Abstract
Ca2+/calmodulin-dependent protein kinase II (CaMKII) promotes trafficking and activation of the GluR1 subunit of α-amino- 3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptors (AMPARs) during synaptic plasticity. GluR1 is also modulated in parallel by multiprotein complexes coordinated by synapse-associated protein 97 (SAP97) that contain A-kinase anchoring protein 79/150 (AKAP79/150), protein kinase A, and protein phosphatase 2B. Here we show that SAP97 is present in CaMKII immune complexes isolated from rodent brain as well as from HEK293 cells co-expressing CaMKIIα and SAP97. CaMKIIα phosphorylated recombinant SAP97 within immune complexes in vitro and in intact cells. Four alternative mRNA splice variants of SAP97 expressing combinations of four inserts (I2, I3, I4, I5) in the U5 region between Src homology 3 (SH3) and guanylyl kinase-like (GK) domains were identified in rat brain at postnatal day 21. CaMKIIα preferentially phosphorylated a full-length SAP97 and a glutathione S-transferase (GST) fusion protein containing the I3 and I5 inserts (SAP97-I3I5 and GST-SH3-I3I5-GK, respectively) and also specifically interacted with GST-SH3-I3I5-GK compared with GST proteins containing other naturally occurring insert combinations. AKAP79/150 also directly and specifically bound only to GST-SH3-I3I5-GK, but CaMKII phosphorylation of GST-SH3-I3I5-GK prevented this interaction. AKAP79-dependent down-regulation of GluR1 AMPAR currents was ablated by overexpression of SAP97-I2I5 (which does not bind AKAP79) or by infusion of active CaMKIIα. Collectively, the data suggest that CaMKIIα targets a specific SAP97 splice variant to disengage AKAP79/150 from regulating GluR1 AMPARs, providing new insight into protein-protein interactions and phosphorylation events that are required for normal regulation of glutamatergic synaptic transmission, learning, and memory.
Introduction
Synaptic plasticity, learning, and memory require a precisely orchestrated series of molecular changes in dendritic spines, including changes in protein-protein interactions and post-translational modifications. Many changes are directly or indirectly driven by Ca2+ signals. Ca2+/calmodulin-dependent protein kinase II (CaMKII)3 plays a central role interpreting dynamic changes in Ca2+ concentrations into appropriate changes in synaptic efficacy. CaMKIIα is highly abundant in dendritic spines, where it regulates synaptic function and morphology and is essential for normal long term potentiation in the hippocampus (for review, see Refs. 1–4). In contrast, recent studies showed that CaMKIIα is essential for long term depression at cerebellar synapses (5). Normal synaptic plasticity requires autophosphorylation of CaMKIIα at Thr-286 to generate an autonomously active form of the kinase with enhanced affinity for binding calmodulin and several CaMKII-associated proteins (6, 7). These findings show the complexity of signals that can originate from CaMKII depending on biological context.
Normal synaptic plasticity requires changes in phosphorylation of Ser-831 and Ser-845 in the GluR1 subunit of the AMPA-type glutamate receptor (AMPAR) (8–16). In general, phosphorylation of these sites by CaMKII or protein kinase C (PKC) and by protein kinase A (PKA), respectively, directly enhances AMPAR activity and is required for long term potentiation, whereas long term depression requires dephosphorylation of these sites mediated by protein phosphatase 2B (PP2B) and/or protein phosphatase 1. Long term depression is also associated with net internalization of GluR1 subunits by a mechanism that requires PP2B-dependent dephosphorylation of Ser-845 (15, 17, 18). In contrast, CaMKII activation enhances GluR1 trafficking to the synapse during long term potentiation. CaMKII-dependent trafficking requires prior PKA phosphorylation of GluR1 at Ser-845 and association of the GluR1 C-terminal motif with a protein (or proteins) containing a PDZ domain but not phosphorylation of Ser-831 in GluR1 (9, 12, 13, 19–21).
SAP97 is a membrane-associated guanylyl kinase (MAGUK) protein that appears to play a role in regulating GluR1 in addition to NMDA receptor NR2A/2B subunits and KV4.2 potassium channels (22–25). SAP97 also interacts with AKAP79/150, thereby targeting PKA, PKC, and/or PP2B to efficiently regulate the phosphorylation of GluR1 (8, 10, 16, 18, 26–28). Alternative mRNA splice variants of SAP97 are differentially distributed in cells (29–35). CaMKII phosphorylation of Ser-39 in the N-terminal L27 domain of SAP97 modulates trafficking of SAP97 and the associated proteins (Fig. 1) (22, 24, 25). In contrast, CaMKII phosphorylation of Ser-232 in the first PDZ domain may modulate binding of other proteins, such as NMDA receptor subunits (23, 24). However, the precise relationship between splicing and phosphorylation in controlling SAP97 function is unclear.
FIGURE 1.
Domain organization of SAP97. SAP97 contains multiple canonical protein-protein interaction domains (L27, three PDZ, SH3, and GK) separated by five unique regions (U1–U5). Asterisks indicate two sites of alternative mRNA splicing in the U1 and U5 regions. CaMKII phosphorylates Ser-39 and Ser-232, as indicated by the P in the L27 and PDZ1 domains, respectively.
Emerging studies are beginning to illustrate the importance of CaMKII assembly with specific substrates in modulating downstream signaling events (36–38). Here we show that CaMKII associates with SAP97 in the brain and preferentially phosphorylates a specific SAP97 splice variant of the U5 region. AKAP79/150 also preferentially interacts with this splice variant, but CaMKII-mediated phosphorylation disrupts the interaction. Moreover, CaMKII disrupts AKAP79-dependent regulation of GluR1 AMPARs, which relies on Ser-845 phosphorylation/dephosphorylation. Our findings provide new insights into the modulation of protein-protein interactions that are required for normal synaptic plasticity, learning, and memory.
EXPERIMENTAL PROCEDURES
DNA Constructs
Rat βSAP97 cDNAs with insert 1B (I1B) in the U1 region and I3 and I5 inserts in the U5 region with either an N-terminal Myc tag or a C-terminal GFP tag were a generous gift from Dr. Craig Garner (Stanford University). The full-length SAP97 and SAP97ΔC (1–595) were shuffled into pFLAG-CMV2 (Sigma) expression vector. Fragments encoding N-terminal region (L27; amino acids 1–190), PDZ1/2 (191–375), PDZ3 (426–519) domains in pGEX-2T (GE Healthcare) were used for bacterial expression of GST proteins. The sequence of SH3-GK domains (542–894, SAP97-ΔN) was inserted into pGEX-4T (GE Healthcare) and pEYFP-Mem vector (Clontech Laboratories, Inc., Mountain View, CA) for bacterial or eukaryotic expression, respectively.
Murine wild type CaMKIIα (GI 142388229) was expressed in HEK293 cells using either pcDNA3.1(+) (Invitrogen) (39) or mCherry (40) (supplied by Dr. Donna Webb (Vanderbilt) from a stock originally provided by Dr. Roger Y. Tsien (University of California, San Diego, CA/Howard Hughes Medical Institute)) expression vectors. Single or double mutants of SAP97 (S39A, S232A, S39A/S232A) and CaMKII (T286D) were created by site-directed mutagenesis. AKAP150 (in pcDNA3.1) and GFP-fused AKAP79 (in pEGFP-N1) expression constructs were generous gifts from Dr. Mark Dell'Acqua (University of Colorado-Denver, Health Science Center). GluR1 and GluR1-S831A cDNAs (both in pRK5) were kindly provided by Dr. Tom Soderling (Vollum Institute, Oregon Health Sciences University).
Identification of Rat Brain SAP97 Variants by Reverse Transcription-PCR
Total RNA was isolated from postnatal day 21 (P21) rat brain using Trizol reagent (Invitrogen) (39, 41). The cDNA pool generated by reverse transcription was amplified with primers (50 pmol) designed to amplify the region encoding the SH3 and U5 domains. Fragments of the expected size (400–500 bp) were purified using the QIAquick PCR purification kit (Qiagen, Valencia, CA), ligated into pGEMT-Easy vector (Promega, Madison, WI), and transformed into Escherichia coli (DH5α). The SAP97-positive plasmids were sequenced to identify U5 splice variants (see the supplemental information). Constructs for bacterial expression of GST-SH3-U5-GK variants and eukaryotic expression of FLAG-tagged full-length variants were generated using the “Experimental Procedures” detailed in the supplemental information. Sequences of all constructs were verified.
Protein Purification
GST fusion proteins were purified following standard protocols (see the supplemental information). Murine CaMKIIα was purified from baculovirus-infected Sf9 cells as previously described (42, 43).
CaMKIIα Autophosphorylation
CaMKII autophosphorylation at Thr-286 was carried out essentially as previously described (42) with minor modifications (see the supplemental information for details). Nonphosphorylated (control) CaMKIIα was incubated and prepared similarly, except ATP was omitted from the reaction, and the concentration of CaMKII in the pooled fractions was determined by Bradford assay (Bio-Rad).
Glutathione-agarose Co-sedimentation Assays
For CaMKII binding, GST fusion proteins or GST alone (250 nm) were incubated for 2 h at 4 °C with 250 nm desalted [32P]Thr-286 or nonphosphorylated CaMKIIα in 50 mm Tris-HCl, pH 7.5, 200 mm NaCl, 0.5% (v/v) Triton X-100. Protein complexes were isolated using glutathione-agarose (25 μl) (Sigma) and resolved by SDS-PAGE for immunoblot analysis.
