significant interaction between pol γA and the distal pol γB monomer by forming a salt bridge with Glu394 of pol γB (Fig. 1A) (10). Although the association of disease with mutations that disrupt the interactions between pol γA and the proximal pol γB monomer is easy to comprehend, any cause and effect relationship between mutations that alter the limited pol γA-distal pol γB monomer interaction is more tenuous without additional biochemical testing.
We report here that disruption of the dimeric structure of pol γB decreases the stability of the human holoenzyme and abolishes the acceleration of polymerization rate. Consequently, a monomeric pol γB confers less processivity to the holoenzyme. Our studies further reveal that each pol γB monomer has a distinct role in promoting processive DNA synthesis.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Protein Purification
All mutants and wild-type pol γB were cloned into pET22b(+), and the C-terminal His-tagged constructs were expressed in E. coli Rosetta (DE3) (Novagen) at 37 °C in LB. Proteins were induced with 0.4 mm isopropyl 1-thio-β-d-galactopyranoside when the cell density reached 0.6 A600, and the culture was subsequently incubated at a reduced temperature of 25 °C for 6 h before harvesting. The deletion mutant ΔI4 was constructed as previously described (14). Other mutant pol γBs were constructed using the following oligonucleotides (mutation sites in bold) as primers for QuikChange (Stratagene) site-directed mutagenesis: D129K, 5′-GCAGGTATTCCCGGTGAAAGCCCTCCACCACAAACC and 5′-GGTTTGTGGTGGAGGGCTTTCACCGGGAATACCTGC; R107E, 5′-CCTTGGGCGTAGAGTTGGAAAAGAACCTGGCCGCAG and 5′-CTGCGGCCAGGTTCTTTTCCAACTCTACGCCCAAGG.
The C-terminal His-tagged, exonuclease-deficient catalytic subunit pol γA was constructed by substituting Glu200 with Ala and by deleting the mitochondrial localization sequence (residues 1–29). The exo− pol γA gene was transferred into the baculovirus genome using the shuttle vector pBacPAK9 (Clontech) and expressed in infected Sf9 insect cells. Proteins were purified by sequential application to nickel-nitrilotriacetic acid, SOURCE S, and Superdex 200 columns (15).
Analytical Ultracentrifugation
All experiments were performed using a Beckman Optima XL-I. Data were analyzed with the program UltraScan v9.9,3 making appropriate hydrodynamic corrections for the buffers used,4 The partial specific volumes of pol γB proteins, estimated from the protein sequence (18), were 0.734, 0.735, 0.736, and 0.736 cm3/g for ΔI4, ΔI4-D129K, D129K, and wt pol γB, respectively. All samples were analyzed in 50 mm NaCl and 25 mm sodium phosphate buffer (pH 7.4).
Sedimentation velocity experiments were conducted at 40,000 rpm, 20 °C, for pol γB wild-type, D129K, ΔI4, and ΔI4-D129K at equal loading concentrations (1.7 μm). Scans were taken at 230 nm in intensity mode. All data, with time invariant noise subtracted, were initially analyzed by the two-dimensional spectrum method (19), and further refined with the genetic algorithm (20). Statistics were subjected to Monte Carlo analysis (21). Sedimentation coefficient distributions were calculated by the method of van Holde-Weischet as previously described (22).
Sedimentation equilibrium experiments were conducted at 4 °C for pol γB wild-type, mutant D129K, ΔI4, and ΔI4-D129K. Two sets of loading concentrations were prepared for each protein: 3.5, 5.8, and 8.1 μm for scanning at 280 nm and 0.8, 1.3, and 1.8 μm for 230 nm. Samples were centrifuged to equilibrium at 15,000, 18,700, 22,500, 26,200, or 30,000 rpm and scanned simultaneously at 230 and 280 nm. The resulting 30 scans were globally fitted to multiple models as described (23).5 The extinction coefficient at 280 nm was determined to be 71,940 absorption unit mol−1cm−1 by amino acid composition (25). The extinction coefficient at 230 nm was estimated to be 323,340 absorption unit mol−1cm−1 by globally fitting wavelength scans from each concentration to sums of Gaussian terms (26). The most appropriate model was chosen based on minimum residual and the best statistics.
Steady-state Polymerization Assay
Polymerization assays used single-stranded M13mp18 DNA annealed to a 26-nucleotide primer (5′-GGATTATTTACATTGGCAGATTCACC). Reactions contained 80 nm pol γA, 200 nm pol γB (or variant), and 50 nm primer/template DNA in 20 μl of 10 mm HEPES, pH 7.5, 80 mm KCl, 12.5 mm NaCl, 50 μg/ml bovine serum albumin, and 3 mm β-mercaptoethanol. The holoenzyme titration experiment used pol γA/pol γB ratios of 40 nm/100 nm, 80 nm/200 nm, and 160 nm/400 nm; after preincubation at 37 °C for 5 min, 500 nm poly(dA-dT)·poly(dA-dT) was added as “trap” DNA. Reactions were then initiated by the addition of MgCl2 (10 mm), dNTPs (50 μm dGTP, dATP, and dTTP, 5 μm dCTP, and 0.1 μm [α-32P]dCTP) and incubated at 37 °C for 10 min. Reactions were stopped by the addition of 1% SDS, 20 mm EDTA, and 0.1 mg/ml Protease K and then incubated at 42 °C for 30 min. After applying reaction mixtures to Micro Bio-Spin 6 columns (Bio-Rad) to remove free nucleotides, DNAs were heat-denatured at 95 °C for 5 min in gel loading buffer (70% formamide, 1× Tris, boric acid, and EDTA, 100 mm EDTA), and were analyzed on a 6% polyacrylamide/7 m urea gel. Reaction products were visualized by autoradiography.
