Abstract
Exagerated immune responses, such as those implicated in severe inflammatory reactions, are costly to the metabolism. Inflammation and pro-inflammatory mediators negatively affect production in the food animal industry by reducing growth, feed intake, reproduction, milk production, and metabolic health. An ever-increasing number of findings have established that antibiotics, macrolides in particular, may generate anti-inflammatory effects, including the modulation of pro-inflammatory cytokines and the alteration of neutrophil function. The effects are time- and dose-dependent, and the mechanisms responsible for these phenomena remain incompletely understood. Recent studies, mostly using the veterinary macrolide tilmicosin, may have shed new light on the mode of action of some macrolides and their anti-inflammatory properties. Indeed, research findings demonstrate that this compound, amongst others, induces neutrophil apoptosis, which in turn provides anti-inflammatory benefits. Studies using tilmicosin model systems in vitro and in vivo demonstrate that this antibiotic has potent immunomodulatory effects that may explain why at least parts of its clinical benefits are independent of anti-microbial effects. More research is needed, using this antibiotic and others that may have similar properties, to clarify the biological mechanisms responsible for antibiotic-induced neutrophil apoptosis, and how this, in turn, may provide enhanced clinical benefits. Such studies may help establish a rational basis for the development of novel, efficacious, anti-microbial compounds that generate anti-inflammatory properties in addition to their antibacterial effects.
Résumé
Les réponses immunes excessives, telles que celles impliquées lors de réactions inflammatoires sévères, sont coûteuses pour le métabolisme. Les médiateurs inflammatoires et pro-inflammatoires affectent négativement la production dans l’industrie animale en réduisant la croissance, la prise de nourriture, la reproduction, la production laitière et la santé métabolique. De plus en plus de découvertes ont établi que les antibiotiques, en particulier les macrolides, peuvent avoir des effets anti-inflammatoires, incluant la modulation de cytokine pro-inflammatoiress et une altération de la fonction des neutrophiles. Les effets sont temps- et dose-dépendant, et le mécanisme responsable de ces phénomènes demeurent non-complètement élucidés. Des études récentes, utilisant principalement le macrolide vétérinaire tilmicosin, pourrait avoir fourni de nouvelles informations sur le mode d’action des macrolides et leurs propriétés anti-inflammatoires. En effet, des trouvailles récentes ont démontré que ce produit avait, entre autres, induit l’apoptose chez les neutrophiles, ce qui avait des effets anti-inflammatoires bénéfiques. Des études utilisant des modèles in vitro et in vivo ont démontré que cet antibiotique à des effets immuno-modulateurs marqués qui pourraient expliquer pourquoi une partie de ses effets cliniques bénéfiques sont indépendants des effets antimicrobiens. En utilisant cet antibiotique et d’autres qui ont des propriétés similaires, plus de recherche sont nécessaires afin de clarifier les mécanismes biologiques responsables de l’apoptose des neutrophiles induite par l’antibiotique, et comment ce fait augmente les effets cliniques bénéfiques. De telles études pourraient aider à fournir une base rationnelle pour le développement de composés anti-microbiens nouveaux et efficaces qui ont des propriétés anti-inflammatoires en plus de leurs effets antibactériens.
