Abstract
The circadian clock-associated 1 (CCA1) gene encodes a Myb-related transcription factor that has been shown to be involved in the phytochrome regulation of Lhcb1*3 gene expression and in the function of the circadian oscillator in Arabidopsis thaliana. By using a yeast interaction screen to identify proteins that interact with CCA1, we have isolated a cDNA clone encoding a regulatory (β) subunit of the protein kinase CK2 and have designated it as CKB3. CKB3 is the only reported example of a third β-subunit of CK2 found in any organism. CKB3 interacts specifically with CCA1 both in a yeast two-hybrid system and in an in vitro interaction assay. Other subunits of CK2 also show an interaction with CCA1 in vitro. CK2 β-subunits stimulate binding of CCA1 to the CCA1 binding site on the Lhcb1*3 gene promoter, and recombinant CK2 is able to phosphorylate CCA1 in vitro. Furthermore, Arabidopsis plant extracts contain a CK2-like activity that affects the formation of a DNA–protein complex containing CCA1. These results suggest that CK2 can modulate CCA1 activity both by direct interaction and by phosphorylation of the CCA1 protein and that CK2 may play a role in the function of CCA1 in vivo.
The phytochromes, a class of plant photoreceptors that has been studied extensively (1), regulate the expression of many genes, including the Lhcb genes that encode the chlorophyll a/b-proteins of photosystem II (2). A promoter region of the Lhcb1*3 gene of Arabidopsis thaliana that is essential for its regulation by phytochrome has been identified (3, 4), and the CCA1 gene, whose product specifically interacts with this promoter region, has been cloned (5). The motif to which CCA1 binds is highly conserved in promoters of Lhcb genes from many species. Transgenic Arabidopsis plants expressing antisense CCA1 RNA show reduced phytochrome induction of the endogenous Lhcb1*3 gene in etiolated seedlings. Furthermore, the increase in CCA1 mRNA in response to light precedes the increase in Lhcb1*3 mRNA (5). These data show that CCA1 is a downstream component of the phytochrome signal transduction pathway leading to increased transcription of the Lhcb1*3 gene in Arabidopsis.
Expression of the Lhcb genes is regulated also by circadian rhythms (6). Characterization of CCA1 has shown that it is involved also in the circadian regulation of the Lhcb1*1 gene and in the control of other physiological rhythms, such as timing of flowering. CCA1 mRNA and protein levels themselves exhibit circadian oscillations, and overexpression of CCA1 represses the expression of the endogenous CCA1 gene. Our results have demonstrated that the function of CCA1 is closely associated with the circadian oscillator itself (7).
To understand how CCA1 may function in the phytochrome signal transduction pathway and in the regulation of circadian rhythms, a yeast two-hybrid system has been used to identify proteins that can interact with the CCA1 protein. A gene designated CKB3, whose product interacts specifically with CCA1, has been identified in this way. CKB3 is a structural and functional homologue of the regulatory (β) subunit of protein kinase CK2 in Arabidopsis. The characteristics of the interaction of CK2 subunits with CCA1 have been examined, and our results suggest that CK2 may play a role in the regulation of CCA1 function.
MATERIALS AND METHODS
Yeast Strains and Expression Plasmid.
Saccharomyces cerevisiae Y190 and pAS2, pAS-SNF1, and pACT were obtained from the Arabidopsis Biological Resource Center (ABRC, Ohio State University, Columbus, OH). YDH8 and pJCR14 have been described (8, 9). pKT-CKB3 was constructed by ligating the Arabidopsis CKB3 cDNA into pKT10 (10).
Yeast Two-Hybrid Screen and cDNA Isolation.
For the yeast two-hybrid screen, the entire coding region for CCA1 was fused to the GAL4 DNA binding domain (GAL4-DB) in pAS2. Y190 was transformed with the resulting plasmid, pAS-CCA1, then with a library made from Arabidopsis cDNAs fused to the GAL4 transactivation domain (GAL4-AD) (ABRC). Transformants (4 × 106) were analyzed as described (11). To obtain a cDNA for the full length CKB3 gene, an Arabidopsis cDNA library in λgt22 (5) was screened with the selected clone 106. DNA sequencing was done with a Sequenase kit (United States Biochemical). The GenBank database was searched by using the blast program (National Center for Biotechnology Information, Bethesda, MD).
Recombinant Proteins.
pGEX-CCA1 contains cDNA encoding CCA1 cloned into pGEX-3X (Pharmacia). pET-CKA1 and pET-CKA2 have been described (12). CKB1 and pT7-CKB3 contain cDNAs encoding Arabidopsis CKB1 (13) and CKB3 in pT7-His (14). Expression and purification of glutathione-S-transferase (GST) and GST-CCA1 and purification of CCA1 by cleavage of GST-CCA1 with factor Xa have been described (5). CKA1 and CKA2 were produced as described (12) and were purified on a heparin–agarose column (Bio-Rad). His-tagged CKB1 and CKB3 were produced in Escherichia coli strain BL21(DE3) and purified on Ni-NTA agarose (Qiagen, Valencia, CA). Protein concentrations were determined by the Bradford assay (Bio-Rad).