Pre-steady-state Kinetics
A 25/45-mer primer-template was prepared by annealing equimolar amounts of 5′-32P-labeled primer (5′-TCCTCGCAGCCGTCCAACCAACTCA) and template (5′-GGACGGCATTGGATCGAGGTTGAGTTGGTTGGACGGCTGCGAGGA) by heating at 95 °C for 5 min and then slowly cooling to 20 °C in 10 mm Tris-HCl (pH 8.0 at 25 °C) and 50 mm NaCl. Single-nucleotide incorporation DNA polymerization assays were performed using a RQF-3 Rapid Chemical Quench Flow instrument (KinTek Co.), where one syringe contained pol γ·DNA complex (140 nm pol γA, 600 nm pol γB (or variant), 400 nm 25/45-mer DNA, 20 mm HEPES (pH 7.5 at 25 °C), 100 mm NaCl), and the other syringe contained a nucleotide-magnesium mix (100 μm dATP, 20 mm MgCl2, 20 mm HEPES (pH 7.5 at 25 °C), 100 mm NaCl). The reaction was initiated by rapidly mixing equal volumes from each syringe at 37 °C for 5, 10, 20, 30, 40, 60, 80, 100, 250, and 500 ms, and 1, 2.5, and 5 s and quenched with 0.5 m EDTA. Quenched reaction samples were applied to a 15% polyacrylamide/7 m urea gel. The 26-mer DNA product was visualized by autoradiography and quantified with software Quantity One (Bio-Rad). The time dependence of the product formation was fit to the burst equation (Equation 1).
Analytical Gel Filtration
Each pol γB variant (2 μm monomer) was analyzed alone, with 1 μm pol γA, or with 1 μm pol γA and 3 μm 25/30-mer (5′-GCATCTACGACCAACTCATACACCT/3′-AAAGGAGGTGTATGAGTTGGTCGTAGATGC) primer/template DNA on a Superdex 200 10/300 GL column. Samples (300 μl) were applied to the column in 20 mm HEPES (pH 7.5 at 25 °C), 140 mm KCl, 1 mm EDTA (pH 8.0), 5 mm β-mercaptoethanol and eluted at a flow rate of 0.65 ml/min. Eluates were monitored at A280 and A260, and proteins were visualized by Coomassie staining after SDS-PAGE.
RESULTS
Construction and Preparation of pol γB Variants
In contrast to the monomeric Drosophila pol γB, the human protein is a homodimer. To investigate the function of each human pol γB monomer, we constructed a monomeric pol γB, expecting to detect differences in activity between pol γA alone and its complex with a monomeric pol γB (heterodimer AB holoenzyme), or with the dimeric pol γB (heterotrimer AB2 holoenzyme).
Guided by bioinformatic, structural, and prior biochemical analyses, we identified two regions that contribute to pol γB dimerization. In comparison to the monomeric Drosophila pol γB, the human protein has two insertions that are located in the dimer interface (Fig. 1, B and D): Insertion I contains residues 165–201, which is part of the four-helical bundle (147–180) formed with the same region from another monomer. The region has been termed I4, and a mutant lacking it has been termed ΔI4 (Fig. 1B) (14). Insertion II contains residues Arg107–Val119 and His133–Ala146. This region in human pol γB harbors cross-dimer hydrogen bonds formed by Asp129–Arg107, His77–Asp198, and His133–Glu233, each of which is duplicated by the 2-fold symmetry axis relating the two pol γB monomers (Fig. 1C). The Asp129–Arg107 salt bridges should be particularly strong, because they include both H-bonding and charge-charge interactions.
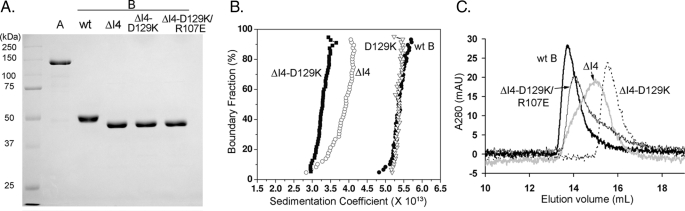
Hypothesizing that both regions are necessary for dimerization, we constructed four human pol γB mutants where the regions are disrupted either individually or jointly: ΔI4 removes the four-helical bundle by replacing residues 147–179 with a Gly-Gly dipeptide. D129K converts the Asp129–Arg107 electrostatic attraction to repulsion by substituting Asp with Lys at position 129. ΔI4-D129K combines the I4 deletion and the D129K substitution. Lastly, anticipating altered activity from mutant ΔI4-D129K, we constructed ΔI4-D129K/R107E, where two substitutions, D129K and R107E, were added to the ΔI4 construct. These substitutions replace the wild-type Asp129–Arg107 pair with a new salt bridge Lys129–Glu107. All proteins were purified to high homogeneity (Fig. 2A).
FIGURE 2.
Variant pol γB oligomeric states. A, purified pol γB proteins (1 μg) analyzed on a SDS-PAGE gel and stained with Coomassie Blue R-250. B, superimposed van Holde-Weischet integral distribution plots of wild-type (filled circles), D129K (open triangles), ΔI4 (open circles), and ΔI4-D129K (filled squares). C, superimposed chromatograms of pol γB variants (2 μm) analyzed on a Superdex 200 10/300 GL column: wt pol γB (thick black line), ΔI4 (gray line), ΔI4-D129K (dotted line), and ΔI4-D129K/R107E (thin black line).
Oligomerization of pol γB Variants
Dimerization of pol γB mutant proteins was first evaluated by analytical ultracentrifugation. We performed sedimentation velocity experiments under identical conditions using pol γB wild-type, D129K, ΔI4, and ΔI4-D129K proteins. Both pol γB wild-type and mutant D129K have the same weight-average sedimentation coefficient of 5.37, indicating that they have identical oligomeric states (Fig. 2B). ΔI4 has a weight-average sedimentation coefficient of 3.74 and shows a typical monomer-dimer equilibrium pattern that is consistent with a weak dimer. Mutant ΔI4-D129K gave a weight-average sedimentation coefficient 3.28, consistent with it being completely monomeric under these conditions.
To obtain quantitative measurements of dimer formation and dissociation, we analyzed the proteins by sedimentation equilibrium centrifugation. Mutant ΔI4 best fit a reversible monomer-dimer equilibrium model with a dissociation constant of 16.6 μm, in agreement with the previously reported value of 7 μm (15). All other pol γB variants were best fit by a single species model, because only a very low level of other species was detected. Wild-type pol γB was calculated to have a molecular mass of 114.1 kDa, and mutant D129K of 99.5 kDa; both values are consistent with the proteins being dimers of a 52.5-kDa protein (by sequence). The mutation D129K therefore appears to have little effect on dimer formation. Conversely, the molecular mass of ΔI4-D129K was estimated to be 50 kDa, consistent with it being monomeric. To estimate the Kd boundary values for these variants, the missing species (monomer for the wild-type and mutant D129K, and dimer for ΔI4-D129K) were assumed to be below the detection limit (A230 < 0.05 absorption units). Data for all pol γB variants are summarized in Table 1.