For binding of AKAP79/150, GST-SAP97 fusion proteins were preincubated under phosphorylation or control (−ATP) conditions (see below). Reactions were stopped by the addition of EDTA (12 mm final), and GST fusion proteins (370 pmol) were incubated for 12 h at 4 °C in IP buffer (Tris-buffered saline (50 mm Tris-HCl, pH 7.4, 150 mm NaCl) containing 1% (v/v) Nonidet P-40, 1 mm EDTA, 1 mm EGTA, 0.1 mm phenylmethylsulfonyl fluoride, 1 mm benzamidine, 5 μg/ml leupeptin) supplemented with phosphatase inhibitors (1 μm microcystin-LR, 1 mm NaVO4, 1 mm NaF) with lysates of HEK293 cells expressing AKAP79 or AKAP150. The complexes were isolated using glutathione-agarose (25 μl), washed in IP buffer, and then analyzed by immunoblotting.
Transfection and Immunoprecipitation from HEK293 Cells
HEK293 cells were maintained in minimum Eagle's medium with 10% fetal bovine serum (Invitrogen), 2 mm glutamine, and penicillin/streptomycin (Sigma) at 37 °C in 5% CO2. Cells (10-cm dish) were transfected at ≈70% confluence using FuGENE 6 (Roche Applied Science) following the manufacturer's protocol. After 48 h the cells were lysed in 700 μl of IP buffer supplemented with phosphatase inhibitors (1 μm microcystin-LR, 1 mm NaVO4, 1 mm NaF). After centrifugation (15,000 × g, 15 min), supernatants were immunoprecipitated as follows. For CaMKII immunoprecipitation, the supernatants were precleared with Gamma Bind G-Sepharose (GE Healthcare) for 1 h at 4 °C and then incubated for 2 h at 4 °C with mouse monoclonal anti-CaMKIIα antibody (5 μg/ml). After the addition of 30 μl of Gamma Bind G-Sepharose, incubation was continued overnight at 4 °C. Alternatively, supernatants were directly incubated with anti-FLAG antibody-conjugated agarose beads (Sigma) (30 μl) for 2 h at 4 °C. The immune complexes were collected by centrifugation (100 × g, 1 min), washed 5 times in IP buffer, eluted with 2× SDS sample buffer, and resolved by SDS-PAGE for immunoblot.
Immunoprecipitation from Rat Hippocampi
Adult rat hippocampi were dissected and stored at −80 °C. Tissue was homogenized using a loose-fitting Teflon glass Dounce homogenizer in Tris-buffered saline containing 1 mm dithiothreitol, 0.2 mm phenylmethylsulfonyl fluoride, 1 mm benzamidine, 1 μm pepstatin, 10 mg/liter leupeptin, 1 μm microcystin-LR, 1% (v/v) Triton X-100. The homogenate was diluted to a protein concentration of 0.5–1 mg/ml, incubated for 1 h at 4 °C, and centrifuged at 9000 × g for 10 min at 4 °C. The supernatant was precleared with Gamma Bind G-Sepharose and incubated with affinity-purified goat anti-CaMKII (42) or preimmune IgG (8 μg/ml each), and immune complexes were collected and analyzed as described above.
In Vitro Phosphorylation of GST-SAP97
GST fusion proteins or GST alone (blank) (1 μm each) were incubated for 20 min at 30 °C with purified CaMKIIα (100 nm subunit) in phosphorylation buffer (50 mm HEPES, pH 7.5, 10 mm magnesium acetate, 0.5 mm CaCl2, 2 μm calmodulin, 1 μm dithiothreitol, 0.4 mm [γ-32P]ATP (300–1000 cpm/pmol)). Aliquots (10 μl) were spotted on P81 phosphocellulose papers, which were washed with tap water and then analyzed in a liquid scintillation counter to measure phosphorylation stoichiometries. The rest of the incubation (15 μl) was mixed with sample buffer and resolved by SDS-PAGE before staining with Coomassie Blue. Dried gels were autoradiographed to visualize 32P-labeled proteins.
In Vitro Phosphorylation and Post Hoc (Back) Phosphorylation of FLAG-SAP97
FLAG-tagged SAP97 splice variants or phosphorylation-deficient mutants were expressed in HEK293 cells alone or with wild type (WT) CaMKIIα as indicated (see above). After 48 h cells were either treated with calcium ionophore (A23187: Sigma) (20 μm) and CaCl2 (2 mm) for 2 min before lysis (for post hoc phosphorylation) or directly lysed in IP buffer in the absence or presence of phosphatase inhibitors (1 mm NaVO4, 1 μm microcystin-LR, 1 mm NaF). FLAG-SAP97 was immunoprecipitated as described above and washed 4 times in the IP buffer and then 1 time in 50 mm HEPES, pH 7.5, 0.1 mm EDTA. Immune complexes were phosphorylated in vitro (see above) with or without the addition of purified CaMKIIα (100 nm subunit) or the selective CaMKII inhibitor peptide CaMKIINtide (5 μm) (44, 45). (CaMKIINtide (KRPPKLGQIGRSKRVVIEDDRIDDVLK) was synthesized by the Macromolecular Resources facility at the University of Colorado, Fort Collins, CO) Reactions were stopped by boiling in SDS sample buffer, and samples were analyzed by SDS-PAGE and autoradiography (see above).
Electrophysiology
HEK293 cells were obtained from ATCC (Manassas, VA) at passage 35 and used for a maximum of 8 passages. Cell cultures were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (Hyclone, Logan, UT) and penicillin/streptomycin. Cells were plated at low density (∼50,000 cells/ml) on 15-mm round glass coverslips in 12-well plates and transfected by the calcium phosphate method with 1 μg of each construct per coverslip. GFP epifluorescence was used to confirm the expression of AKAP79. Control cells (i.e. lacking AKAP79) were transfected with pEGFP (0.3 μg). Whole-cell recordings from fluorescent cells were made with patch pipettes (2–4 megaohms) containing 140 mm cesium methanesulfonate, 10 mm HEPES, 5 mm adenosine triphosphate (sodium salt), 5 mm MgCl2, 0.2 mm CaCl2, and 1 mm BAPTA, pH 7.4. The constitutively active CaMKII-(1–290) was prepared as previously described (10) and added from frozen stocks. The extracellular solution contained 150 mm NaCl, 5 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, 10 mm glucose. Glutamate (1 mm) was added from frozen concentrated stock solutions. Cyclothiazide (Ascent Scientific; 100 μm) was used to block AMPAR desensitization. Solution exchanges were accomplished through a series of flow pipes controlled by solenoid valves (Warner, Hamden, CT) and moved into position by a piezoelectric bimorph. HEK293 cells were lifted off the coverslip to speed the solution exchange time. Currents were digitally acquired at 5 kHz and filtered at 1 kHz using a Axopatch 200B amplifier, Digidata 1322A board, and Clampex 9 software (Molecular Devices, Sunnyvale, CA). Series resistance (90–95%) and whole-cell capacitance compensation were employed. Series resistance was monitored throughout the experiments by 10-mV hyperpolarizing jumps before each application of glutamate. Only cells with series resistance of <6 megaohms that were stable throughout the recording were included for analysis. All experiments were initiated within 1 min of establishing the whole-cell configuration and were performed at a holding potential of −60 mV at 20 °C. Currents were normalized to the amplitude of current from the initial agonist application for each experiment. All data are expressed as the means ± S.E. and were subjected to statistical analysis using a one-way ANOVA followed by Student's t test.
Immunoblotting
Standard protocols were used, as described in the supplemental information.
Antibodies
Primary antibodies and dilutions for immunoblotting were as follows: mouse anti-SAP97 (NeuroMab, 1:1000), goat anti-CaMKII (Ref. 42; 0.2–0.4 μg/ml), mouse monoclonal anti-CaMKIIα, (Affinity Bioreagents, Golden, CO; 1:1000), mouse monoclonal anti-GFP (Santa Cruz Biotechnology, Santa Cruz, CA; 1:2000), rabbit anti-GFP (Invitrogen). Horseradish peroxidase-conjugated donkey anti-goat, anti-mouse, and anti-rabbit secondary antibodies (Santa Cruz Biotechnologies) were used at 1:3000.
RESULTS
Co-assembly of SAP97 with CaMKII in Brain and Heterologous Cells
CaMKII often forms stable multiprotein complexes containing substrates (see the Introduction). Therefore, we used affinity-purified goat CaMKII antibodies (raised to the purified brain holoenzyme) to isolate CaMKII from a Triton-soluble subcellular fraction of rat hippocampus. Immunoblot analysis of the immune complexes showed that microgram quantities of CaMKIIα were readily detected by staining nitrocellulose membranes with Ponceau S (not shown) or using a mouse antibody to CaMKIIα (Fig. 2A). As expected, CaMKIIβ coprecipitated with CaMKIIα (data not shown). However, neither CaMKII isoform was detected in control samples obtained using preimmune IgG. Parallel immunoblotting studies revealed that SAP97, a known CaMKII substrate, was present in the CaMKII immune complex but not in control samples isolated using preimmune IgG (Fig. 2A). Interestingly, multiple forms of SAP97 with differing electrophoretic mobilities were detected in the CaMKII immune complex, perhaps representing splice variants (in U1 and/or U5 domains) or post-translationally modified forms. SAP97 was also present in CaMKII immune complexes isolated from extracts of rat cortex (data not shown).