(Traduit par Docteur Serge Messier)
Introduction
Infection-induced inflammatory reactions directly and indirectly affect growth, feed intake, milk production, reproduction, and metabolic health. Therapeutic compounds that generate both antibacterial as well as anti-inflammatory effects are therefore likely to be most effective at treating bacteria-induced inflammatory diseases. Over the past 2 decades, there has been increasing interest in the potential anti-inflammatory effects of macrolides, as well as those of azalides, in which a methyl-substituted nitrogen atom is added into the lactone ring. During infection, both bacterial virulence factors as well as the host inflammatory response, which implicates local infiltration by polymorphonuclear leukocytes (neutrophils), are responsible for pathophysiology, tissue destruction and, in some cases, death. Neutrophils are bacterial killers that migrate to the site of infection and therefore constitute a critical line of host defense. Uncontrolled infiltration and necrosis of neutrophils at the site of inflammation, however, leads to the release, by these cells, of large amounts of toxic compounds that target the invading bacteria, but concurrently contribute to the self-perpetuating inflammatory injury. In contrast, neutrophil death by apoptosis helps resolve inflammation and avoid immunopathology. Recent findings indicate that some antibiotics, such as the 16-membered macrolide tilmicosin, may generate anti-inflammatory benefits by modulating the production of pro-inflammatory mediators, and by inducing neutrophil apoptosis. The aim of this review is to discuss how antibiotics with such immunomodulatory and anti-inflammatory effects may improve animal health and production. Using tilmicosin and the treatment of respiratory disease as an example, this article also critically reviews how induction of neutrophil apoptosis at the site of inflammation may confer anti-inflammatory properties to an antibiotic.
Immunity and metabolic costs
The interplay between the immune, endocrine, and nervous systems is at the core of the homeostatic network (1). Immune output influences overall physiological balance and metabolism. Conversely, neural pathways mediate immune reactivity in health and disease. For example, stress may be responsible for immunosuppression in some instances, or promote immune hyper-reactivity such as in intestinal anaphylaxis in others (2–4). It is also well known that the immune system is compromised in stressed cattle, which contributes to the high incidence of respiratory disease in feedlot cattle during their first 45 days on feed (5). The reality of these tight interactive processes at least in part explains why fighting infection may be associated with significant costs to metabolic rates, protein synthesis, and growth. Animals rely on energy and proteins to mount and maintain immune functions, whether cellular or humoral (6–8). These resources are limited, particularly in the young and growing animal, forcing trade-offs to occur between immune, metabolic, and physiological processes. While some findings indicate that basal immune responsiveness is not necessarily detrimental to nutrient-dependent body demands (9,10), excess immune reactions implicated in the immunopathololgy of various inflammatory disorders impair metabolic performance. For example, excessive immune responses occur at the expense of antioxidant nutrients, trace minerals, and vitamins, similarly to what occurs in stress (11). In other words, an exagerated investment in immune reactions negatively affects growth and development.
A wide variety of molecules regulate the critical communications between cellular and humoral immune responses. As these may be proteins or lipids (cytokines or eicosanoids for example), these mediators also require an adequate balance between dietary input and immunological synthesis. Dietary fats for example may alter the composition of membrane phospholipids, which are the precursors of eicosanoid synthesis. Indeed, bioactive eicosanoids are released upon cleavage of membrane phospholipids by the enzyme phospholipase A2, an important factor in diseases that involve infection and inflammation (12). The inflammatory response may be altered by dietary fats, which can cause Th1–Th2 T helper lymphocyte switching (13). These changes are mediated by effects on the various cytokines, prostaglandins, leukotrienes, and other eicosanoids, that are released to coordinate inflammatory reactions (13). Indeed, fish oil supplementation has been found to attenuate the production of IL-1β, prostaglandin E2, and cortisol, in pigs challenged with Escherichia coli lipopolysaccharide (14). Conversely, the collateral damage due to exaggerated inflammation primarily driven by IL-1, and TNFα cytokines, and by the eicosanoid leukotriene B4, may cause decreased food intake, nutrient loss, and reduced weight gain (15). The reduction in growth caused by excessive immune reactions is often greater than can be explained by decreases in feed intake alone (16). Indeed, there is evidence to suggest that cytokines like IGF-1 or IL-1β, may directly alter growth hormone receptor signaling (17). The current hypothesis for the decreased muscle mass gain during intense immune reactions proposes that because of reduced amino acids from decreased feed intake, amino acids shunt the skeletal muscle to go to the liver and other sites, to support the synthesis of pro-inflammatory mediators (18–20). Moreover, some inflammatory mediators, such as IL-1 and TNFα, have direct anorectic properties (18). As illustrated in Table I, severe inflammatory reactions may therefore negatively affect growth, reproduction, milk production, and general metabolic health (21). These observations stem from scientific evidence mostly related to mastitis and bovine respiratory disease (BRD). During BRD caused by Mannheimia (Pasteurella) haemolytica, production of acute phase proteins, antibodies, and inflammatory cells was found to decrease dry matter intake, which in turn reduces nitrogen balance and causes metabolic stress (22). These events, at least in part, contribute to the decreased performance and carcass quality of animals that had a BRD event (22). Thus, the complexities associated with animal production, where feed conversion and carcass quality are paramount, also depend on optimally managing immune processes. This becomes particularly crucial in cases of inflammatory diseases, and in conditions of high intensity housing, where disease transmission and stress are common. The most logical approach to minimize the detrimental effects of immune hyperactivity on metabolism and production is to minimize the incidence and severity of infection and inflammation. Therefore, it is likely that antibiotics with potent anti-inflammatory properties will optimize our ability to support maximal growth and efficiency in meat- and milk-producing animals.