In Vitro Binding Assays.
CK2 subunits and GBF4 labeled with [35S]methionine were synthesized by coupled transcription translation with wheat germ extract (Promega). For in vitro binding, 20 μl of the reactions was added to 200 μl of binding buffer (20 mM Hepes pH 7.6/100 mM KCl/10% glycerol/5 mM EDTA/0.02% NP40/1 mM DTT/5 mg/ml BSA) followed by 10 μl of glutathione–agarose beads with bound GST or GST-CCA1 and was incubated at 4°C. The beads were washed with binding buffer, then with binding buffer without BSA. Bound proteins were eluted with 1X SDS/PAGE sample buffer and were resolved by 12.5% SDS/PAGE. 35S-labeled bands were detected by autoradiography, and quantitation was done with a PhosphorImager (Molecular Dynamics).
In Vitro Kinase Assays.
GST-CCA1 bound to glutathione–agarose beads was resuspended in 50 μl of CK2 buffer (50 mM Tris⋅HCl, pH 7.5/10 mM MgCl2/100 mM NaCl/1 mM DTT/0.1 mM ATP or GTP) in the presence of 5–10 μCi of [γ-32P]ATP or GTP. The reaction was started by adding CK2 or whole-cell extracts (WCE) and incubating samples at 30°C for 30 min. WCE were prepared as described (3), except that the phosphatase inhibitor mixture (5 mM NH4VO3/0.2 mM ammonium molybdate/1 mM EGTA/50 mM NaF) was added to the extraction buffer. The beads were washed with PBS containing 1% Triton X-100 and were resuspended in 1X SDS/PAGE sample buffer. The phosphorylated samples were separated by 10% SDS/PAGE. 32P-labeled bands were detected by autoradiography and quantitated with a PhosphorImager.
Electrophoretic Mobility-Shift Assays (EMSAs).
CCA1 was incubated with 0.1 ng of end-labeled A2 fragment of the Arabidopsis Lhcb 1*3 gene (3) in the presence of 0.5 μg of poly(dI-dC) at 25°C for 15 min. WCE were incubated in preincubation buffer (50 mM Tris⋅HCl, pH 7.5/2 mM MnCl2/5 mM DTT/0.1 mM EDTA/0.01% Brij 35) at 30°C for 45 min, then incubated with the A2 probe in the presence of 1 μg of poly(dI-dC). The EMSA buffer and electrophoresis conditions have been described (3). DNA–protein complexes were detected by autoradiography.
RESULTS
Isolation and Analysis of the CKB3 cDNA.
To isolate proteins that interact with CCA1, the yeast two-hybrid system that uses GAL4 recognition sites to regulate expression of both HIS3 and lacZ genes was used (11). The GAL4-DB-CCA1 fusion protein did not itself activate transcription of the reporter genes. Four positive colonies were obtained that contained plasmid that activated HIS3 and lacZ transcription only in the presence of GAL4-DB-CCA1. These colonies fell into two classes, based on sequence analysis, and one of them, clone 106, was fully characterized.
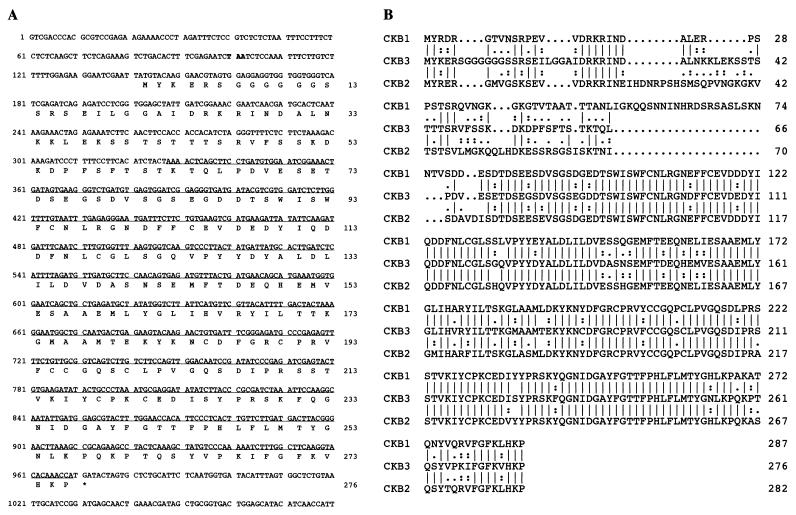
The reading frame of clone 106 encoded a 214-aa fragment. Because a putative translation initiation codon was missing in this cDNA clone, corresponding clones were isolated from an Arabidopsis cDNA library. The sequence of the full length cDNA insert is shown in Fig. 1A. The 276-aa residue ORF encodes an estimated 30.8-kDa protein. The first ATG codon of the ORF starts at 142 bp and is preceded by an in-frame stop codon at the −42 to −40 position and by a purine (A) at the −3 position. This is a favorable context for an initiation codon in plants (15). The deduced amino acid sequence is highly homologous to the β-subunit of protein kinase CK2, in particular to Arabidopsis CKB1 and CKB2. Thus, the gene corresponding to this cDNA clone was designated CKB3.