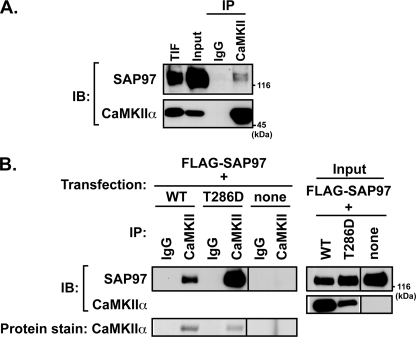
FIGURE 2.
Isolation of SAP97-CaMKII complexes from rat hippocampus and heterologous cells. A, Triton X-100 soluble fraction of adult rat hippocampus (Input) was immunoprecipitated (IP) using antibodies to CaMKII or control (preimmune) IgG. Aliquots of immune complexes, input (∼0.5% of total tissue lysate), and Triton X-100-insoluble fraction (TIF) were immunoblotted (IB) for SAP97 and CaMKIIα. These data are representative of three similar experiments. B, lysates of HEK293 cells expressing FLAG-SAP97 alone or with mCherry-CaMKIIα (wild type or T286D; apparent molecular mass ≈ 80 kDa) were immunoprecipitated with CaMKIIα or control antibodies. Inputs (0.1% of total cell lysate) and immune complexes were resolved by SDS-PAGE for immunoblotting as indicated (top). The immunoprecipitated mCherry-CaMKIIα was detected by staining nitrocellulose membranes with Ponceau S (bottom). These data are representative of four similar experiments.
Interactions of CaMKIIα with several CaMKII-associated proteins are modulated by autophosphorylation at Thr-286 (41, 46–50). Therefore, to further explore the assembly of complexes containing SAP97 and CaMKIIα, FLAG-tagged SAP97 was expressed in HEK293 cells alone or with wild type or T286D-mutated mCherry-CaMKIIα. Detergent-soluble cell extracts were immunoprecipitated with a mouse monoclonal antibody to CaMKIIα or a control mouse IgG1. The mCherry-CaMKIIα was highly and specifically enriched in immune complexes isolated from cells expressing either form of CaMKII, as detected by protein staining (Fig. 2B). In addition, immunoblotting revealed specific co-immunoprecipitation of FLAG-SAP97 with WT CaMKIIα that was strongly enhanced by the T286D mutation (which mimics the effects of Thr-286 autophosphorylation). No FLAG-SAP97 was detected in CaMKII immune complexes isolated from cells expressing FLAG-SAP97 alone. In combination, these data show that SAP97 specifically assembles with CaMKII in intact cells and suggest that CaMKII autophosphorylation at Thr-286 may enhance binding to SAP97.
Phosphorylation of SAP97 by CaMKII
To investigate whether CaMKII can phosphorylate SAP97 within this protein complex, FLAG immune complexes isolated from HEK293 cells expressing FLAG-SAP97 and/or CaMKIIα were then incubated with Ca2+/calmodulin (to activate CaMKII), Mg2+, and [γ-32P]ATP (see “Experimental Procedures”). After fractionation by SDS-PAGE, autoradiography revealed that FLAG-SAP97 was readily phosphorylated in complexes isolated from cells expressing both FLAG-SAP97 and CaMKIIα but not in complexes isolated from cells expressing either protein alone (Fig. 3A). Moreover, in vitro phosphorylation of SAP97 in these complexes was essentially blocked by inclusion of a selective CaMKII inhibitor peptide (CaMKIINtide) in the in vitro phosphorylation reactions. In combination, these data confirm that CaMKII associates with FLAG-SAP97 in intact cells and show that the bound CaMKII can phosphorylate SAP97.
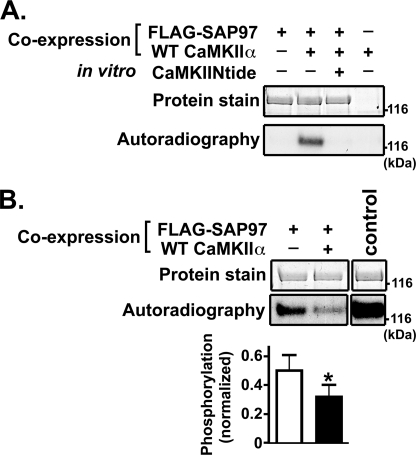
FIGURE 3.
CaMKII phosphorylates SAP97 in vitro and in intact cells. A, lysates of HEK293 cells expressing FLAG-SAP97 and/or CaMKIIα as indicated were immunoprecipitated with an anti-FLAG antibody. Immune complexes were incubated in a phosphorylation reaction using [γ-32P]ATP (see “Experimental Procedures”) in vitro in the absence or presence of CaMKIINtide as indicated. Samples were resolved by SDS-PAGE, and stained/dried gels were autoradiographed. These data are representative of at least three similar experiments. B, post hoc (back) phosphorylation of SAP97. Left and center lanes, lysates of HEK293 cells expressing FLAG-SAP97 with or without CaMKIIα and treated with Ca2+ ionophore were prepared in the presence of protein phosphatase inhibitors and immunoprecipitated with anti-FLAG antibody. Right lane, for the control sample, FLAG-SAP97 was expressed alone, and cell lysates were immunoprecipitated in the absence of phosphatase inhibitors. All immune complexes were phosphorylated by exogenous purified CaMKIIα in the presence of [γ-32P]ATP. Samples were resolved by SDS-PAGE; gels were stained, dried, and then autoradiographed. Phosphorylation in each lane was normalized to the corresponding amount of SAP97 and then expressed relative to phosphorylation of the control sample. The bar graph shows mean ± S.E. phosphorylation from five similar experiments; the in vitro 32P phosphorylation of SAP97 was significantly reduced by co-expression of CaMKIIα (p < 0.05), indicating that the co-expressed CaMKII phosphorylated SAP97 in the intact cells.
We developed a post hoc (back) phosphorylation assay to investigate whether CaMKII phosphorylates SAP97 in intact cells. HEK293 cells expressing FLAG-SAP97 with or without CaMKIIα were incubated with a calcium ionophore (A23187) and then lysed in the presence of protein phosphatase inhibitors to prevent SAP97 dephosphorylation (see “Experimental Procedures”). A control sample was prepared in the absence of protein phosphatase inhibitors from cells expressing FLAG-SAP97 alone (and not treated with A23187) so that the immunoprecipitated FLAG-SAP97 should be mostly dephosphorylated. All of the FLAG-SAP97 immune complexes were incubated in parallel with Ca2+/calmodulin, Mg2+, and [γ-32P]ATP in the presence of excess exogenous CaMKII. As expected, control FLAG-SAP97 samples were robustly 32P-phosphorylated in vitro by CaMKIIα. The 32P phosphorylation of experimental FLAG-SAP97 samples was reduced, presumably because CaMKII phosphorylation sites had been phosphorylated by nonradioactive phosphate in the intact cells before cell lysis. Notably, co-expression of CaMKIIα significantly reduced subsequent in vitro 32P phosphorylation of FLAG-SAP97 by ∼40% (Fig. 3B). These data demonstrate that CaMKII can phosphorylate FLAG-SAP97 in intact cells.
Characterization of CaMKII Binding Domains in SAP97
Because CaMKII forms stable complexes with SAP97 in brain and in heterologous cells, we tested the hypothesis that CaMKII bound directly to a specific domain(s) in SAP97. Full-length or truncated SAP97 (mYFP-SAP97ΔN or FLAG-SAP97ΔC) proteins were co-expressed with mCherry-CaMKIIα(T286D) in HEK293 cells. CaMKII immune complexes were probed using rabbit GFP antibodies (that recognize all GFP variants) or anti-SAP97 antibodies. Surprisingly, both the N- and C-terminal truncation variants of SAP97 independently associated with CaMKIIα(T286D) (Figs. 4, A and B). Similarly, SAP97ΔC and SAP97ΔN also associated with WT CaMKIIα, albeit at lower levels than observed with CaMKIIα(T286D) (data not shown). These data suggest that CaMKIIα directly or indirectly interacts with multiple regions of SAP97.
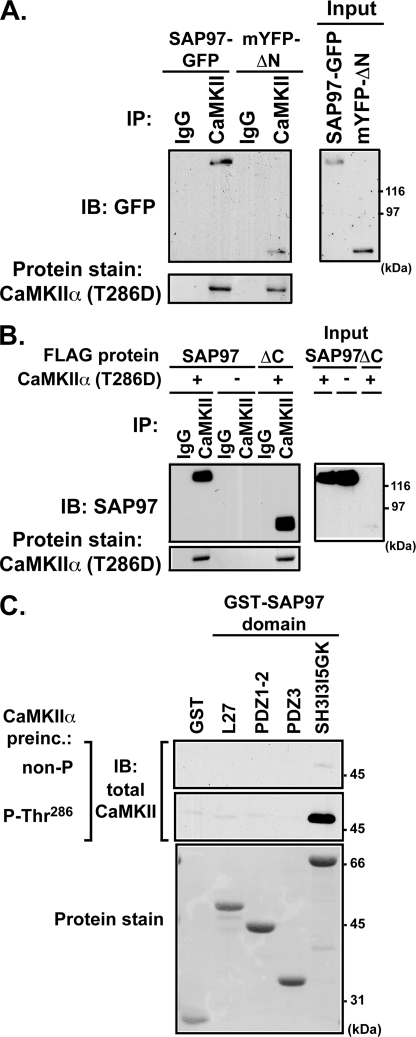
FIGURE 4.