Table I.
Examples of metabolic costs due to inflammation. Inflammatory mediators may inhibit growth directly and indirectly
| Physiological parameter affected | Mediator implicated | References |
|---|---|---|
| Growth | TNFα, IL-1β, IGF-1, IL-6, cortisol | 16–22,90,94 |
| Feed intake | TNFα, IL-1β, IGF-1, cortisol | 16–18,21,22 |
| Reproduction | cortisol, PGF-2alpha | 21,91,92 |
| Milk production | unclear | 21,93,94 |
| Periparturient metabolic health | decreased plasma Ca++ | 21 |
Infection, neutrophil death, and inflammation
Alveolar macrophages represent the first line of innate immune defense in the lung. Upon inhalation of foreign particles, including infectious bacteria, macrophages initiate phagocytic elimination of these materials, and, in the process, release chemoattractants for neutrophils to migrate to the site. This migration is fast and extensive, as illustrated in the M. haemolytica-infected lung, where neutrophils may represent over 90% of the alveolar cell population very early post-infection (23). While bacterial leukotoxin evidently affects these cells, at least part of these pro-inflammatory effects is mediated by M. haemolytica lipopolysacharide, a potent inducer of IL-1β and TNFα in bovine pasteurellosis (24). This characteristic, severe, inflammatory reaction can be readily observed in bronchoalveolar lavages collected from infected cattle (Figure 1). In homeostatic conditions, migration of neutrophils to the inflamed site serves to protect the host from disease-causing microbes or other foreign materials. However, excessive and sustained recruitment of these cells to the lung leads to extensive inflammatory damage (Figure 1), pulmonary failure, and death during BRD. Similar pathophysiological processes can be observed in all mucosal surfaces, including in the mammary gland during mastitis (25,26). Indeed, infiltration by neutrophils is at the core of tissue inflammation. The mode of clearance and death of these cells from the site dictates how inflammation will be resolved. Neutrophil death may occur in two ways: via apoptosis or necrosis. When neutrophils at an inflammatory site die via necrosis, the cells swell and burst, spilling pathogenic compounds such as oxygen radicals, proteolytic enzymes, and cationic proteins into the surrounding tissues (27,28). In turn, this amplifies local inflammatory injury. In contrast to necrosis, neutrophil apoptosis is a key mechanism for the nonphlogistic removal of extravasated neutrophils, and contributes to the resolution of inflammation (29,30). In the M. haemolytica- or Actinobacillus pleuropneumoniae — infected lung, the self-regulatory control of neutrophil infiltration and apoptosis is overwhelmed and pathogenic accumulation of viable and necrotic neutrophils in pulmonary tissue ensues (23,31). This results in persistent inflammation and severe tissue injury, thereby causing the often fatal fibrinous pneumonia seen in cattle or swine infected with M. haemolytica or A. pleuropneumoniae. As discussed above, inflammation is also responsible for the detrimental effects on growth and metabolism in animals with BRD. Therefore, a better understanding of this immunopathological cascade will help establish a rational basis for developing more effective pharmaceutical interventions that control both the microbial invasion as well as the host inflammatory reaction.