TABLE 1.
Dissociation constants measured by analytical ultracentrifugation
| pol γB proteins | Kda | Molecular massb | Oligomeric state |
|---|---|---|---|
| μm | kDa | ||
| Wild-type | <0.1 | 92.9 (89.5, 94.7) | Dimer |
| D129K | <0.1 | 91.1 (90.8, 92.0) | Dimer |
| ΔI4 | 16.6 | 90.9 (90.7, 91.3) | Monomer/dimer mixture |
| 78.1 (77.8, 79.1) | |||
| ΔI4-D129K | >200 | 43.8 (43.6, 44.1) | Monomer |
| ΔI4-D129K/R107E | NDc | Monomer/dimer mixture |
a Measured by analytical ultracentrifugation using the sedimentation equilibrium method.
b Based on genetic algorithm-Monte Carlo analysis of sedimentation velocity data. Values in parenthesis are 95% confidence intervals.
c ND, not determined.
The oligomeric states of pol γB and variants were independently confirmed by gel-filtration chromatography, extrapolating from their elution volumes and their calculated molecular weights. All proteins were analyzed at 2 μm, a minimum concentration that is dictated by the system's UV detection limit (20 absorption units at 280 nm). Wild-type pol γB elutes with an apparent molecular mass of 100 kDa (Fig. 2C), consistent with it being a dimer. ΔI4 elutes as a broadened peak, suggestive of it being a mixture of 50- and 100-kDa species that correspond to monomers and dimers, and ΔI4-D129K behaves as a 50-kDa monomer. However, when ΔI4-D129K bears the additional R107E substitution, it chromatographs as a dimer. Thus, the D129K-R107E combination, which restores the salt-bridge interaction between two pol γB monomers, also restores the ability to form a dimer that may be even stronger than ΔI4. This result therefore clearly demonstrates the importance of the salt bridge between residues 129 and 107 in pol γB dimer formation.
These analyses suggest that alteration of either dimer-stabilization region alone is insufficient to dissociate dimeric pol γB completely under the conditions of our analyses, but together they abolish all significant intermolecular interactions. The Kd for dimerization of pol γB ΔI4-D129K is more than 2000 times higher than that of the wild-type, and the mutant protein can therefore be considered monomeric under our conditions of analysis. Because the construction of pol γB ΔI4-D129K was predicated on the structure of the dimeric human protein and sequence alignment differences with Drosophila pol γB, these data also explain why the latter protein is a monomer.
Effects of pol γB Dimerization on Processive DNA Synthesis
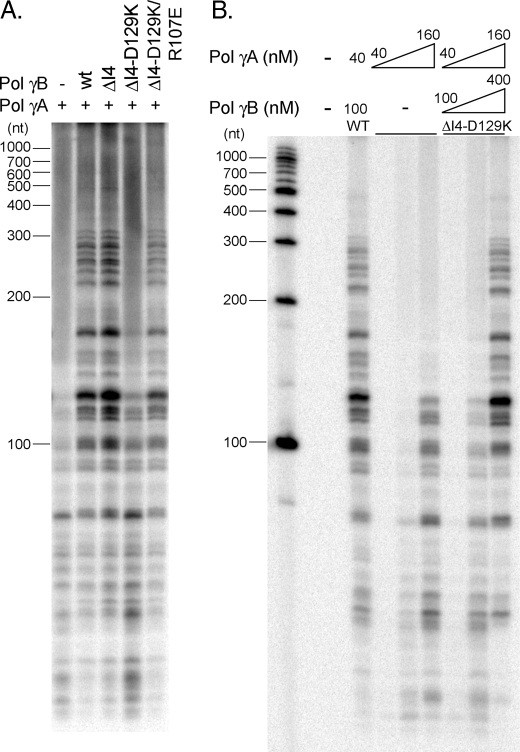
On primed M13 DNA, most products synthesized by pol γA are <100 nt, but, as expected for a processivity factor, when pol γA forms a holoenzyme with wild-type pol γB, they increase in length severalfold and become more abundant (Fig. 3A). Similar results are seen when pol γA complexes with pol γB ΔI4, suggesting that the latter is fully competent to stimulate DNA synthesis in this system. Removal of the four-helical bundle, thereby weakening formation of the pol γB dimer, has little effect. These results are comparable to previous studies comparing wild-type and ΔI4 pol γB (15).
FIGURE 3.
Steady-state DNA polymerization assays. pol γA with or without pol γB (wild-type or a variant) were analyzed using M13mp18 DNA annealed to a 26-nt primer. A, reaction products were visualized on a polyacrylamide denaturing gel. Reactions contained 80 nm pol γA, 200 nm pol γB or a variant, 50 nm primer-template DNA, and 10-fold excess of trap DNA. B, reactions were performed with pol γA either alone at 40, 80, and 160 nm, or in the presence of ΔI4-D129K at concentrations of pol γA/ΔI4-D129K 40 nm/100 nm, 80 nm/200 nm, or 160 nm/400 nm. pol γA/wild-type pol γB 40 nm/100 nm served as the control.
In contrast, the monomeric pol γB ΔI4-D129K is much less effective; although a small increase in total products was observed relative to pol γA alone, there was little increase in product length (Fig. 3A). These data suggest that loss of the distal pol γB monomer diminishes holoenzyme processivity. In agreement with this conclusion, when using pol γB ΔI4-D129K/R107E, where the salt bridge and dimerization capability is restored, the resulting holoenzyme exhibits activity comparable to the wild-type or ΔI4-containing enzyme.
To test whether the deficiency of the monomeric pol γB is due to an intrinsic lack of activity or to a weakened interaction between subunits, holoenzyme containing monomer pol γB was titrated in a polymerization assay. Activities comparable to the wild-type holoenzyme were observed at concentration 4-fold higher concentration (160 nm, Fig. 3B). ΔI4-D129K at this concentration remains a monomer, and we show below that the corresponding holoenzyme is an AB heterodimer. This indicates that loss of function in ΔI4-D129K is likely caused either by impaired subunit interactions or by impaired interactions of holoenzyme with DNA.