Figure 1.
The structure of Arabidopsis CKB3 and its homology with Arabidopsis CKB1 and CKB2. (A) cDNA and deduced amino acid sequence of Arabidopsis CKB3. The predicted amino acid sequence is indicated in single-letter code. The clone 106 cDNA sequence is underlined. An upstream in-frame stop codon is shown in bold. Nucleotide numbers are on the left, and amino acid numbers are on the right. (B) Alignment of predicted amino acid sequences of Arabidopsis CKB1, CKB2, and CKB3. Identical amino acid residues are indicated by vertical lines, and conservative amino acid replacements are indicated by single and double dots. Dashes represent gaps introduced to give maximal identity.
Fig. 1B shows an alignment of the amino acid sequences of Arabidopsis CKB1, CKB2, and CKB3. The amino acid identities between CKB3 and CKB1, CKB3 and CKB2, and CKB1 and CKB2 are 75%, 71%, and 80%, respectively. The similarity is greatest over the carboxyl-terminal two-thirds of the three proteins. The CKB3 protein shares most of the structural features of CKB1 and CKB2 at the level of primary structure (13). First, CKB3 contains a potential metal binding motif CPX3C-X22-CPXC (9). Second, although the conserved autophosphorylation site SSSEE is missing in the amino-terminal region of CKB3, there are two CK2 recognition phosphorylation sites, 81SGSEGD and 83SEGDD, in about the same location as in the animal β-subunits. Third, CKB3 has an N-terminal extension preceding the putative phosphorylation sites that exhibits a moderate level of similarity to the N-terminal extension of the other Arabidopsis β-subunits. Neither yeast nor animal β-subunits contain such an N-terminal extension, and this region bears no extensive similarity to other proteins.
CKB3 Has Functional Similarity to CKB1 and CKB2.
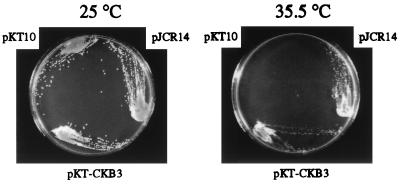
S. cerevisiae has two genes coding for the catalytic (α) subunits of CK2, and at least one of the two genes is required for vegetative growth. YDH8, which carries the cka1-Δ1 cka2–8 mutation, grows at 25°C but not at 35.5°C (8), and this temperature sensitivity can be compensated for by overexpressing CK2 β-subunits, including Arabidopsis CKB1 and CKB2 (13). We tested whether CKB3 also could compensate for the temperature sensitivity of the mutation. As controls, pKT10 and pJCR14, which contains the S. cerevisiae CKB2 gene, also were transformed into YDH8. Fig. 2 shows that YDH8 cells expressing either S. cerevisiae CKB2 or Arabidopsis CKB3 could grow both at 25°C and 35.5°C, whereas transformants with pKT10 could grow only at 25°C. These results demonstrate that CKB3 shares functional similarity with CKB1 and CKB2.
Figure 2.
Compensation of the cka1-Δ1 cka2–8 temperature-sensitive mutation by Arabidopsis CKB3 cDNA. YDH8 (cka1-Δ1, cka2–8) was transformed with yeast expression vectors and was incubated at 25°C or 35.5°C. Transformants harboring pKT10 vector only, pJCR14 carrying the S. cerevisiae CKB2 gene, and pKT-CKB3 carrying the Arabidopsis CKB3 gene are shown.
CCA1 Can Interact with Both α- and β-Subunits of CK2.
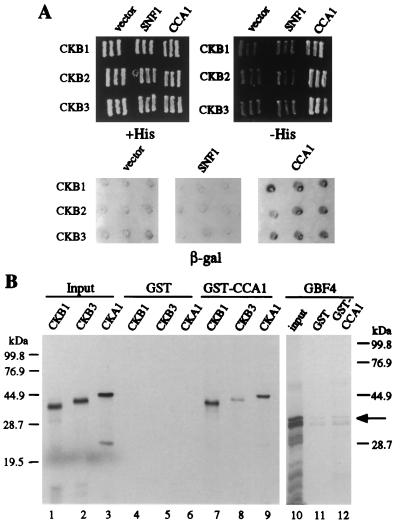
Although CKB1, CKB2, and CKB3 share a high degree of structural and functional homology, it might be that CKB3 is the only β-subunit that associates with CCA1 specifically. Therefore, we tested whether CKB1 and/or CKB2 also are able to interact with CCA1 in the yeast two-hybrid system and confirmed that the full length CKB3 could interact with CCA1 in the same way as the product of the original cDNA clone. Fig. 3A demonstrates that CKB1, CKB2, and CKB3 each can interact specifically with CCA1 in yeast.