Characterization of CaMKII interactions with SAP97. A, lysates of HEK293 cells co-expressing mCherry-CaMKIIα(T286D) with full-length SAP97-GFP or mYFP-SAP97ΔN were immunoprecipitated (IP) with mouse anti-CaMKIIα antibody or IgG (control). IB, immunoblot. B, CaMKIIα immunoprecipitation from lysates of HEK293 cells co-expressing FLAG-SAP97 or FLAG-SAP97ΔC with or without mCherry-CaMKIIα(T286D). For panels A and B, immune complexes were resolved by SDS-PAGE for immunoblotting with an anti-SAP97 antibody. The mCherry-CaMKIIα was detected by staining membranes with Ponceau S. C, bacterially expressed GST fusion proteins containing the indicated domains from SAP97 were incubated with inactive nonphosphorylated or activated Thr-286 autophosphorylated CaMKIIα (CaMKIIα preinc.). Complexes isolated using glutathione-agarose were analyzed by immunoblotting for CaMKII. GST proteins were detected by staining for total protein using Ponceau S. The data in all three panels are representative of four similar experiments.
To further probe the mechanism(s) of CaMKII binding to SAP97, we created a family of GST fusion proteins containing previously defined structural domains from SAP97 (Fig. 1). Purified GST fusion proteins were incubated with purified CaMKIIα (nonphosphorylated or Thr-286-autophosphorylated: see “Experimental Procedures”). Under these conditions, only the C-terminal region of SAP97 (GST-SH3-GK) reliably interacted with Thr-286-autophosphorylated CaMKIIα in co-sedimentation assays (Fig. 4C) as well as in “slot-blot” assays (supplemental Fig. S1). Side-by-side comparison showed that this interaction is >10-fold weaker than the binding of Thr-286-autophosphoryated CaMKIIα to a GST-NR2B-(1260–1316) fusion protein (54) (data not shown). However, a weak, but specific, direct interaction of nonphosphorylated CaMKII with GST-SH3-GK was often detected when immunoblots were overexposed (detected very weakly in Fig. 4C). The weak interaction of nonphosphorylated kinase with GST-SH3-GK was essentially unaffected by excess Ca2+/calmodulin and/or adenine nucleotide (supplemental Fig. S3B). Thus, in contrast to CaMKII binding to NR2B, which is supported by binding of Ca2+/calmodulin and adenine nucleotides without autophosphorylation (48), the strong direct interaction of CaMKII with the C-terminal domain of SAP97 requires Thr-286 autophosphorylation. However, it appears that domains in the N-terminal half of SAP97 may also interact directly or indirectly with CaMKIIα.
Identification of SAP97 Splice Variants
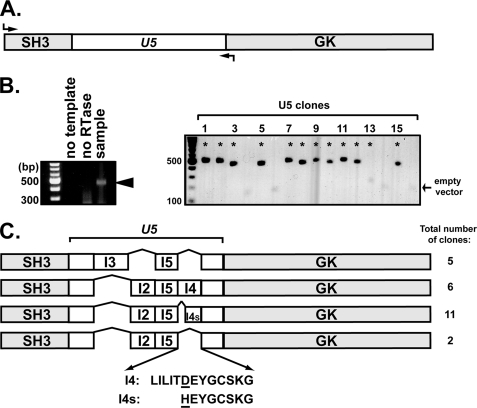
The UniProtKB/SWISS-PROT data base reports four splice variants of the human Dlg1/SAP97 U5 region (Q811DO) and three U5 splice variants of mouse Dlg1/SAP97 (Q12959); this alternative splicing regulates SAP97 function (30–33). Although multiple rat SAP97 splice variants of the U5 region have been reported in the literature (51, 52), only one is documented in UniProtKB/SWISS-PROT (Q62696), and this variant is distinct from the I3I5 variant used in our initial studies (Figs. 2–4). As an initial step toward testing the hypothesis that alternative splicing of rat SAP97 may modulate the effects of CaMKII, we used reverse transcription-PCR to characterize SAP97 splice variants of the U5 region that are expressed in rat hippocampus at P21, an age typically associated with peak synaptic maturation (see “Experimental Procedures”) (Fig. 5). A total of 24 clones containing sequences derived from SAP97 mRNAs were analyzed, all of which were 100% identical to the rat SAP97 nucleotide sequence in the region encoding the SH3 domain. In contrast, four distinct U5 region variants were present in this population, containing different combinations of I2, I3, and/or I5 alternative splicing inserts as well as full-length and truncated variants of the I4 insert (Fig. 5, B and C). The truncated I4 insert also contains an alteration of Asp to His and was designated as I4s (“short”). Eleven clones were identical to Q62696 in the U5 region, containing I2, I5, and I4s inserts. The U5 regions of the other 13 clones were similar to variants of mouse and/or human SAP97. Together, the 24 rat SAP97 clones characterized in this study could be assigned to one of four U5 region variants: I2I5, I3I5, I2I5I4, and I2I5I4s (Fig. 5C). Thus, at least four distinct SAP97 variants are expressed in the hippocampus during a peak period of synaptic plasticity.
FIGURE 5.
Identification of SAP97 alternative splice variants in rat brain at P21. A, primers (shown by arrows) were designed to amplify mRNAs encoding the SH3 domain and the entire U5 region of SAP97. B, left, analysis of the reverse transcription-PCR reaction using RNA from P21 rat brain as template; the expected 400–500-bp DNA band (arrowhead) is missing from controls lacking either reverse transcriptase (RTase) or template mRNA. Right, representative agarose gel showing individual clones of different sizes chosen for sequence analysis (asterisks). C, schematic illustrating four U5 splice variants detected among the total of 24 clones analyzed that contained distinct combinations of the I2, I3, I5, and/or full-length and short forms of the I4 insert (I4 and I4s, respectively). Amino acid sequences of I4 and I4s inserts are shown below.
Alternative Splicing at the U5 Region Regulates CaMKII Binding to SAP97
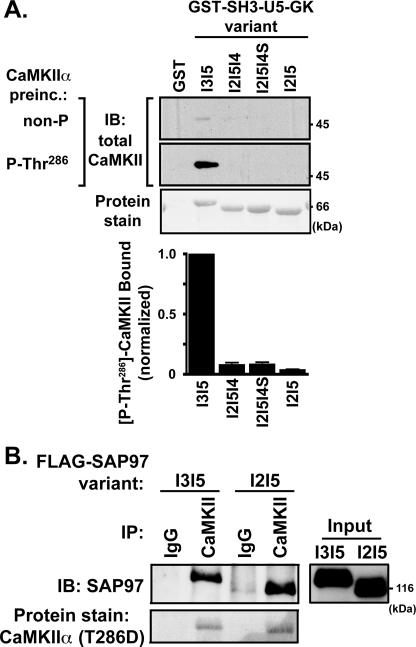
Because Thr-286 autophosphorylated CaMKII bound directly to the C-terminal region of SAP97-I3I5 (Fig. 4C), we investigated whether alternative splicing in the U5 region could modulate the interaction in glutathione-agarose cosedimentation assays. Approximately 10-fold more Thr-286 autophosphorylated CaMKII bound to GST-SH3-I3I5-GK than to any of the other splice variants (Fig. 6A), and binding of inactive CaMKII to these variants was not significant under these conditions. Thus, autophosphorylated CaMKII preferentially and directly binds to the C-terminal domain of SAP97-I3I5.
FIGURE 6.
Alternative splicing of SAP97 in the U5 region modulates CaMKIIα binding. A, GST fusion proteins containing the indicated C-terminal SH3-U5-GK domains of the four alternative splice variants were incubated with purified CaMKIIα (nonphosphorylated or prephosphorylated at Thr-286). Protein complexes collected using glutathione-agarose beads were immunoblotted (IB) using a pan-CaMKII antibody. The bar graph indicates the amount of Thr-286-autophosphorylated CaMKIIα bound to each splice variant, normalized to that bound to GST-SH3-I3I5-GK, revealing significantly less binding to all other splice variants (p < 0.001; n = 4). B, CaMKIIα was immunoprecipitated from lysates of HEK293 cells co-expressing mCherry-CaMKIIα(T286D) with either FLAG-SAP97-I3I5 or -I2I5. Protein complexes were immunoblotted with an anti-SAP97 antibody, and mCherry-CaMKIIα was detected by staining nitrocellulose membranes with Ponceau S. The panel is representative of three similar experiments.
We next explored whether alternative splicing modulates CaMKII binding to full-length SAP97. HEK293 cell lysates containing mCherry-CaMKIIα(T286D) co-expressed with either FLAG-SAP97-I3I5 or -I2I5 were immunoprecipitated using mouse anti-CaMKIIα. Immunoblotting for SAP97 showed that similar amounts of the two splice variants associated with mCherry-CaMKIIα(T286D) (Fig. 6B). Thus, despite selective and direct interaction of activated CaMKII with the SH3-I3I5-GK domains, there is no apparent selectivity for interaction with full-length SAP97 variants, presumably because of the direct or indirect interaction of CaMKII with the N-terminal region of SAP97, which is identical in all U5 variants (Fig. 4B).