Pre-steady-state Kinetics Analysis of pol γB Variants
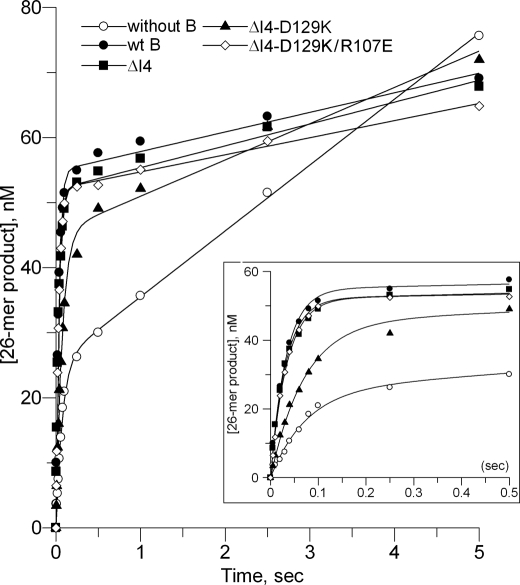
To gain a better mechanistic insight of pol γB oligomerization on the polymerization reaction, we used pre-steady-state kinetics to examine single nucleotide incorporation to a 25-mer primer annealed to a 45-mer template (25/45-mer). Identical experiments were carried out with 70 nm pol γA, either with or without 300 nm pol γB wild-type, ΔI4, ΔI4-D129K, or ΔI4-D129K/R107E proteins, 200 nm DNA substrate 25/45-mer. A pre-equilibrated pol·DNA complex was rapidly mixed with 50 μm dATP and 10 mm MgCl2. The time dependence for formation of the 26-mer product was plotted against time (Fig. 4), and data were then fitted to the burst equation: [product_26-mer] = A(1 − e−kpol·t) + kss·t, where A, the burst amplitude, reflects the amount of productive protein·DNA complex that can be turned over in the first cycle of the reaction, and kpol, the burst rate, denotes the fast polymerization rate in the first cycle of the reaction. We should note that kpol is not the initial slope, or the first order derivative of the curve, because (d[product])/dt = A·kpol when t→0, before steady-state conditions apply. The initial slope is thus the product of the two parameters A and kpol. Finally, kss, the steady-state turnover rate, is the slope of the linear steady-state phase of the reaction. Other parameters are computed from the primary experimental data: the off-rate, koff = kss/A, reflects the frequency at which polymerase dissociates from its template, and the equation, processivity = kpol/koff, gives the number of nucleotides incorporated before dissociation. The kinetic parameters for pol γB, both wild-type and variants, are summarized in Table 2.
FIGURE 4.
Time-dependent product formation in pre-steady-state assays. The 26-mer products were quantified from reactions of pol γA without or with pol γB variants and plotted against time; without pol γB (open circles), wt pol γB (filled circles), ΔI4 (filled squares), ΔI4-D129K (filled triangles), and ΔI4-D129K/R107E (open diamonds). Shorter time points are shown as an inset. Reactions contained 70 nm pol γA, ±300 nm pol γB wild-type or a variant, 200 nm 25/45-mer DNA, 10 mm MgCl2, and 50 μm dATP.
TABLE 2.
Pre-steady state kinetic parameters for pol γB variants
| pol γA |
|||||
|---|---|---|---|---|---|
| Wild-type pol γB | pol γB ΔI4 | pol γB ΔI4-D129K | pol γB ΔI4-D129K/R107E | ||
| Amplitude A (nm) | 25.40 ± 0.70 | 54.83 ± 0.70 | 52.00 ± 0.80 | 45.40 ± 1.06 | 52.09 ± 0.39 |
| Burst rate kpol (s−1) | 13.38 ± 0.91 | 30.99 ± 1.24 | 31.56 ± 1.52 | 14.07 ± 0.81 | 29.32 ± 0.68 |
| Steady state rate kss (nm·s−1) | 10.13 ± 0.26 | 3.01 ± 0.30 | 3.36 ± 0.34 | 5.58 ± 0.40 | 2.62 ± 0.16 |
| koffa (s−1) | 0.40 | 0.05 | 0.06 | 0.12 | 0.05 |
| Processivityb (nt) | 33 | 620 | 526 | 117 | 586 |
a Calculated as koff = kss/A.
b Processivity was calculated as kpol/koff. The standard deviations are residual errors from least-square model fitting.
In the presence of wild-type pol γB, the burst amplitude of pol γA increases from 25 to 55 nm, showing that pol γB increases the formation of a productive protein·DNA complex 2-fold; the burst rate increases from 13 to 31 s−1, indicating that pol γB also accelerates the polymerization rate (Table 2). In addition, kss is reduced 3-fold, indicating a lower steady-state turnover rate. The lower the value of kss, the less likely is polymerase to dissociate from its template. This means that the polymerase can catalyze more rounds of nucleotide incorporation, thereby becoming more processive, before it dissociates from the template. The combination of an increased polymerization rate and a reduced koff, due to the presence of a wild-type dimeric pol γB in holoenzyme, results in an increase in processivity from 33 nt by pol γA alone to 650 nt, a 20-fold enhancement. Pre-steady-state data (Table 2) using pol γB-ΔI4 and pol γB-ΔI4-D129K/R107E show that these proteins have similar properties to wild-type.
The monomeric pol γB ΔI4-D129K confers different kinetic properties from a dimeric pol γB. Although pol γB ΔI4-D129K increases the amplitude of the reaction to nearly the same level as the wild-type protein (from 25 to 45 nm), it is unable to accelerate the burst rate (Table 2). Accordingly, the monomeric pol γB increases processivity only from 33 to 117 nt, a mere ∼3.5-fold. To test whether the slightly reduced amplitude of ΔI4-D129K, which is ∼90% of wild-type, was caused by a reduced interaction with pol γA, we repeated the experiment at double the ΔI4-D129K concentration. The amplitudes are the same for ΔI4-D129K at 300 or 600 nm, (45.4 and 45.5 nm, respectively), suggesting the reduction is not due to a reduced interaction with pol γA, rather that a monomeric pol γB is slightly inferior to a dimer in stimulating formation of a productive pol·DNA complex. Importantly, no change of burst rate was observed (14.1 s−1 and 16.3 s−1 at 300 nm and 600 nm, respectively) compared with pol γA alone (13.4 s−1), showing that monomeric pol γB has little or no ability to accelerate the rate of synthesis by pol γA.