Figure 3.
Interactions of CCA1 with CK2 subunits. (A) Interactions of CCA1 and CKB1, CKB2, and CKB3 in yeast. Each panel shows triplicate patches of yeast expressing GAL4-DB (Left), GAL4-DB-SNF1 (Center), or GAL4-DB-CCA1 (Right) transformed with GAL4-AD-CKB1 (Top), GAL4-AD-CKB2 (Middle), or GAL4-AD-CKB3 (Bottom). (Top, Left) +His, control plate containing histidine, (Top, Right) -His, plate lacking histidine (selective for the HIS3 reporter gene expression), (Bottom) β-gal, β-galactosidase assay performed on a filter. Dark color shows β-galactosidase activity accumulated after a 3-hr incubation with substrate. (B) Autoradiograph of SDS/PAGE analysis showing in vitro interactions among CKB1, CKB3, CKA1, and CCA1. GST-CCA1 or GST immobilized on glutathione–agarose beads was mixed with 35S-labeled CKB1, CKB3, CKA1, or GBF4. The amount of proteins bound to GST (lanes 4–6, 11) or GST-CCA1 (lanes 7–9, 12) is shown. Lanes 1–3 and 10 represent 5% of the 35S-labeled proteins used.
To further investigate the direct interaction of CCA1 with β-subunits of CK2, the ability of GST-CCA1 to bind to CK2 β-subunits in vitro also was tested. Fig. 3B shows that CKB1 and CKB2 bound to GST-CCA1 efficiently but not to GST alone (Fig. 3B, lanes 4 and 7 and additional data not shown). CKB3 also interacted with GST-CCA1 but apparently less efficiently than did CKB1 and CKB2 (Fig. 3B, lane 8). Similar analyses showed that CKA1 and CKA2, the two α-subunits of CK2 (12), also bound to GST-CCA1 (lane 9 and data not shown). While it is possible that CK2 β-subunits that could be present in the wheat germ extract might mediate the interaction of CCA1 with the CK2 α-subunit, our results suggest that CCA1 can interact with both CK2 α-subunits and β-subunits in vitro. As a negative control in these experiments, GBF4, a bZIP transcription factor (16), was used. GBF4 did not show a specific interaction with GST-CCA1 (Fig. 3B, lanes 11 and 12), confirming that the interaction of CCA1 with CK2 subunits is specific.
CK2 Can Stimulate Binding of CCA1 to the Lhcb1*3 Promoter in Vitro.
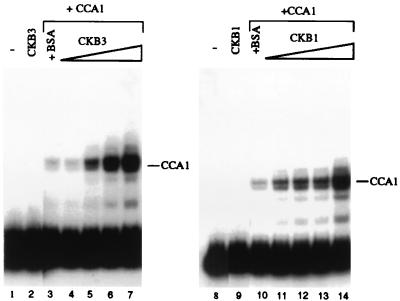
The possible biochemical consequences of the interaction of CK2 β-subunits with CCA1 were examined. First, we tested whether DNA binding activity of CCA1 was affected by CCA1 interaction with CK2 β-subunits. As shown in Fig. 4, binding of CCA1 to the A2 fragment was stimulated specifically by CKB3 at low concentrations of CCA1 (Fig. 4, lanes 3–7). CKB3 did not itself show any binding to the probe (Fig. 4, lane 2). CKB1 also enhanced DNA binding activity of CCA1 in the same way (Fig. 4, lanes 10–14).
Figure 4.
CK2 β-subunits enhance the binding of CCA1 to the Lhcb1*3 promoter. Autoradiographs of the EMSA are shown. The 32P-labeled A2 fragment was incubated with 0.5 ng of CCA1 in the presence of increasing amounts of His-tagged CKB3 (Left) or CKB1 (Right) (lanes: 4 and 11, 1 ng; 5 and 12, 2 ng; 6 and 13, 5 ng; 7 and 14, 10 ng). Lanes: 1 and 8, probe alone; 2 and 9, the A2 fragment with 50 ng of CKB3 and CKB1; and 3 and 10, the A2 fragment with 0.5 ng of CCA1 and 50 ng of BSA.
CK2 Can Phosphorylate CCA1 in Vitro.
A second approach to understanding the function of the CK2-CCA1 interaction was to determine whether recombinant CK2 can phosphorylate CCA1 in vitro. CCA1 has several putative sites for phosphorylation by CK2. We initially tested phosphorylation of CCA1 by CKA1, one of the α-subunits of CK2. Fig. 5A shows that a large amount of CKA1 (280 ng) phosphorylates GST-CCA1 but does not phosphorylate GST (Fig. 5A, lanes 1 and 3). It has been shown that CK2 β-subunits stimulate the catalytic activity of α-subunits toward most substrates (17–19). Fig. 5B shows that when a smaller amount (14 ng) of CKA1 was used, a strong stimulation of the CCA1 phosphorylation was observed by adding either CKB1 or CKB3 (Fig. 5B, lanes 2–4). Similar results were obtained for CKA2, the other α-subunit of CK2 (data not shown). Fig. 5B also shows that CCA1 could be phosphorylated in the presence of GTP as well as in the presence of ATP (Fig. 5B, lanes 5–7). These data confirm that the phosphorylation can be attributed to CK2 activity because CK2 is unique among protein kinases in that it can use both ATP and GTP as phosphodonors.
