Abstract
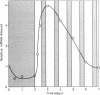
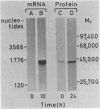
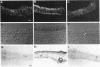
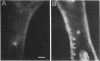
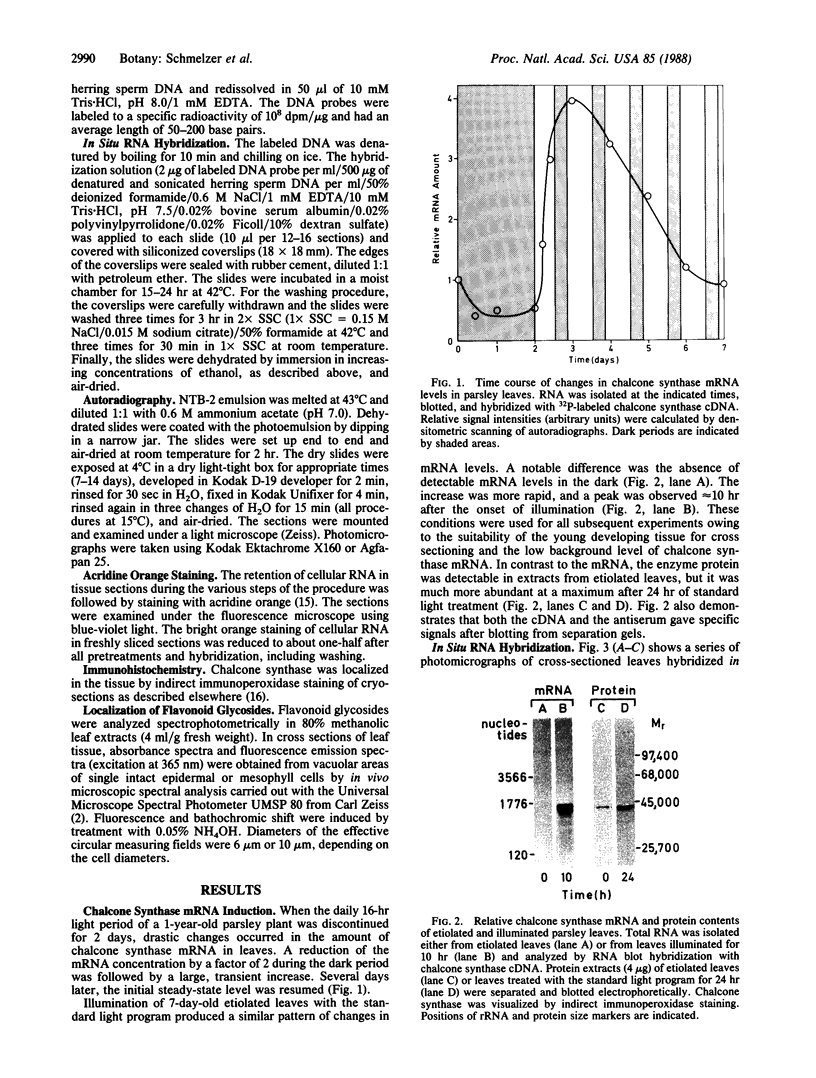
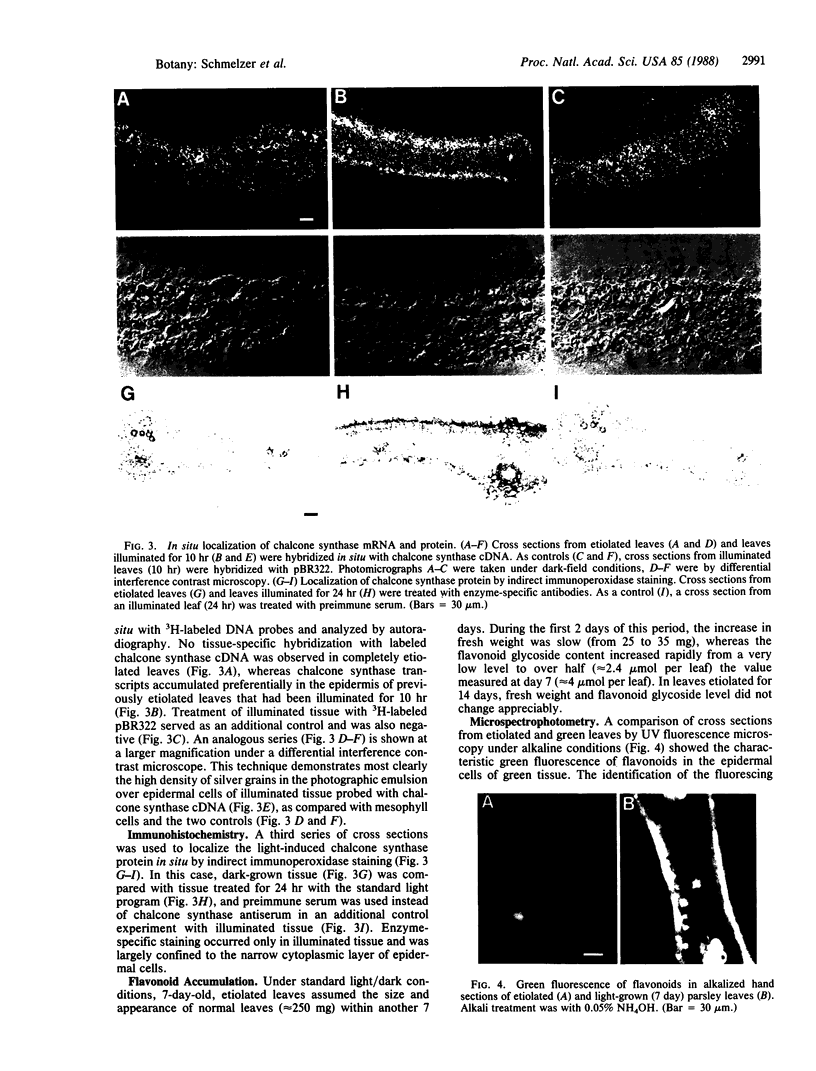
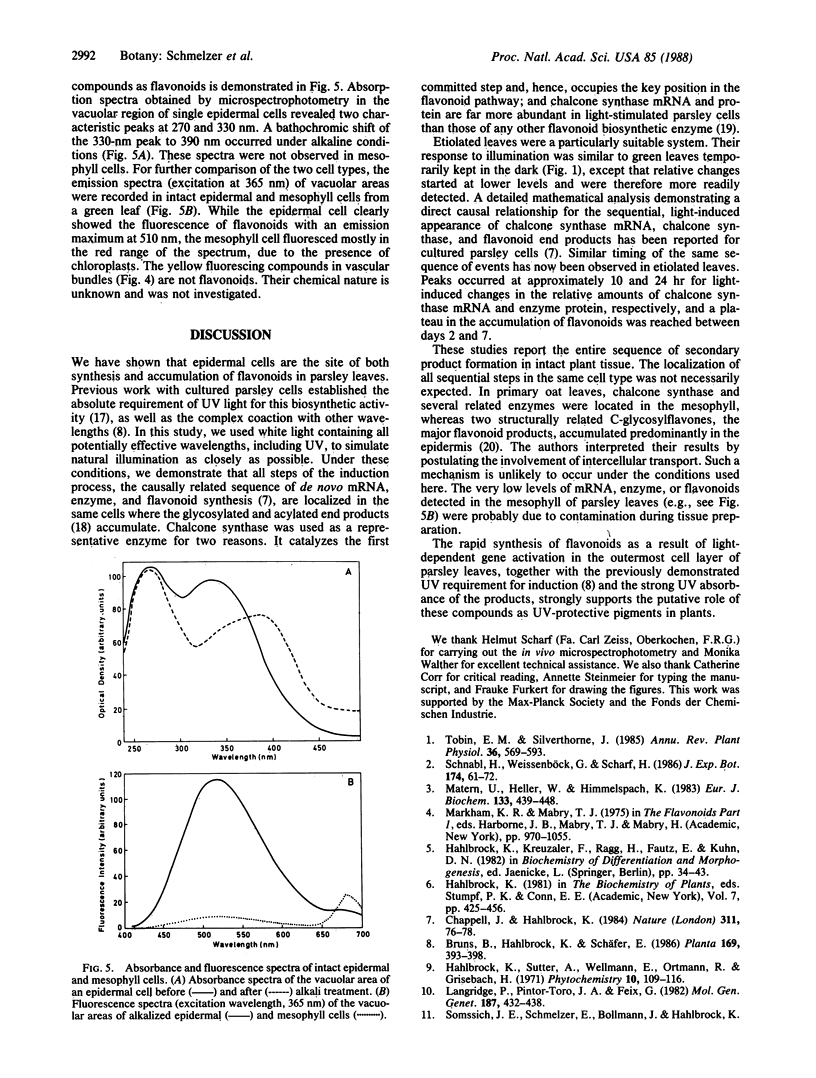
Methods involving in situ RNA hybridization, immunohistochemistry, and microspectrophotometry of individual cells were used to localize the mRNA encoding chalcone synthase (the key enzyme of flavonoid biosynthesis), the enzyme protein, and the biosynthetic end products in cross sections of parsley leaves (Petroselinum crispum). The light-dependent, sequential occurrence of all three components was restricted to epidermal cells. The results are in agreement with the putative function of flavonoids (transcriptionally inducible, UV-protective pigments) and suggest that all biosynthetic steps occur in those cells in which the products accumulate.
Keywords: in situ RNA hybridization, immunohistochemistry, microspectrophotometry, UV-protective pigments, transcriptional regulation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- HOOKER W. J., SUMMANWAR A. S. INTRACELLULAR ACRIDINE ORANGE FLUORESCENCE IN PLANT VIRUS INFECTIONS. Exp Cell Res. 1964 Feb;33:609–612. doi: 10.1016/0014-4827(64)90033-3. [DOI] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern U., Heller W., Himmelspach K. Conformational changes of apigenin 7-O-(6-O-malonylglucoside), a vacuolar pigment from parsley, with solvent composition and proton concentration. Eur J Biochem. 1983 Jun 15;133(2):439–448. doi: 10.1111/j.1432-1033.1983.tb07483.x. [DOI] [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Wellmann E. UV dose-dependent induction of enzymes related to flavonoid biosynthesis in cell suspension cultures of parsley. FEBS Lett. 1975 Mar 1;51(1):105–107. doi: 10.1016/0014-5793(75)80863-5. [DOI] [PubMed] [Google Scholar]