Effect of pol γA on pol γB Dimerization
All the activity assays for holoenzyme containing pol γB-ΔI4 were conducted at concentrations far below the measured Kd for the dimer. pol γB ΔI4 would therefore be expected to be completely monomeric in these reactions, yet it functions as effectively as the dimeric wild-type pol γB and distinctly more effective than pol γB ΔI4-D129K, which is clearly a monomer. The apparent discrepancy in the properties of pol γB ΔI4 suggests that the oligomeric state of the protein may be affected by its association with pol γA, either in the form of holoenzyme or in a holoenzyme·DNA complex.
We used analytical gel-filtration chromatography to reveal the oligomeric state of pol γB variants. Experiments were carried out using 2 μm pol γB wild-type, ΔI4, ΔI4-D129K, or ΔI4-D129K/R107E, either in the absence or presence of pol γA (1 μm). These concentrations were expected to allow detection of changes in dimer-monomer equilibrium of ΔI4, because they are near its Kd of 7–17 μm (this work and Ref. 15) but far from the Kd for ΔI4-D129K and wild-type pol γB (this work), so that the ΔI4-D129K protein is essentially entirely monomeric and the wild-type is dimeric.
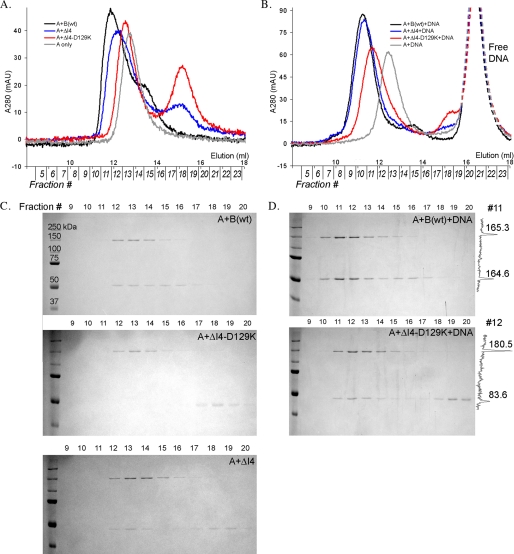
Wild-type pol γB (a predicted 52.5-kDa monomer) alone elutes as a molecule of ∼100 kDa and as a singular ∼220-kDa species when mixed with pol γA (135 kDa) (Fig. 5A), consistent with complete formation of the trimeric AB2 holoenzyme. In contrast, pol γB ΔI4-D129K (50 kDa) elutes in the position expected for a 50-kDa monomer. When pol γB ΔI4-D129K is mixed with pol γA and chromatographed, two individual peaks, whose apparent molecular weights correspond to each protein alone, were observed. There was no evidence for the formation of any complex, indicating that the monomeric pol γB ΔI4-D129K does not interact with pol γA at this concentration. These conclusions were confirmed by analysis of the column eluate by SDS-PAGE (Fig. 5C). No pol γB ΔI4-D129K could be detected in column fractions containing pol γA. Only when the concentrations of pol γA and pol γB ΔI4-D129K were both raised to 4–5 μm could any subunit interaction be detected. At this concentration, ΔI4-D129K is still monomeric and binds to pol γA to form an AB heterodimer (data not shown).
FIGURE 5.
Effects of pol γA and DNA on pol γB dimerization. A, superimposed analytical gel-filtration elution profiles of 1 μm pol γA in the presence of 2 μm wt pol γB (black), ΔI4 (blue), or ΔI4-D129K (red). The protein contents of peak fractions were visualized on SDS-PAGE gels for wt pol γB, ΔI4, and ΔI4-D129K (C). B, the same elution profiles as in A except that 3 μm 25/30-mer duplex DNA was included. The contents of peak fractions were analyzed on SDS gels for pol γA+DNA with pol γB wild-type or ΔI4-D129K (D). Densitometry profiles of the gels are shown on the right (fraction 11 for top panel and fraction 12 for bottom panel, respectively).
At a concentration of 2 μm, pol γB ΔI4 is a mixture of monomer and dimer (∼50 and ∼100 kDa) species (Fig. 2C). The concentration of pol γB ΔI4 dimer can be estimated from the Kd to be 0.2–0.3 μm. In the presence of pol γA, a protein species appears with an apparent molecular mass of ∼200 kDa; this is larger than either pol γA or the pol γB dimer. Because ΔI4 is a mixture of monomers and dimers, this apparent complex could be an AB heterodimer or an AB2 heterotrimer. As shown above, the monomeric ΔI4-D129K does not bind to pol γA under these conditions, and so an AB heterodimer would not be expected. The new peak, therefore, most likely contains a mixture of AB2 heterotrimeric holoenzyme and free pol γA, the latter because of the substoichiometric amounts of ΔI4 dimer. This conclusion is supported both by the facts that the new peak contains both pol γA and pol γB and by the presence of substantial amounts of uncomplexed pol γB ΔI4 (Fig. 5, A and C).
These observations suggest that pol γA has higher affinity for the pol γB dimer than the monomer. pol γA would thus preferentially associate with a dimeric protein in the monomer-dimer mixture, and in doing so would bias the monomer-dimer equilibrium toward dimer formation. However, these gel-filtration experiments do not explain how pol γB ΔI4-D129K can increase the polymerization amplitude of pol γA in pre-steady-state kinetic analysis. Because pol γA is unable to interact with monomeric ΔI4-D129K at 2 μm concentration and yet stimulates pol γA activity at a lower concentration, we considered the possibility that formation of a pol γA-pol γB holoenzyme may be affected by a primer-template DNA.
DNA-dependent Subunit Interaction
To examine the effect of primer-template DNA on the interaction between pol γA and pol γB, we repeated the analytical gel-filtration analyses in the presence of 3 μm primer-template DNA. In contrast to the DNA-free experiments, the presence of primer-template DNA promotes complete formation of holoenzyme for both pol γB ΔI4 (Fig. 5B) and ΔI4-D129K/R107E (not shown). The estimated molecular mass of the complex is 282 kDa, similar to that of the wild-type complex (290 kDa), indicating in both cases the formation of trimeric AB2-holoenzyme (calculated molecular masses of 235 and 240 kDa, respectively) complexed to the 16-kDa primer-template DNA.