Phosphorylation of SAP97 by CaMKII
To compare the phosphorylation of the four full-length SAP97 U5 region splice variants, FLAG-tagged U5 variants were isolated from transfected HEK293 cell lysates. CaMKIIα 32P-phosphorylated all four variants in vitro, but phosphorylation of the SAP97-I3I5 variant was ∼5 times stronger than any other variant (Fig. 7A). This strong preference for SAP97-I3I5 was somewhat surprising because CaMKII was previously shown to phosphorylate SAP97 in the L27 and PDZ1 domains (Ser-39 and Ser-232, respectively) (22, 23), and these domains are identical in all four SAP97 variants. To ascertain contributions of Ser-39 and/ or Ser-232 to total SAP97-I3I5 phosphorylation, either or both serine residues were mutated to Ala. WT and mutated FLAG-SAP97-I3I5 was then isolated and phosphorylated in vitro using exogenous purified CaMKIIα and [γ-32P]ATP. Autoradiography revealed that removal of either or both of the previously identified sites had no significant effect on total phosphorylation (Fig. 7B). Thus, FLAG-SAP97-I3I5 appears to be predominantly phosphorylated by CaMKII at sites other than Ser-39 and Ser-232 under these conditions.
FIGURE 7.
Preferential phosphorylation of the C-terminal region of SAP97-I3I5 by CaMKII. A, the four natural SAP97 U5 splice variants with a FLAG tag were expressed in HEK293 cells. FLAG immune complexes were phosphorylated by exogenous purified CaMKIIα in the presence of [γ-32P]ATP and analyzed by SDS-PAGE followed by staining of the gel and autoradiography. These data are representative of three experiments. B, wild type or mutated FLAG-SAP97 (S39A, S232A, S39A/S232A) were isolated from lysates of transfected HEK293 cells and then phosphorylated by purified CaMKIIα in the presence of [γ-32P]ATP (see “Experimental Procedures”). Samples were resolved by SDS-PAGE, and stained/dried gels were autoradiographed. Phosphorylation signals for each mutant were normalized to wild type SAP97. The bar graph shows the mean ± S.E. of data collected from at least six similar experiments. C, GST alone or GST fusion proteins containing the indicated domains from SAP97 splice variants (1 μm each) were phosphorylated by purified CaMKII (0.1 μm) in the presence of [γ-32P]ATP. Phosphorylated protein bands were detected by autoradiography of SDS-polyacrylamide gels. The bracket indicates 32P-phosphorylated CaMKII bands that were also observed in the GST blank (data not shown). These data are representative of >5 similar experiments. D, GST-SH3-U5-GK variants were phosphorylated as in panel C, and aliquots of the reactions were spotted on P81-phosphocellulose papers to estimate phosphorylation stoichiometries after subtracting the GST blank. Data are plotted as the mean ± S.E. from >6 experiments.
To identify SAP97 domains that can be phosphorylated at potentially novel sites by CaMKII, GST fusion proteins containing different domains from SAP97 or GST alone were incubated with activated CaMKII and [γ-32P]ATP. SDS-PAGE and autoradiography revealed a poorly defined series of 50–60-kDa bands in all lanes, including the GST blank, representing various autophosphorylated forms of CaMKII itself. GST-PDZ3 was not significantly phosphorylated. As expected, GST-L27 and GST-PDZ1/2 proteins containing Ser-39 and Ser-232, respectively, were 32P-phosphorylated by CaMKII, but GST-SH3-I3I5-GK was phosphorylated much more strongly (Fig. 7C, top). Quantitative analysis using P81 phosphocellulose papers revealed that CaMKII phosphorylated GST-SH3-I3I5-GK to a stoichiometry of ≈2.6 mol/mol, but other GST fusion proteins were phosphorylated to much lower stoichiometries (≤0.2 mol/mol). Similar analyses showed that other U5 region splice variants also were phosphorylated to a much lower stoichiometry than GST-SH3-I3I5-GK (Fig. 7D). Collectively, these data suggest that CaMKII preferentially phosphorylates several residues in the C-terminal domain of SAP97-I3I5.
Consistent with this idea, phosphorylation of GST-SH3-I3I5-GK was significantly reduced by 67 ± 10% by deletion of the I3 insert (n = 7, p < 0.005). Moreover, a GST fusion protein containing only the U5 region of the I3I5 variant was readily phosphorylated by CaMKII in vitro, and deletion of the I3 insert significantly reduced this phosphorylation by 54 ± 8% (n = 5, p < 0.005) (supplemental Fig. S2). Taken together, these data show that CaMKII can phosphorylate multiple sites in the U5 region of SAP97-I3I5 but that these sites may be distributed between the I3 insert and the remainder of the U5 region.
Because CaMKII preferentially phosphorylates GST-SH3-I3I5-GK, we wondered whether enzyme-substrate interactions contribute to the stable binding of CaMKII to these domains (see Fig. 6A). However, an excess of the classical substrate peptide, syntide-2, had no effect on binding of Thr-286-autophosphorylated CaMKII to GST-SH3-I3I5-GK in slot-blot assays (supplemental Fig. S3A). Interestingly, NR2B is also a potent CaMKII substrate (53) but forms stable complexes with a site in the CaMKII catalytic domain that is distinct from the syntide-2 substrate binding site (54). A synthetic peptide analog of the core CaMKII binding domain in NR2B (residues 1290–1309) competitively reduces the binding of Thr-286-autophosphorylated CaMKII to GST-NR2B by 80–90% but is somewhat less effective at blocking CaMKII binding to GST-SH3-I3I5-GK (≈60% reduction in binding) even though CaMKII binds with higher affinity to GST-NR2B (see above). Moreover, the R1299N mutation of the NR2B peptide completely prevents the competition with GST-NR2B but fails to fully relieve competition for CaMKII binding to GST-SH3-I3I5-GK (supplemental Fig. S3). Thus, although C-terminal domains of SAP97-I3I5 can be phosphorylated by CaMKII, a binding site that is distinct from the classical substrate binding site but that may overlap a binding site occupied by NR2B is required for formation of stable complexes with CaMKII.
CaMKII Modulates AKAP79/150 Interactions with SAP97 Variants
AKAP79/150 was shown to interact with the C-terminal half of SAP97 (26). However, the impact of alternative splicing in the U5 region on this interaction is unknown. Therefore, GST-SH3-U5-GK splice variants were incubated with extracts of HEK293 cells expressing GFP-AKAP79. Strong association of AKAP79 with GST-SH3-I3I5-GK, but not with other SAP97 splice variants, was detected by immunoblotting using a GFP antibody (Fig. 8, left). A similar splice variant selectivity was observed for SAP97 interaction with AKAP150, the rodent ortholog of human AKAP79 (data not shown). Because CaMKII preferentially phosphorylates SAP97-I3I5, we tested whether CaMKII phosphorylation of SAP97-I3I5 could alter the interaction of AKAP79 with this SAP97 variant. Thus, GST-SH3-U5-GK fusion proteins were maximally phosphorylated with CaMKII before incubation with HEK293 cells lysates containing AKAP79. Pre-phosphorylation of GST-SH3-I3I5-GK substantially reduced the association of AKAP79 (Fig. 8, right) and of AKAP150 (not shown). These data suggest that AKAP79/150 may bind only to the SAP97-I3I5 splice variant and that CaMKII activation and phosphorylation of SAP97 may disrupt the interaction in intact cells.
FIGURE 8.
Binding of AKAP79 to SAP97 is regulated by alternative mRNA splicing and CaMKII phosphorylation. GST fusion proteins containing SH3-U5-GK domains of the four SAP97 splice variants were preincubated with CaMKIIα in the absence or presence of ATP (Control and Phospho., respectively). After stopping the phosphorylation, lysates of HEK293 cells expressing GFP-AKAP79 were added. Protein complexes were collected on glutathione-agarose beads and immunoblotted (IB) with a GFP antibody.
SAP97 Splicing and CaMKII Modulate AKAP79-dependent Regulation of GluR1 AMPARs
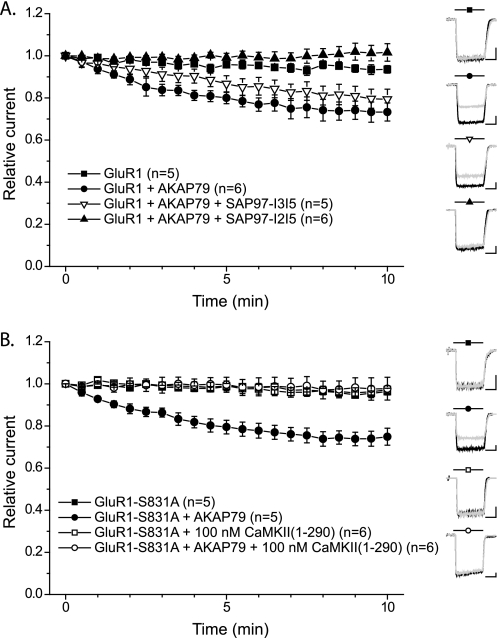
Interaction of AKAP79/150 with SAP97 targets PKA and PP2B to GluR1 to bidirectionally regulate GluR1 phosphorylation at Ser-845 and AMPAR currents (see the Introduction). In agreement with previous studies (8, 55), AMPAR currents were relatively stable when GluR1 alone was expressed in HEK293 cells but exhibited a significant time-dependent reduction of activity (rundown) when AKAP79 was co-expressed (Fig. 9A). In cells expressing GluR1 alone, the current amplitude after 10 min of recording (I10) was 93.5 ± 1.8% (n = 5) of the initial current amplitude (I0), whereas in cells co-expressing GluR1 and AKAP79 the I10/I0 ratio was 73.3 ± 4.2% (n = 6) (p < 0.01). This rundown requires the interaction of both GluR1 and AKAP79 with SAP97 endogenous to HEK293 cells (8, 27). Reverse transcription-PCR analyses indicated that HEK293 cells express both the I2 and I3 variants of SAP97; comparisons with parallel control PCR amplifications of purified cDNAs using the same primers suggested that I3-containing mRNAs appeared to be more abundant than I2-containing mRNAs (supplemental Fig. S4). To compare the role of SAP97 splice variants in the AKAP79-dependent rundown of GluR1 receptor currents, we co-expressed GluR1 and AKAP79 in HEK293 cells with either FLAG-SAP97-I2I5 or FLAG-SAP97-I3I5. The time course of GluR1-AMPAR rundown in the presence of AKAP79 was unaffected by co-expression of FLAG-SAP97-I3I5 (I10/I0 = 79.4 ± 4.7%, n = 5), but co-expression of FLAG-SAP97-I2I5 essentially abrogated AMPAR rundown (I10/I0 = 101.6 ± 4.2%, n = 6; p < 0.01 compared with GluR1+AKAP79 in the absence of SAP97-I2I5) (Fig. 9A). These data indicate that FLAG-SAP97-I3I5 effectively substitutes for the endogenous HEK293 cell SAP97. In contrast, FLAG-SAP97-I2I5 exerts a dominant negative effect to prevent effective targeting and regulation of GluR1 by the AKAP79 signaling complex.