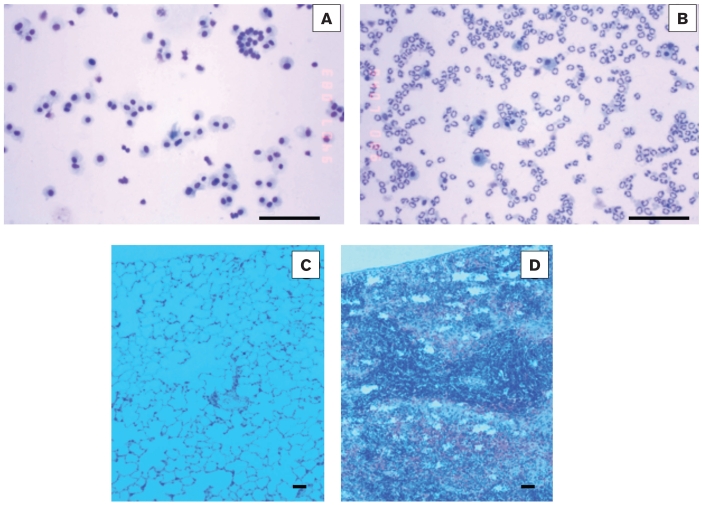
Figure 1.
Light micrographs from cytospin slides of bronchoalveolar lavages (A, B) or from histological preparations (eosin and hematoxylin; C,D) from calves 24 h after intratracheal inoculation with sterile endotoxin-free saline (controls, A, C), or with 2 × 108 live M. haemolytica in saline (infected, B, D). Infection induces a rapid migration of neutrophils to the bronchoalveolar space (B). In homeostatic conditions, neutrophils serve to protect the lung against the invading bacteria; however, when unchecked, this accumulation of neutrophils is responsible for severe inflammatory injury. In lesional areas (D), inflammation during this infection produces classical pathological signs of bovine respiratory disease (BRD), including alveolar obstruction by inflammatory infiltrates and fibrin deposition. Bars = 100 μm.
Resolution of inflammation: The role of neutrophil apoptosis
Apoptosis in neutrophils, as well as in other cell types, is associated with characteristic morphological changes (29,32). These include cell shrinking and membrane blebbing, vacuolation of the cytoplasm, nuclear membrane delamination, and chromatin condensation. In contrast to necrosis, the cell retains the integrity of its membrane and organelles. Eventually, nuclear DNA breaks down to form mono/oligo-nucleosomes, and the cell tears itself apart in membrane-bound apoptotic bodies, which remain sealed from the environment. Throughout this process, again in contrast to necrosis, the cell does not spill its contents into the extracellular milieu. These morphological characteristics are recognized as classical markers of apoptosis. In addition, a number of biochemical events accompany apoptotic death. Perhaps one of the best known early biochemical change during apoptosis is the loss of membrane phospholipid asymmetry, whereby phosphatidylserine translocates onto the outer portion of the membrane bi-layer (33). Other biochemical changes include the loss of sialic acid residues on cell membrane components, and the decreased expression of the glycosylphosphatidylinositol-linked protein CD16 (FcϒRIII) (34,35). Ultimately, apoptotic cells and fragments are removed by cells such as macrophages (Figure 2) and, in some instances, other “nonprofessional” phagocytes, including neighboring epithelial cells (29,36). This phagocytic elimination is facilitated by receptors on the macrophages which recognize targets that are newly expressed on apoptotic target cells (37–43). For example, macrophages have phosphatidylserine receptors that allow the selective elimination of apoptotic cells (29,36); hence, apoptotic neutrophils are cleared before they are given the opportunity to undergo secondary necrosis.