The most dramatic changes occurred to the holoenzyme containing monomeric pol γB. As described above, the monomeric pol γB ΔI4-D129K is unable to bind to pol γA at 2 μm concentration. However, a singular peak of ∼239-kDa molecular mass was observed in the presence of DNA, and the A260:A280 ratio indicated that the peak contained DNA. Assaying column fractions by SDS-PAGE shows the presence of both pol γA and pol γB (Fig. 5D). The relative amounts of pol γB in holoenzyme were estimated by densitometry scans of the bands corresponding to pol γA and pol γB on the SDS gels. The ratio of pol γB:polγA for ΔI4-D129K is about one-half that of the wild-type pol γB-containing holoenzyme, providing strong evidence that ΔI4-D129K forms an AB heterodimer. (Note that apparent molecular weights of the holoenzyme·DNA complexes are systematically overestimated, presumably due to the elongated DNA.) Increased holoenzyme formation in the presence of a primer-template fully explains the ability of the monomeric pol γB ΔI4-D129K to stimulate pol γA in the pre-steady-state polymerization reaction.
DISCUSSION
Mitochondrial DNA polymerase accessory subunits pol γB are structurally and functionally different from other accessory proteins. This divergence of mitochondrial DNA replicase from prokaryotic and eukaryotic enzymes inspires many interests in evolution and structural-functional relationship of mitochondrial replication system. In contrast to the monomeric protein in lower eukaryotes, mammalian pol γBs dimerize to become a larger protein, thereby raising the question whether the extra pol γB monomer yields any additional functions relating to DNA synthesis processivity.
Contribution Factors to Processivity
Processivity is defined as the length of DNA synthesized per enzyme binding event. It is a distance that a polymerase travels before dissociating from the template, and therefore can be expressed by two parameters, d = vt, functionally the same as kpol/koff, where v (or kpol) is the rate of single nucleotide incorporation reaction, t (or 1/koff) is the duration of the enzyme-DNA interaction per binding event.
An accessory factor can increase processivity of a holoenzyme by either accelerating the rate of polymerization or prolonging the enzyme-DNA interaction. Several accessory proteins, such as the ring-shaped accessory proteins for DNA pol II and III superfamily members, and thioredoxin for T3 and T7 DNA polymerase, increase protein-DNA affinity. These processivity factors prolong the duration of holoenzyme binding to DNA but have no effect on catalysis rate (e.g. see Ref. 27). In contrast, human pol γB both strengthens the binding of holoenzyme to DNA and simultaneously accelerates the rate of polymerization.
Interestingly, the catalytic subunit pol γA is more processive than other polymerases, evidenced by its ability to synthesize DNA up to ∼100 nt (28), in comparison to the 1–15 nt of other enzymes (29, 30). From a crystal structure, this high level of intrinsic processivity was attributed to a subdomain (IP) of the spacer domain that is not found in other DNA polymerases (10). However, the rate of synthesis by pol γA is low, and forming a holoenzyme with pol γB provides a significant rate enhancement.
Some estimates of DNA polymerase processivity have been obtained by direct visualization of product length following synthesis on a long single-stranded template in steady-state reactions where multiple cycles of nucleotide incorporation occur. Other estimates have used the ratio of the polymerization rate kpol to the off-rate koff, which can be obtained from pre-steady-state kinetics experiments. This method breaks processivity into two simple parameters, enabling a more detailed mechanistic dissection of processivity.
Distinct Roles of Each pol γB Monomer in Processivity
It is conceivable that a single mode of processivity enhancement, i.e. strengthening the affinity of polymerase for DNA, can increase processivity usefully only to a certain level. For example, T7 DNA polymerase and E. coli pol III holoenzyme exhibit a comparable processivity, despite the great difference in the nature of their accessory subunits (16). In addition, DNA polymerases must retain some ability to dissociate from a template, and thus, if additional stimulation of processivity is needed, another mechanism may be necessary.
We have shown here that, although the proximal monomer of the human pol γB dimer is solely responsible for increasing the affinity of the holoenzyme to DNA, the distal monomer is essential for the polymerization rate enhancement. These results suggest that the monomeric pol γB in Drosophila and perhaps other lower multicellular eukaryotic organisms should have only the former activity, whereas the additional pol γB monomer of mammals confers a new mode of processivity enhancement.
One reason for lack of rate enhancement by a monomeric pol γB could be that an AB holoenzyme has lower affinity for dNTP. At low dNTP concentrations, the rate of synthesis by a heterodimeric AB-holoenzyme would then be slower than a heterotrimeric AB2. However, the pre-steady-state data we report were performed at a dNTP concentration (50 μm) high enough to compensate for any theoretical increased Kd of the AB-holoenzyme. The Kd values for wild-type pol γA and pol γ AB2 holoenzyme for dNTP are 4.7 μm and 0.9 μm, respectively (8, 28), and it is difficult to imagine how the Kd for an AB-holoenzyme could be outside that range. The reduced rate of synthesis by the AB-holoenzyme is therefore most likely due to other reasons.
Enhancement in rate of DNA synthesis occurs only when pol γB is a dimer. The pol γB distal monomer contacts the catalytic subunit at the exonuclease (exo) domain, in the vicinity of the DNA-binding channel but some distance away from the polymerization (pol) active site. It is therefore improbable that the distal monomer directly affects the pol active site conformation. Instead, its enhancement of polymerization rate is achieved either through other protein elements of pol γA or by optimizing alignment of the template DNA. A modeled human pol γ·DNA complex suggests that binding of dimeric pol γB preferentially positions the primer terminus in the pol active site, thereby facilitating an in-line nucleophilic attack of the primer 3′-OH on the incoming dNTP. If this model is correct, a dimeric pol γB would provide a more rigid scaffold for the primer-template than could be provided by a monomer. The latter would be less effective in restricting movement of the DNA. This idea also rationalizes why the small accessory subunit thioredoxin and the toroidal sliding clamps that form flexible interactions with the catalytic subunit lack the ability to accelerate the rate of synthesis.