FIGURE 9.
SAP97 splicing and CaMKII regulate AKAP79-dependent GluR1 AMPAR current rundown. A, whole-cell GluR1 AMPAR currents were recorded every 30 s from HEK293 cells expressing wild type GluR1 alone (filled squares) or GluR1 and AKAP79 (filled circles) in the absence or presence of either FLAG-SAP97(I3I5) (open triangles) or FLAG-SAP97(I2I5) (closed triangles) (see “Experimental Procedures”). The amplitude of each response was normalized to the peak amplitude in response to the initial application of glutamate to that cell. Insets show representative traces for each condition at time 0 (black) and 10 (gray) min (scale bars, 1 nA, 200 ms). B, CaMKII effect on AKAP79-dependent GluR1 AMPAR rundown. Whole-cell GluR1 AMPAR currents were recorded every 30 s from HEK293 cells expressing the CaMKII/PKC phosphorylation site mutant GluR1 (GluR1-S831A) alone (squares) or co-expressing AKAP79 (circles). Recordings were performed with (open symbols) or without (filled symbols) constitutively active CaMKII(1–290) in the patch pipette. Insets show representative traces for each condition at times 0 (black) and 10 (gray) min (scale bars, 500 pA, 200 ms).
We investigated the effects of CaMKII on AKAP79-dependent regulation of AMPARs using S831A-mutated GluR1 to avoid potentially confounding effects of CaMKII via the direct phosphorylation of Ser-831 (56). GluR1-S831A AMPARs exhibited normal AKAP79-dependent rundown in HEK293 cells (GluR1-S831A alone, I10/I0 = 96.3 ± 1.6%, n = 5; GluR1-S831A+AKAP79, I10/I0 = 74.9 ± 4.0%, n = 5; p < 0.01) (Fig. 9B) (8). As expected in cells expressing GluR1-S831A alone, inclusion of constitutively active CaMKII in the patch pipette had little effect on the stability of AMPAR currents I10/I0 = 97.2 ± 4.7%, n = 6). However, in cells co-expressing GluR1-S831A and AKAP79, CaMKII completely abrogated the AKAP79-dependent rundown of AMPAR activity (I10/I0 = 97.7 ± 5.4%, n = 6; p < 0.01 compared with GluR1-S831A+AKAP79 without CaMKII). Collectively, our data suggest that CaMKII-mediated phosphorylation of SAP97 disrupts the ability of AKAP79 to target PP2B for effective dephosphorylation of Ser-845 in GluR1-AMPARs.
DISCUSSION
The present studies show that SAP97 is associated with CaMKII in the brain and define new functional relationships between CaMKII, AKAP79/150, and GluR1 that appear to be coordinated by a single mRNA splice variant of the U5 region of SAP97. Four SAP97 variants were identified in mRNA isolated from rat hippocampus at postnatal day 21, with U5 regions containing different combinations of the I2, I3, and I5 inserts along with full-length or truncated versions of the I4 insert (Fig. 5). The I4s insert appears to arise from use of alternative cryptic splice acceptor sites (see the supplemental information for a discussion). Multiple splice variants in this region of the human and mouse SAP97 mRNAs are documented in SWISS-PROT and other databases. However, only one rat mRNA splice variant is listed (I2I5I4s) even though multiple rat variants have been described in the literature (30, 51, 52, 57, 58). It is likely that additional rat splice variants can be expressed because we did not detect a variant containing only an I2 insert even though this variant has been identified in human mRNA pools (Q811DO). It should also be noted that splice variants of SAP97 in the U1 domain and at the extreme N terminus have also been identified (32, 52, 57–59). It will be interesting to investigate whether splicing is coordinated to link certain combinations of N- and C-terminal inserts. Moreover, given the functional differences (see below), it will be important to investigate the cellular specificity and relative abundance of splice variant expression in addition to potential changes during development and in disease processes.
Using a combination of in vitro and heterologous cell experiments with protein fragments and full-length proteins (Figs. 2, 3, 4, and 6 and supplemental Fig. S1), we found that autophosphorylated CaMKII forms a stable complex with SAP97. Activated CaMKII can directly and selectively interact with the C-terminal SH3-I3I5-GK domains in vitro, and a complex of CaMKII with the SH3-I3I5-GK domains can be co-immunoprecipitated from transfected HEK293 cells. The intact N-terminal “half” of SAP97 also associates with CaMKII in HEK293 cells, presumably explaining the lack of selectivity for CaMKII co-immunoprecipitation with full-length U5 region splice variants. However, we were unable to detect substantial direct interactions of purified CaMKII with isolated domains from the N-terminal portion of SAP97 in vitro. Thus, the association of CaMKII with the N-terminal half of SAP97 may be mediated by another protein present in HEK cells. Alternatively, longer contiguous segments of the N-terminal portion of SAP97 may be required for direct interaction. Additional studies will be required to dissect the molecular basis for CaMKII interaction with N-terminal domain(s) in SAP97. Nevertheless, the selective interaction of CaMKII with C-terminal domains of the I3I5-containing variant may confer unique regulatory properties.
Demonstration of a direct interaction between activated CaMKII and the SH3-I3I5-GK domains means that SAP97 can be added to the growing list of cellular proteins that can interact with CaMKII (for review, see Ref. 6). Some proteins favor interaction with activated forms of CaMKII, whereas others prefer inactive forms. By this criterion, the C-terminal fragment of SAP97-I3I5 appears to fit in the former group, which includes the NR2B subunit of the NMDA receptor, densin, and the β2a and β1b subunits of voltage-gated calcium channels (41, 46, 47, 49, 50, 60, 61). NR2B, β2a, and β1b contain a conserved CaMKII binding domain (Kd = 0.1–0.2 μm CaMKII subunit) with amino acid sequence similarity to the autoinhibitory domain of CaMKII itself (46, 47, 54, 61), but the CaMKII binding domain in densin exhibits no amino acid sequence similarity to these domains (41). The U5 region of SAP97-I3I5 has no detectable amino acid sequence similarity to any previously identified CaMKII binding domain. However, the binding sites for SAP97 and NR2B in the CaMKII catalytic domain may partially overlap (supplemental Fig. S3) even though the differential effects of Ca2+/calmodulin and nucleotide on binding of nonphosphorylated kinase to GST-SH3-I3I5-GK and GST-NR2B suggest unique aspects to these interactions. Additional studies will be required to understand the structural basis for CaMKII interactions with these CaMKII-associated proteins.
Previous studies defining the interaction of AKAP79/150-PKA-PP2B complexes with C-terminal domains of SAP97 did not examine the impact of alternative splicing. Our findings significantly extend these studies by demonstrating that the SAP97-I3I5 variant is specifically involved (Fig. 8). In a well established HEK293 cell model for AKAP79-dependent regulation of GluR1-AMPARs via endogenous SAP97 (8, 27, 55), we showed that overexpression of SAP97-I2I5 functioned as a dominant negative protein to disrupt AKAP79-dependent rundown of AMPAR currents (Fig. 9A), whereas overexpression of SAP97-I3I5 had no significant effect on AMPAR rundown. These data may be explained by the apparent predominance of SAP97 variants containing the I3 insert in HEK293 cells (supplemental Fig. S4). Notably, SAP97-I3I5 is selectively trafficked to dendritic spines in neurons, whereas variants containing I2 are typically localized to cytosolic/perinuclear/nuclear compartments (29, 31, 32). In combination, these data suggest a unique role for SAP97-I3I5 in regulation of synaptic AMPARs.
In addition to CaMKII and AKAP79/150, C-terminal domains of SAP97-I3I5 selectively interact with the Band 4.1 actin-binding protein (33, 35). Splicing in the U5 region also modulates intramolecular SAP97 domain interactions that can interfere with the binding to other targeting proteins (30). The apparently unique properties conferred by I3 may result from differences in the isoelectric point (pI) of the U5 region. Whereas the I3I5 insert combination has an overall pI of 6.49, the pI values of other U5 splicing inserts examined here range from 4.6 to 4.9. Thus, we hypothesize that the unique lack of acidity in the U5 region of SAP97-I3I5 contributes to the selectivity of the entire C-terminal half for binding CaMKII, AKAP79/150, and Band 4.1.