Figure 5.
Phosphorylation of CCA1 by CK2 in vitro. (A) Autoradiograph of SDS/PAGE analysis showing that CKA1 can phosphorylate CCA1. GST (lane 1) or GST-CCA1 (lanes 2 and 3) incubated with 280 ng of CKA1 (lanes 1 and 3) or without CKA1 (lane 2) in the presence of [γ-32P]ATP. (B) CK2 β-subunits enhance the phosphorylation of CCA1 by CKA1. GST-CCA1 (lanes 1–7) or GST alone (lanes 8 and 9) was incubated with 14 ng of CKA1 (lanes 2–9) or without CKA1 (lane 1) in the presence of [γ-32P]ATP (lanes 1–4, 8, and 9) or [γ-32P]GTP (lanes 5–7). Lanes: 2 and 5, CKA1 alone; 3, 6, and 8, with 35 ng of CKB1; and 4, 7, and 9, with 35 ng of CKB3. Arrows in both panels indicate the position of the full length GST-CCA1 protein. Other bands might be degradation products of GST-CCA1.
Phosphorylation by CK2 has been shown to affect the DNA binding activity of many transcription factors. Therefore, the possible effect of CK2 phosphorylation on the DNA binding activity of CCA1 was examined. When recombinant CCA1 was phosphorylated by CK2, no effect on its DNA binding activity was observed in the EMSA assay (data not shown).
Arabidopsis Plants Contain a CK2-Like Activity That Can Phosphorylate CCA1 in Vitro.
We next examined whether plants contain a CK2-like protein kinase activity that can phosphorylate CCA1 in vitro. Fig. 6 shows that GST-CCA1, but not GST alone, was phosphorylated by a kinase activity in Arabidopsis WCE in vitro (Fig. 6, lanes 1 and 2). Fig. 6 also shows that this kinase activity was able to use both ATP and GTP as phosphodonors (Fig. 6, lane 5). Furthermore, addition of 2,3-diphosphoglycerate, which is an inhibitor of CK2 (20), reduced the incorporation of ATP into GST-CCA1 by 63% (Fig. 6, lane 3). When this inhibitor was added to recombinant CK2, the phosphorylation of CCA1 was reduced by 77% (Fig. 6, lane 7). These results demonstrate that Arabidopsis plants contain a CK2-like activity that phosphorylates CCA1 in vitro and that this kinase activity is responsible for much of the phosphorylating activity on CCA1 in the extracts.
Figure 6.
Arabidopsis plants contain a CK2-like activity that phosphorylates CCA1 in vitro. GST (lanes 1 and 4) or GST-CCA1 (lanes 2, 3, 5–7) were incubated with 160 μg of WCE in the absence (lanes 1, 2, 4, and 5) or presence (lane 3) of 10 mM 2,3-diphosphoglycerate (D.G.), with recombinant CK2 (rCK2) in the absence (lane 6) or presence of 10 mM 2,3-diphosphoglycerate (lane 7) together with [γ-32P]ATP (lanes 1–3, 6, and 7) or [γ-32P]GTP (lanes 4 and 5). The arrow indicates the position of the full length GST-CCA1 protein.
Phosphorylation by CK2 Is Required for Formation of the DNA–Protein Complex Containing CCA1 in Plant Extracts.
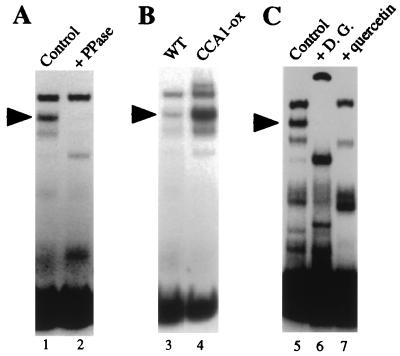
Fig. 7A shows that the DNA binding activity of the major CCA1-containing complex in plant extracts depends on phosphorylation. When the extracts were treated with λ protein phosphatase, formation of the complex was inhibited completely. The major DNA–protein complex containing CCA1 (Fig. 7A, marked with arrows) migrated more slowly than that formed with recombinant CCA1, suggesting that other proteins in the plant extracts are included in this complex, interacting with CCA1 and/or binding to the DNA. The A2 fragment used as a probe includes known binding sites for G-box and CAAT binding proteins (3). Fig. 7B shows that this complex is more abundant in extracts of plants expressing CCA1 under the control of a constitutive promoter (7). Additional evidence that the marked complex contains CCA1 includes the observations that this complex is absent in extracts prepared from CCA1-null mutant plants and that addition of anti-CCA1 antibodies inhibits the formation of this complex (R.M.G. and E.M.T., unpublished data). Fig. 7C demonstrates that the CK2-like phosphorylation activity in the extracts is important for this binding activity. Formation of the complex was abolished when CK2 inhibitors, either 2,3-diphosphoglycerate or quercetin, were added to the preincubation reactions. 2,3-diphosphoglycerate inhibits both CK1 and CK2, whereas inhibition by quercetin is specific to CK2 (20, 21). The components of the new bands that appear in the inhibitor-treated samples are not yet known, but these bands presumably represent a change in the composition of the CCA1-containing complex in the absence of CK2-mediated phosphorylation. Taken together, these results demonstrate that phosphorylation by CK2 is required in the plant extracts for formation of the major DNA–protein complex containing CCA1.