pol γA Promotes pol γB Dimerization
Mammalian pol γB has an unusually large dimer interface of ∼4000 Å2, more than two times the size of an average protein-protein interface (1600 ± 400 Å2) (17). We identified two regions that are critical for human pol γB dimerization. Deletion of I4 in one region removes nearly half the surface contact area. However, the remaining contact area of pol γB ΔI4 is sufficiently large to support dimer formation at moderate concentrations. The estimated binding energy remaining for dimerization of ΔI4, using a converting factor of 25 cal/Å2, is ∼50 kcal/mol. A substitution in the second region breaks two salt bridges, removing at least 8 kcal/mol binding energy. This value is probably an underestimation for the pol γB D129K substitution, because the change introduces a repulsive interaction in replacement of the attractive interaction at the dimer interface. Nevertheless, D129K is not sufficient by itself to force pol γB into a monomer, and both it and ΔI4 are necessary in combination. In Drosophila pol γB, the corresponding I4 region is partly missing, as are the residues that can make a salt bridge. We conclude that the lack of these dimerization regions result in Drosophila pol γB being a monomer. By the same token, pol γB from either mosquito or Caenorhabditis elegans is also predicted to be a monomer. These monomeric accessory proteins are further predicted to be able to enhance the polymerase-DNA interaction but to lack the ability to accelerate the rate of polymerization.
Dimerization of pol γB is also affected by the presence of pol γA. Perhaps due to the additional interactions with the distal pol γB monomer, pol γA preferentially binds to the dimer to form the more stable trimeric AB2, and in doing so, shifts the pol γB dimer-monomer equilibrium toward dimer formation. pol γA may also directly strengthen the pol γB dimer. The proximal pol γB monomer becomes sandwiched between the distal monomer and pol γA in the holoenzyme (Fig. 1A); by interacting with the distal pol γB monomer, pol γA also reinforces its interaction with the proximal monomer. Some clinical symptoms associated with the pol γA R232 mutations (12, 13) may be a consequence of this lack of reinforcement. Consequently, the patients may have less efficient mitochondrial DNA synthesis.
Regardless of the oligomeric state of pol γB, the presence of a primer-template DNA further stabilizes the interactions between subunits in the holoenzyme. The effect is unlikely to be caused by pol γB and pol γA interacting with DNA independently, because pol γB does not bind to DNA of this length (24). Rather, the interaction is most likely mediated by pol γA, whose biphasic AID subdomain simultaneously binds both to the upstream DNA via a positively charged surface and to the pol γB proximal monomer via a hydrophobic surface (10). The observation, that both monomeric and dimeric pol γBs show DNA-dependent holoenzyme stabilization, is consistent with the proximal monomer being solely responsible for DNA-dependent subunit interaction. Combining results from our biophysical and biochemical assays, ΔI4 has a Kd value of 16.6 μm but shows comparable activity to the dimeric wild-type activity at 70 nm. At this concentration the amount of dimer should be ∼1 nm, and we can then estimate that dimerization of pol γB ΔI4 is increased by at least 70-fold by pol γA and a primer-template DNA.
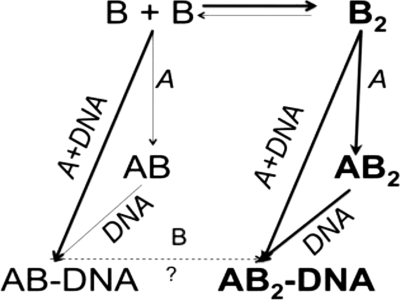
The various interactions between pol γA, pol γB, and DNA can be summarized in Scheme 1. The catalytic subunit pol γA alters the monomer-dimer equilibrium of pol γB in favor of dimer formation by selectively interacting, and thus sequestering, dimeric pol γB, driving more monomer into the dimer state (indicated by the thicker arrows). Primer-template DNA also enhances the interaction between pol γA and pol γB, which may then allow formation of a ternary complex to be independent of the oligomeric state of pol γB. A consequence of this may be that certain mutations that destabilize the pol γB dimer may have less severe clinical consequences than those that destabilize the pol γA-pol γB interface. The AB heterodimer preserves some capacity for processive DNA synthesis, albeit without the rate enhancement, whereas the lack of any interaction between the A and B subunits precludes all processivity enhancement. An interesting question is whether AB·DNA can be converted to the AB2·DNA species by binding an additional monomeric pol γB. With pol γB ΔI4-D129K (and perhaps Drosophila pol γB), we imagine that it could, but at much higher concentrations than we have examined. However, the reverse reaction is more difficult to predict, because the affinities of pol γB for itself (i.e. forming a homodimer) and for pol γA (forming a heterodimer) are comparable (Refs. 15, 28, and this work). It may then be possible for AB2·DNA to dissociate to (AB-DNA) + B, (A-DNA) + B2, or simply to A + B2 + DNA.
SCHEME 1.
We present here a rare example of two identical proteins that perform different functions. The species-dependent oligomeric states are thought-provoking on the evolutional pathways leading to pol γB. pol γB shows obvious similarity to Class II aminoacyl-tRNA synthetases. Although modern Class II aaRSs are dimeric, the primordial enzymes are thought to be monomeric, which matches their much simpler primordial stem-loop-structured tRNA. If pol γBs indeed evolved from Class II aaRS, their ancestors may be the primordial monomeric aaRS, as reflected by the monomeric pol γB of lower eukaryotes. Subsequently, both aaRSs and the mammalian pol γB independently became dimers to perform more sophisticated functions. Alternatively, pol γB might have evolved after aaRSs had become dimers; in this scenario lower eukaryote pol γBs subsequently lost a monomer, whereas their mammalian counterparts remained unchanged. Dimerization of aaRSs not only accommodates the larger modern tRNA, but also potentially allows more regulation of activity. Dimerization of pol γB, as we have shown in this work, may also enable an additional mechanism of processivity enhancement to the mitochondrial DNA pol γA.
Acknowledgment
We thank Taewung Lee for constructing the pol γB D129K mutant.
This work was supported, in whole or in part, by National Institutes of Health Grants GM032095 (to I. J. M.), GM044613 (to K. A. J.), and GM083703 (to Y. W. Y.). This work was also supported by Welch Foundation Grants F-1604 and F-1592 (to K. A. J. and Y. W. Y., respectively). Continuing development of the UltraScan software is supported by NIH Grant RR022200 (to B. D.).