Proteins that form stable complexes with CaMKII are often good substrates (46, 47, 54, 61). Consistent with this idea, SAP97-I3I5 is selectively phosphorylated by CaMKII in vitro in the context of full-length protein (Fig. 7A) and can be phosphorylated by co-precipitating CaMKII (Fig. 3A). Interestingly, a post hoc (back) phosphorylation assay showed that relative to an internal “dephosphorylated” control, SAP97-I3I5 is ≈50% phosphorylated at CaMKII sites even in the absence of co-expressed CaMKIIα in HEK293 cells, perhaps due to the activities of endogenous CaMKII isoforms or other kinases that target the same sites. However, co-expression of CaMKIIα significantly enhanced the phosphorylation of SAP97-I3I5 in intact cells (Fig. 3B). Thus, our data suggest that CaMKII associates with and selectively phosphorylates SAP97-I3I5 in intact cells.
CaMKII was shown to phosphorylate Ser-39 and Ser-232 but not C-terminal domains of SAP97 (22, 23), but we found that the known sites were poorly phosphorylated under our conditions in the context of both full-length SAP97-I3I5 mutants and isolated L27 and PDZ1/2 domains. GST-SH3-I3I5-GK was a substantially better in vitro substrate for CaMKII than GST fusion proteins containing the other U5 variants (Fig. 7C). CaMKII can phosphorylate at least three Ser/Thr residues in GST-SH3-I3I5-GK based on a maximal phosphorylation stoichiometry of ≈2.6 mol/mol. In fact, the U5 region of the I3I5 splice variant contains 11 serines and 4 threonines. Deletion of the I3 insert removes 5 of the Ser/Thr residues and reduces the phosphorylation stoichiometry by ≈33% (supplemental Fig. S2), suggesting that the I3 insert can be phosphorylated at 1 or more residues. Residual phosphorylation of the artificial GST-SH3-I5-GK variant (≈1.5 mol/mol) must occur at sites present in the C-terminal domains of the other natural SAP97 variants. Nevertheless, GST-SH3-U5-GK proteins containing other natural splice variants (I2I5, I2I5I4, or I2I5I4s) are rather poor CaMKII substrates (stoichiometries of <0.1 mol/mol). Thus, the I2 and/or I4 inserts appears to interfere with CaMKII phosphorylation of the conserved sites in the U5 domain, presumably due to changes in amino acid sequences surrounding Ser/Thr residues that are located close to splice insert junctions.
CaMKII phosphorylation of SAP97-I3I5 will reduce the pI of the U5 region closer to that of the other U5 region variants, perhaps explaining why phosphorylation of GST-SH3-I3I5-GK essentially abrogated AKAP79/150 binding (Fig. 8). This suggests that CaMKII can uncouple AKAP79/150 from SAP97-I3I5 in cells. Indeed, our electrophysiological studies using an S831A-GluR1 mutant (to avoid confounding effects of CaMKII via direct phosphorylation at Ser-831) showed that infusion of constitutively active CaMKII abrogated the rundown of AMPAR currents (Fig. 9B). Similarly, previous studies showed that disruption of the SAP97·AKAP79/150·PKA·PP2B complex using peptide competitors for specific interactions within the complex or by knocking out/down AKAP150 or SAP97 has profound effects on AMPAR trafficking and activity (8, 26–28, 55, 62). These findings are consistent with a model in which CaMKII phosphorylation of SAP97-I3I5 displaces AKAP79 to limit further changes in GluR1 phosphorylation at Ser-845 (Fig. 10). This might preserve previously induced high levels of phosphorylation at Ser-845, sustaining the activity of AMPAR currents as observed in our HEK293 cell model (Fig. 9B). Alternatively, under other cellular conditions CaMKII phosphorylation of SAP97-I3I5 might uncouple increased phosphorylation at Ser-845 from PKA activation. Notably, some studies indicate that Ser-845 must first be phosphorylated before CaMKII can be engaged to promote GluR1 trafficking to the synapse (9, 13, 21). Interestingly, although other mechanisms contribute to the localization of AKAP79/150 to the cortical cytoskeleton and dendritic spines (18, 63), NMDA receptor activation has been shown to dissociate MAGUK·AKAP complexes and promote translocation of AKAP79/150 and PKA away from synapses (17, 63). In parallel, our model predicts that CaMKII bound to SAP97-I3I5 can phosphorylate Ser-831 in wild type GluR1 (Fig. 10). Thus, CaMKII association with SAP97-I3I5 may coordinate GluR1 phosphorylation at Ser-831 and Ser-845, representing a novel pathway to provide flexibility of downstream CaMKII signaling to regulate AMPARs in different biological contexts and during synaptic plasticity.
FIGURE 10.
Working model for CaMKII-dependent modulation of SAP97-I3I5 signaling complexes. White/open symbols containing black text indicate the phosphorylated Ser-845 residue of GluR1 as well as PKA and PP2B, the opposing enzymes that control Ser-845 phosphorylation. Black symbols containing white text represent CaMKII itself as well as CaMKII phosphorylation sites in SAP97-I3I5, GluR1, and CaMKII. In the basal state the SAP97-I3I5 isoform assembles synaptic GluR1-AMPARs with AKAP79/150, facilitating PKA phosphorylation at Ser-845. Ca2+ influx activates both CaMKII and PP2B. CaMKII binding and phosphorylation of SAP97-I3I5 in the U5 region triggers dissociation of AKAP79/150, uncoupling PP2B from GluR1 and protecting Ser-845 in GluR1 from dephosphorylation. CaMKII binding to SAP97-I3I5 also may facilitate phosphorylation of GluR1 at Ser-831. The dual phosphorylation of GluR1 at Ser-831 and Ser-845 favors long term potentiation. Other splice variants of SAP97 (not shown) do not bind AKAP79/150.
In summary, our data suggest that multiprotein complexes formed by SAP97 splice variants can be differentially regulated, increasing the flexibility and fidelity of neuronal signaling. First, alternative splicing in the U5 region can modulate the selectivity of SAP97 association with other signaling proteins, such as the selective binding of AKAP79/150 to SAP97-I3I5. Second, SAP97-I3I5 may provide a scaffold to selectively target CaMKII to substrates like GluR1 subunits of AMPARs, NR2A/2B subunits of NMDA receptors, and KV4.2 potassium channel subunits (22–25), facilitating precise CaMKII-dependent control of a specific pool of ion channels. Finally, phosphorylation of SAP97-I3I5 by CaMKII disrupts the binding of AKAP79/150 to promote divergence of downstream signaling pathways in response to Ca2+ influx. These findings emphasize the nuances and specificity involved in the regulation of signaling protein complexes that control synaptic plasticity, learning, and memory.
Supplementary Material
Acknowledgments
We greatly appreciate the generous gifts of cDNA constructs from Drs. Mark Dell'Acqua, Craig Garner, Tom Soderling, and Donna Webb (see “Experimental Procedures”). Excellent assistance was provided by Martha Bass (purified CaMKII) and Christopher Arnette (generation of mCherry-CaMKIIα (wild type) expression construct).
This work was supported, in whole or in part, by National Institutes of Health Grants RO1-MH63232 and PO1-NS044282 (to R. J. C.) and R01-NS046661 (to S. J. T.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- CaMKII
- calcium/calmodulin-dependent protein kinase II
- AKAP
- A-kinase anchoring protein
- AMPAR
- α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid-type glutamate receptor
- GK
- guanylyl kinase-like
- MAGUK
- membrane-associated guanylyl kinase
- NMDA
- N-methyl-d-aspartic acid
- PDZ
- PSD-95/Disc Large/Zona Occludens 1
- PP2B
- protein phosphatase 2B
- PKA
- protein kinase A (also known as cAMP-dependent protein kinase and A-kinase)
- PKC
- protein kinase C
- SAP97
- synapse-associated protein 97 (also known as hDLG1)
- SH3
- Src homology 3
- GST
- glutathione S-transferase
- IP
- immunoprecipitation
- WT
- wild type
- GFP
- green fluorescent protein.