Figure 7.
CK2 phosphorylation is required for the formation of a DNA–protein complex containing CCA1. (A) Phosphatase treatment abolishes CCA1 binding. WCE were incubated in the absence (lane 1) or presence (lane 2) of λ protein phosphatase (200 units added to 20 μl) for 30 min at 30°C. (B) The CCA1-containing complex is more abundant in plants overexpressing CCA1 than in wild type. WCE from wild type (WT) and a CCA1-ox line (CCA1-ox) (7) grown in 12:12 photoperiods were used in the EMSA. (C) Inhibition of CK2 activity in plant extracts abolishes CCA1 binding. WCE were incubated with the indicated inhibitor for 45 min at 30°C. Lanes: 1, no inhibitors; 2, 5 mM 2,3-diphosphoglycerate (D.G.); and 3, 100 μM quercetin. Arrows indicate the position of the major DNA–protein complex containing CCA1.
DISCUSSION
The protein kinase CK2 is a Ser/Thr kinase that is expressed ubiquitously and highly conserved (17–19). CK2 consists of two catalytic (α) and two regulatory (β) subunits, which form an α2β2 heteromeric holoenzyme. Although most organisms have two genes encoding α-subunits and one gene encoding the β-subunit, two genes encoding β-subunits have been reported in S. cerevisiae and Arabidopsis (9, 13, 22). The CKB3 protein exhibits significant amino acid sequence identity with Arabidopsis CKB1 and CKB2. Two lines of evidence confirm that CKB3 does indeed function as a third CK2 β-subunit in Arabidopsis. First, CKB3 was able to compensate for the temperature-sensitive growth defect of an S. cerevisiae cka1-Δ1 cka2–8 mutation. Second, recombinant CKB3 was able to stimulate the catalytic activity of CKA1 when CCA1 was used as a substrate. CKB3 is the only reported example of a third CK2 β-subunit in any organism. Of interest, it had been suggested previously that there might be a third CK2 α-subunit in Arabidopsis (12). It is yet to be determined whether there are several forms of the holoenzyme with different subunit compositions or whether different subunits confer different substrate specificities and/or tissue specificities.
We have shown that CK2 β-subunits specifically interact with CCA1 both in yeast and in an in vitro interaction assay. We did not isolate clones for the two other CK2 β-subunits in the initial screen, but we have observed that the growth of yeast containing the constructs for these subunits is slower than that of cells expressing the GAL4-AD-CKB3 construct, and this may account for our failure to identify them along with CKB3.
We have found that recombinant His-tagged CK2 β-subunits stimulate binding of CCA1 to a fragment of the Lhcb1*3 gene. This effect is likely to be specific because OBP1, a DNA binding protein that stimulates interaction of OBF4 and OBF5 with ocs elements, did not affect binding of CCA1 to the A2 fragment (23). Also, both recombinant GST-CKB1 and GST-CKB3 stimulated DNA binding of CCA1, whereas GST alone had no effect (S.S. and E.M.T., unpublished data), demonstrating that CK2 β-subunits are responsible for the enhancement. The fact that the mobility of the complex was not affected suggests that the interaction of the proteins might be transient or unstable under the conditions for the EMSA. There have been other such reports of enhancement of DNA binding by a second protein without altering the mobility of the DNA–protein complex (23–26).
The fact that CK2 β-subunits associate with CCA1 and stimulate its binding to the Lhcb1*3 promoter suggests a different mechanism for regulation of CCA1–DNA binding activity other than phosphorylation. In fact, the α-subunit is not required for this stimulation, and CK2 β-subunits cannot themselves phosphorylate CCA1. It is possible that, in addition to being the regulatory subunit of CK2, the β-subunit might play other roles in the cell. Overexpression of the CK2 β-subunit in Schizosaccharomyces pombe causes multiple septation and inhibits cell growth and cytokinesis (27). These phenotypes appear to result from the production of free β-subunit rather than excess holoenzyme. In Xenopus oocytes, the β-subunit interacts with Mos, a germ cell-specific Ser/Thr kinase that is required for oocyte maturation, and this interaction negatively regulates Mos-mediated, mitogen-activated protein kinase activation, resulting in repression of oocyte maturation (28, 29). Recently, it also was shown that cyclin D, which is a regulatory component of complexes of cyclin with cyclin-dependent kinase, stimulates transcriptional activity of estrogen receptor independent of interaction with cyclin-dependent kinases (30). Therefore, it is intriguing to speculate that direct interaction of CK2 β-subunits with CCA1 stimulates binding of CCA1 to promoter sequences and can affect CCA1-mediated transcription.