B. Demeler (2009) An Integrated Data Analysis Software Package for Sedimentation Experiments. University of Texas Health Science Center at San Antonio, Dept. of Biochemistry.
T. M. Laue, B. D. Shah, T. M. Ridgeway, and S. L. Pelletier (1992) Computer-aided Interpretation of Analytical Sedimentation Data for Proteins. Analytical Ultracentrifugation in Biochemistry and Polymer Science, Royal Society of Chemistry, Cambridge, United Kingdom.
B. Demeler (2005) UltraScan: A Comprehensive Data Analysis Software Package for Analytical Ultracentrifugation Experiments. Modern Analytical Ultracentrifugation: Techniques and Methods, Royal Society of Chemistry, United Kingdom.
- pol
- polymerase
- nt
- nucleotide(s)
- aaRS
- aminoacyl-tRNA synthetase.
REFERENCES
- 1.Kong X. P., Onrust R., O'Donnell M., Kuriyan J. (1992) Cell 69, 425–437 [DOI] [PubMed] [Google Scholar]
- 2.Appleton B. A., Loregian A., Filman D. J., Coen D. M., Hogle J. M. (2004) Mol. Cell 15, 233–244 [DOI] [PubMed] [Google Scholar]
- 3.Zuccola H. J., Filman D. J., Coen D. M., Hogle J. M. (2000) Mol. Cell 5, 267–278 [DOI] [PubMed] [Google Scholar]
- 4.Krishna T. S., Kong X. P., Gary S., Burgers P. M., Kuriyan J. (1994) Cell 79, 1233–1243 [DOI] [PubMed] [Google Scholar]
- 5.Doublié S., Tabor S., Long A. M., Richardson C. C., Ellenberger T. (1998) Nature 391, 251–258 [DOI] [PubMed] [Google Scholar]
- 6.Hamdan S. M., Marintcheva B., Cook T., Lee S. J., Tabor S., Richardson C. C. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5096–5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan L., Sanschagrin P. C., Kaguni L. S., Kuhn L. A. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 9527–9532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson A. A., Johnson K. A. (2001) J. Biol. Chem. 276, 38097–38107 [DOI] [PubMed] [Google Scholar]
- 9.Farge G., Pham X. H., Holmlund T., Khorostov I., Falkenberg M. (2007) Nucleic Acids Res. 35, 902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y. S., Kennedy W. D., Yin Y. W. (2009) Cell 139, 312–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wernette C. M., Kaguni L. S. (1986) J. Biol. Chem. 261, 14764–14770 [PubMed] [Google Scholar]
- 12.Ferrari G., Lamantea E., Donati A., Filosto M., Briem E., Carrara F., Parini R., Simonati A., Santer R., Zeviani M. (2005) Brain 128, 723–731 [DOI] [PubMed] [Google Scholar]
- 13.Kollberg G., Moslemi A. R., Darin N., Nennesmo I., Bjarnadottir I., Uvebrant P., Holme E., Melberg A., Tulinius M., Oldfors A. (2006) J. Neuropathol. Exp. Neurol. 65, 758–768 [DOI] [PubMed] [Google Scholar]
- 14.Carrodeguas J. A., Theis K., Bogenhagen D. F., Kisker C. (2001) Mol. Cell 7, 43–54 [DOI] [PubMed] [Google Scholar]
- 15.Yakubovskaya E., Chen Z., Carrodeguas J. A., Kisker C., Bogenhagen D. F. (2006) J. Biol. Chem. 281, 374–382 [DOI] [PubMed] [Google Scholar]
- 16.Lee J. B., Hite R. K., Hamdan S. M., Xie X. S., Richardson C. C., van Oijen A. M. (2006) Nature 439, 621–624 [DOI] [PubMed] [Google Scholar]
- 17.Lo Conte L., Chothia C., Janin J. (1999) J. Mol. Biol. 285, 2177–2198 [DOI] [PubMed] [Google Scholar]
- 18.Durchschlag H. (1986) in Specific Volumes of Biological Macromolecules and Some Other Molecules of Biological Interest (Hinz H.-J., ed) pp. 45–128, Springer-Verlag, New York [Google Scholar]
- 19.Brookes E., Cao W., Demeler B. (2009) Eur. Biophys. J., Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brookes E., Cao W., Demeler B. (2007) GECCO Proceedings, 978-1-59593-697-4/07/0007, ACM, New York [Google Scholar]
- 21.Demeler B., Brookes E. (2008) Colloid Polym. Sci. 286, 129–137 [Google Scholar]
- 22.Demeler B., van Holde K. E. (2004) Anal. Biochem. 335, 279–288 [DOI] [PubMed] [Google Scholar]
- 23.Johnson M. L., Correia J. J., Yphantis D. A., Halvorson H. R. (1981) Biophys. J. 36, 575–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrodeguas J. A., Pinz K. G., Bogenhagen D. F. (2002) J. Biol. Chem. 277, 50008–50014 [DOI] [PubMed] [Google Scholar]
- 25.Gill S. C., von Hippel P. H. (1989) Analyt. Biochem. 182, 319–326 [DOI] [PubMed] [Google Scholar]
- 26.Yan L., Ge H., Li H., Lieber S. C., Natividad F., Resuello R. R., Kim S. J., Akeju S., Sun A., Loo K., Peppas A. P., Rossi F., Lewandowski E. D., Thomas A. P., Vatner S. F., Vatner D. E. (2004) J. Mol. Cell Cardiol. 37, 921–929 [DOI] [PubMed] [Google Scholar]
- 27.Huber H. E., Tabor S., Richardson C. C. (1987) J. Biol. Chem. 262, 16224–16232 [PubMed] [Google Scholar]
- 28.Johnson A. A., Tsai Y., Graves S. W., Johnson K. A. (2000) Biochemistry 39, 1702–1708 [DOI] [PubMed] [Google Scholar]
- 29.Hori K., Mark D. F., Richardson C. C. (1979) J. Biol. Chem. 254, 11591–11597 [PubMed] [Google Scholar]
- 30.McHenry C., Kornberg A. (1977) J. Biol. Chem. 252, 6478–6484 [PubMed] [Google Scholar]