REFERENCES
- 1.Lisman J., Schulman H., Cline H. (2002) Nat. Rev. Neurosci. 3, 175–190 [DOI] [PubMed] [Google Scholar]
- 2.Asrican B., Lisman J., Otmakhov N. (2007) J. Neurosci. 27, 14007–14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colbran R. J., Brown A. M. (2004) Curr. Opin. Neurobiol. 14, 318–327 [DOI] [PubMed] [Google Scholar]
- 4.Griffith L. C., Lu C. S., Sun X. X. (2003) Mol. Interv. 3, 386–403 [DOI] [PubMed] [Google Scholar]
- 5.Hansel C., de Jeu M., Belmeguenai A., Houtman S. H., Buitendijk G. H., Andreev D., De Zeeuw C. I., Elgersma Y. (2006) Neuron 51, 835–843 [DOI] [PubMed] [Google Scholar]
- 6.Colbran R. J. (2004) Biochem. J. 378, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudmon A., Schulman H. (2002) Biochem. J. 364, 593–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tavalin S. J., Colledge M., Hell J. W., Langeberg L. K., Huganir R. L., Scott J. D. (2002) J. Neurosci. 22, 3044–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H. K., Barbarosie M., Kameyama K., Bear M. F., Huganir R. L. (2000) Nature 405, 955–959 [DOI] [PubMed] [Google Scholar]
- 10.Tavalin S. J. (2008) J. Biol. Chem. 283, 11445–11452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisman J. E., Zhabotinsky A. M. (2001) Neuron 31, 191–201 [DOI] [PubMed] [Google Scholar]
- 12.Boehm J., Ehrlich I., Hsieh H., Malinow R. (2006) Learn Mem. 13, 562–565 [DOI] [PubMed] [Google Scholar]
- 13.Oh M. C., Derkach V. A., Guire E. S., Soderling T. R. (2006) J. Biol. Chem. 281, 752–758 [DOI] [PubMed] [Google Scholar]
- 14.Derkach V., Barria A., Soderling T. R. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3269–3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shepherd J. D., Huganir R. L. (2007) Annu. Rev. Cell Dev. Biol. 23, 613–643 [DOI] [PubMed] [Google Scholar]
- 16.Lu Y., Allen M., Halt A. R., Weisenhaus M., Dallapiazza R. F., Hall D. D., Usachev Y. M., McKnight G. S., Hell J. W. (2007) EMBO J. 26, 4879–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder E. M., Colledge M., Crozier R. A., Chen W. S., Scott J. D., Bear M. F. (2005) J. Biol. Chem. 280, 16962–16968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dell'Acqua M. L., Smith K. E., Gorski J. A., Horne E. A., Gibson E. S., Gomez L. L. (2006) Eur. J. Cell Biol. 85, 627–633 [DOI] [PubMed] [Google Scholar]
- 19.Hayashi Y., Shi S. H., Esteban J. A., Piccini A., Poncer J. C., Malinow R. (2000) Science 287, 2262–2267 [DOI] [PubMed] [Google Scholar]
- 20.Shi S., Hayashi Y., Esteban J. A., Malinow R. (2001) Cell 105, 331–343 [DOI] [PubMed] [Google Scholar]
- 21.Esteban J. A., Shi S. H., Wilson C., Nuriya M., Huganir R. L., Malinow R. (2003) Nat. Neurosci. 6, 136–143 [DOI] [PubMed] [Google Scholar]
- 22.Mauceri D., Cattabeni F., Di Luca M., Gardoni F. (2004) J. Biol. Chem. 279, 23813–23821 [DOI] [PubMed] [Google Scholar]
- 23.Gardoni F., Mauceri D., Fiorentini C., Bellone C., Missale C., Cattabeni F., Di Luca M. (2003) J. Biol. Chem. 278, 44745–44752 [DOI] [PubMed] [Google Scholar]
- 24.Mauceri D., Gardoni F., Marcello E., Di Luca M. (2007) J. Neurochem. 100, 1032–1046 [DOI] [PubMed] [Google Scholar]
- 25.Gardoni F., Mauceri D., Marcello E., Sala C., Di Luca M., Jeromin A. (2007) J. Biol. Chem. 282, 28691–28699 [DOI] [PubMed] [Google Scholar]
- 26.Colledge M., Dean R. A., Scott G. K., Langeberg L. K., Huganir R. L., Scott J. D. (2000) Neuron 27, 107–119 [DOI] [PubMed] [Google Scholar]
- 27.Hoshi N., Langeberg L. K., Scott J. D. (2005) Nat. Cell Biol. 7, 1066–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tunquist B. J., Hoshi N., Guire E. S., Zhang F., Mullendorff K., Langeberg L. K., Raber J., Scott J. D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12557–12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts S., Calautti E., Vanderweil S., Nguyen H. O., Foley A., Baden H. P., Viel A. (2007) Exp. Cell Res. 313, 2521–2530 [DOI] [PubMed] [Google Scholar]
- 30.Wu H., Reissner C., Kuhlendahl S., Coblentz B., Reuver S., Kindler S., Gundelfinger E. D., Garner C. C. (2000) EMBO J. 19, 5740–5751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rumbaugh G., Sia G. M., Garner C. C., Huganir R. L. (2003) J. Neurosci. 23, 4567–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaughlin M., Hale R., Ellston D., Gaudet S., Lue R. A., Viel A. (2002) J. Biol. Chem. 277, 6406–6412 [DOI] [PubMed] [Google Scholar]
- 33.Lue R. A., Marfatia S. M., Branton D., Chishti A. H. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 9818–9822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godreau D., Vranckx R., Maguy A., Goyenvalle C., Hatem S. N. (2003) J. Biol. Chem. 278, 47046–47052 [DOI] [PubMed] [Google Scholar]
- 35.Hanada T., Takeuchi A., Sondarva G., Chishti A. H. (2003) J. Biol. Chem. 278, 34445–34450 [DOI] [PubMed] [Google Scholar]
- 36.Okamoto K., Narayanan R., Lee S. H., Murata K., Hayashi Y. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6418–6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sessoms-Sikes S., Honse Y., Lovinger D. M., Colbran R. J. (2005) Mol. Cell. Neurosci. 29, 139–147 [DOI] [PubMed] [Google Scholar]
- 38.Tsui J., Inagaki M., Schulman H. (2005) J. Biol. Chem. 280, 9210–9216 [DOI] [PubMed] [Google Scholar]
- 39.Jiao Y., Robison A. J., Bass M. A., Colbran R. J. (2008) J. Neurochem. 105, 1746–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. (2004) Nat. Biotechnol. 22, 1567–1572 [DOI] [PubMed] [Google Scholar]
- 41.Strack S., Robison A. J., Bass M. A., Colbran R. J. (2000) J. Biol. Chem. 275, 25061–25064 [DOI] [PubMed] [Google Scholar]
- 42.McNeill R. B., Colbran R. J. (1995) J. Biol. Chem. 270, 10043–10049 [DOI] [PubMed] [Google Scholar]
- 43.Brickey D. A., Colbran R. J., Fong Y. L., Soderling T. R. (1990) Biochem. Biophys. Res. Commun. 173, 578–584 [DOI] [PubMed] [Google Scholar]
- 44.Chang B. H., Mukherji S., Soderling T. R. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 10890–10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang B. H., Mukherji S., Soderling T. R. (2001) Neuroscience 102, 767–777 [DOI] [PubMed] [Google Scholar]
- 46.Bayer K. U., De Koninck P., Leonard A. S., Hell J. W., Schulman H. (2001) Nature 411, 801–805 [DOI] [PubMed] [Google Scholar]
- 47.Grueter C. E., Abiria S. A., Dzhura I., Wu Y., Ham A. J., Mohler P. J., Anderson M. E., Colbran R. J. (2006) Mol. Cell 23, 641–650 [DOI] [PubMed] [Google Scholar]
- 48.Robison A. J., Bartlett R. K., Bass M. A., Colbran R. J. (2005) J. Biol. Chem. 280, 39316–39323 [DOI] [PubMed] [Google Scholar]
- 49.Strack S., Colbran R. J. (1998) J. Biol. Chem. 273, 20689–20692 [DOI] [PubMed] [Google Scholar]
- 50.Walikonis R. S., Oguni A., Khorosheva E. M., Jeng C. J., Asuncion F. J., Kennedy M. B. (2001) J. Neurosci. 21, 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu H., Reuver S. M., Kuhlendahl S., Chung W. J., Garner C. C. (1998) J. Cell Sci. 111, 2365–2376 [DOI] [PubMed] [Google Scholar]
- 52.Müller B. M., Kistner U., Veh R. W., Cases-Langhoff C., Becker B., Gundelfinger E. D., Garner C. C. (1995) J. Neurosci. 15, 2354–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Omkumar R. V., Kiely M. J., Rosenstein A. J., Min K. T., Kennedy M. B. (1996) J. Biol. Chem. 271, 31670–31678 [DOI] [PubMed] [Google Scholar]
- 54.Strack S., McNeill R. B., Colbran R. J. (2000) J. Biol. Chem. 275, 23798–23806 [DOI] [PubMed] [Google Scholar]
- 55.Dell'Acqua M. L., Dodge K. L., Tavalin S. J., Scott J. D. (2002) J. Biol. Chem. 277, 48796–48802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barria A., Derkach V., Soderling T. (1997) J. Biol. Chem. 272, 32727–32730 [DOI] [PubMed] [Google Scholar]
- 57.Schlüter O. M., Xu W., Malenka R. C. (2006) Neuron 51, 99–111 [DOI] [PubMed] [Google Scholar]
- 58.Waites C. L., Specht C. G., Härtel K., Leal-Ortiz S., Genoux D., Li D., Drisdel R. C., Jeyifous O., Cheyne J. E., Green W. N., Montgomery J. M., Garner C. C. (2009) J. Neurosci. 29, 4332–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mori K., Iwao K., Miyoshi Y., Nakagawara A., Kofu K., Akiyama T., Arita N., Hayakawa T., Nakamura Y. (1998) J. Hum. Genet. 43, 123–127 [DOI] [PubMed] [Google Scholar]
- 60.Robison A. J., Bass M. A., Jiao Y., MacMillan L. B., Carmody L. C., Bartlett R. K., Colbran R. J. (2005) J. Biol. Chem. 280, 35329–35336 [DOI] [PubMed] [Google Scholar]
- 61.Grueter C. E., Abiria S. A., Wu Y., Anderson M. E., Colbran R. J. (2008) Biochemistry 47, 1760–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakagawa T., Futai K., Lashuel H. A., Lo I., Okamoto K., Walz T., Hayashi Y., Sheng M. (2004) Neuron 44, 453–467 [DOI] [PubMed] [Google Scholar]
- 63.Gomez L. L., Alam S., Smith K. E., Horne E., Dell'Acqua M. L. (2002) J. Neurosci. 22, 7027–7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.