We have demonstrated that CKA1 phosphorylates CCA1 in vitro and that both CKB1 and CKB3 stimulate this phosphorylation. Although CKA1CKB1 showed a higher activity of CCA1 phosphorylation than did CKA1CKB3, the possibility that this difference was caused by differing relative activities of CKB1 and CKB3 in their corresponding preparations cannot be excluded. We have demonstrated also that Arabidopsis plants contain a CK2-like protein kinase activity that can phosphorylate CCA1 in vitro and that this is a major kinase activity for CCA1 phosphorylation in the extracts. The identity of this kinase was confirmed in two ways. First, the kinase activity phosphorylated CCA1 in the presence of GTP as well as ATP, a unique characteristic that distinguishes CK2 from other Ser/Thr kinases. Second, addition of 2,3-diphosphoglycerate, an inhibitor of CK2, inhibited most of the CCA1 phosphorylating activity in the plant extracts.
Phosphorylation of transcription factors by CK2 has been reported to modulate their DNA binding activity, cellular localization, metabolism, and interaction with other proteins (19, 31–37). It was shown recently that the Lhcb1*1 RNA level in transgenic plants overexpressing CCA1 decreased steadily when plants were transferred from light–dark cycles into constant dark, even though CCA1 was expressed at a high level (7). We also have observed that the Lhcb1*1 RNA level in etiolated transgenic plants overexpressing CCA1 was as low as that in etiolated wild-type plants (Z.-Y.W. and E.M.T., unpublished data). These observations suggest that CCA1 activity is regulated by light through posttranslational modifications, one of which could be phosphorylation. In this regard, our finding that plant extracts contain a CK2-like activity that is required for formation of the major DNA–protein complex containing CCA1 is especially noteworthy. The CCA1-containing complex is likely to contain a protein or proteins in addition to CCA1 (see Results). Although CK2 phosphorylation of recombinant CCA1 did not affect its DNA binding activity in vitro, it is possible that, in the plants, the other proteins affect the relative binding affinities of the phosphorylated and nonphosphorylated forms of CCA1 for CCA1 binding sites. Alternatively, the phosphorylation state of CCA1 might be important for protein–protein interactions of CCA1 with other protein(s) in the complex. It is also possible that phosphorylation of other protein(s) by CK2 is essential for the CCA1 complex formation.
In summary, interaction of subunits of CK2 with CCA1 and phosphorylation of CCA1 by CK2 may modulate CCA1 activities that are required for phytochrome regulation of Lhcb1*3 gene expression and for circadian clock function. In light of the involvement of CCA1 in circadian rhythms (7), it is of particular interest that the clock gene affected in the double-time mutant of Drosophila, which is required for circadian rhythmicity, recently has been cloned and has been found to be closely related to human casein kinase Iɛ (38). Whereas the physiological significance of the CK2-CCA1 association remains to be elucidated, our findings should be important steps toward understanding the regulation of CCA1 function in Arabidopsis.
Acknowledgments
We thank Dr. J. Walker for the Arabidopsis cDNA library and plasmids and Drs. A. R. Cashmore, S. J. Elledge, C. V. C. Glover, T. Mizoguchi, N. Nakayama, Y. Shibagaki, K. Shinozaki, and F. Tamanoi for yeast strains and plasmids and for valuable suggestions. We are grateful to Drs. C. Lin and S. C. Weatherwax for comments on the manuscript. This work was supported by National Institutes of Health Grant R01-GM-23167.
ABBREVIATIONS
- CCA1
circadian clock-associated 1
- GAL4-DB
GAL4 DNA binding domain
- GAL4-AD
GAL4 transactivation domain
- GST
glutathione-S-transferase
- WCE
whole-cell extracts
- EMSAs
electrophoretic mobility-shift assays
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF068318).
References
- 1.Quail P H, Boylan M T, Parks B M, Short T W, Xu Y, Wagner D. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- 2.Tobin E M, Kehoe D M. Semin Cell Biol. 1994;5:335–346. doi: 10.1006/scel.1994.1040. [DOI] [PubMed] [Google Scholar]
- 3.Sun L, Doxsee R A, Harel E, Tobin E M. Plant Cell. 1993;5:109–121. doi: 10.1105/tpc.5.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenigsbuch D, Tobin E M. Plant Physiol. 1995;108:1023–1027. doi: 10.1104/pp.108.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z-Y, Kenigsbuch D, Sun L, Harel E, Ong M S, Tobin E M. Plant Cell. 1997;9:491–507. doi: 10.1105/tpc.9.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millar A J, Kay S A. BioEssays. 1997;19:209–214. doi: 10.1002/bies.950190306. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z-Y, Tobin E M. Cell. 1998;93:1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
- 8.Hanna D E, Rethinaswamy A, Glover C V C. J Biol Chem. 1995;43:25905–25914. doi: 10.1074/jbc.270.43.25905. [DOI] [PubMed] [Google Scholar]
- 9.Reed J C, Bidwai A P, Glover C V C. J Biol Chem. 1994;269:18192–18200. [PubMed] [Google Scholar]
- 10.Tanaka K, Nakafuku M, Tamanoi F, Kaziro Y, Matsumoto K, Toh-e A. Mol Cell Biol. 1990;10:4303–4313. doi: 10.1128/mcb.10.8.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 12.Mizoguchi T, Yamaguchi-Shinozaki K, Hayashida N, Kamada H, Shinozaki K. Plant Mol Biol. 1993;21:279–289. doi: 10.1007/BF00019944. [DOI] [PubMed] [Google Scholar]
- 13.Collinge M A, Walker J C. Plant Mol Biol. 1994;25:649–658. doi: 10.1007/BF00029603. [DOI] [PubMed] [Google Scholar]
- 14.Shibagaki Y, Holmes M L, Appa R S, Chow S A. Virology. 1997;230:1–10. doi: 10.1006/viro.1997.8466. [DOI] [PubMed] [Google Scholar]
- 15.Lütcke H A, Chow K C, Mickel F S, Moss K A, Kern H F, Scheele G A. EMBO J. 1987;6:43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menkens A E, Cashmore A R. Proc Natl Acad Sci USA. 1994;91:2522–2526. doi: 10.1073/pnas.91.7.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinna L A. Biochim Biophys Acta. 1990;1054:267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- 18.Issinger O-G. Pharmacol Ther. 1993;59:1–30. doi: 10.1016/0163-7258(93)90039-g. [DOI] [PubMed] [Google Scholar]
- 19.Allende J E, Allende C C. FASEB J. 1995;9:313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 20.Gonzatti-Haces M I, Traugh J A. J Biol Chem. 1982;257:6642–6645. [PubMed] [Google Scholar]
- 21.Cochet C, Feige J J, Pirollet F, Keramidas M, Chambaz E M. Biochem Pharmacol. 1982;31:1357–1361. doi: 10.1016/0006-2952(82)90028-4. [DOI] [PubMed] [Google Scholar]
- 22.Bidwai A P, Reed J C, Glover C V C. J Biol Chem. 1995;270:10395–10404. doi: 10.1074/jbc.270.18.10395. [DOI] [PubMed] [Google Scholar]
- 23.Zhang, B., Chen, W., Foley, R. C., Büttner, M. & Singh, K. B. Plant Cell 7, 2241–2252. [DOI] [PMC free article] [PubMed]
- 24.Wagner S, Green M R. Science. 1993;262:395–399. doi: 10.1126/science.8211160. [DOI] [PubMed] [Google Scholar]
- 25.Leger H, Sock E, Renner K, Grummt F, Wegner M. Mol Cell Biol. 1995;15:3738–3747. doi: 10.1128/mcb.15.7.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Currie R A. J Biol Chem. 1997;272:30880–30888. doi: 10.1074/jbc.272.49.30880. [DOI] [PubMed] [Google Scholar]
- 27.Roussou I, Draetta G. Mol Cell Biol. 1994;14:576–586. doi: 10.1128/mcb.14.1.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M, Li D, Krebs E G, Cooper J A. Mol Cell Biol. 1997;17:1904–1912. doi: 10.1128/mcb.17.4.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen M, Cooper J A. Proc Natl Acad Sci USA. 1997;94:9136–9140. doi: 10.1073/pnas.94.17.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zwijsen R M, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides R J A M. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
- 31.Datta N, Cashmore A R. Plant Cell. 1989;1:1069–1077. doi: 10.1105/tpc.1.11.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klimczak L J, Schindler U, Cashmore A R. Plant Cell. 1992;4:87–98. doi: 10.1105/tpc.4.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molkentin J D, Li L, Olson E N. J Biol Chem. 1996;271:17199–17204. doi: 10.1074/jbc.271.29.17199. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong S A, Barry D A, Leggett R W, Mueller C R. J Biol Chem. 1997;272:13489–13495. doi: 10.1074/jbc.272.21.13489. [DOI] [PubMed] [Google Scholar]
- 35.Jans D A, Jans J. Oncogene. 1994;9:2961–2968. [PubMed] [Google Scholar]
- 36.McElhinny J A, Trushin S A, Bren G D, Chester N, Paya C V. Mol Cell Biol. 1996;16:899–906. doi: 10.1128/mcb.16.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Reilly D, Hanscombe O, O’Hare P. EMBO J. 1997;16:2420–2430. doi: 10.1093/emboj/16.9.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kloss B, Price J L, Saez L, Blau J, Rothenfluh A, Wesley C S, Young M W. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]