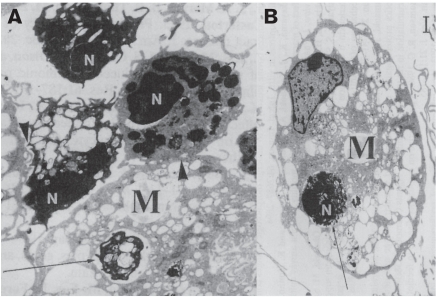
Figure 2.
Transmission electron micrographs (A and B) of bovine neutrophils (N) exposed for 2 h to tilmicosin (0.5 μg/mL), and co-incubated for 2 h with bovine monocyte-derived esterase-positive macrophages (M). Neutrophils exposed to the antibiotic exhibit characteristic signs of apoptosis, including nuclear membrane delamination, chromatin condensation, and cytoplasmic vaculation, while keeping their plasma membrane and cytoplasmic organelles intact. Exposure to tilmicosin significantly enhanced phagocytic uptake of apoptotic neutrophils by macrophages, as illustrated by tight membrane contacts (arrowhead) and full phagocytic inclusion of neutrophils in advanced stages of apoptosis withtin the macrophages (arrows). Bar = 1 μm.
(Modified from reference 83).
Most significantly, the phagocytic elimination of apoptotic cells does not induce the release of pro-inflammatory compounds otherwise associated with macrophage phagocytosis, and instead triggers the production of anti-inflammatory mediators. For example, this phagocytic clearance process is not associated with the release of pro-inflammatory IL-1β, TNFα, IL-8, Thromboxane B2, or Monocyte Chemoattractant Protein-1 (44–47). Moreover, uptake of apoptotic cells triggers the production of anti-inflammatory mediators such as TGFβ, PAF and PGE2 by the macrophages (44,46). Results from recent studies indicate that these signals could be overcome by proteases, including neutrophil elastase, which are released during lysis of necrotic cells (48). Together, these findings have established that the clearance mechanism of apoptotic neutrophils by phagocytes represents a central feature of the resolution of inflammation, and that the final outcome of an inflammatory reaction will be dictated by which cell elimination predominates (apoptosis or necrosis). Of course, other mechanisms actively regulate the resolution of inflammation, including the production of various mediators, such as the newly discovered Resolvin E1 (49–52). Regardless, anti-inflammatory mechanisms and microenvironmental signals that promote apoptosis in neutrophils and their clearance by phagocytes offer promising research grounds from which to develop new therapeutics (49,52–54). For example, it was recently suggested that cyclin-dependent kinase inhibitors could be used to enhance the resolution of inflammation by inducing neutrophil apoptosis, which was proposed as a novel therapeutic strategy for inflammatory diseases (55).
Immunomodulatory effects of macrolides
Traditional knowledge had based the efficacy of a given antibiotic on its direct anti-microbial effects. However, an ever-increasing body of research evidence suggests that there may be another central component to the mode of action of some antibiotics, that is, their capability to generate anti-inflammatory effects. The anti-inflammatory potential of macrolides has been the topic of a number of recent reviews (56–61). Macrolides exhibit a broad spectrum of antibacterial activity against gram-negative and gram-positive (Streptococcus pneumoniae) pathogens. This combines with their good tissue penetration, including significant uptake into neutrophils, giving macrolides excellent pharmacodynamic properties. Macrolides also have a broad spectrum of non-antibiotic properties, including motilin receptor stimulation (62), anticancer activity (63,64), and anti-angiogenesis effects (64). Moreover, it is now well-established that macrolides modulate host immune cell function, and tissue inflammation. The numerous immunomodulatory effects that have been described include the reduced accumulation of pro-inflammatory mediators and the modulation of neutrophil function and apoptosis (Table II). Macrolides such as erythromycin and clarithromycin have also been shown to modify mucosal function, for example by directly reducing mucus secretion and/or decreasing lipopolysaccharide-stimulated goblet cell secretion (65,66). Finally, some macrolides have been found to modulate vaccine-induced humoral immune responses (67).
Table II.
Examples illustrating the anti-inflammatory effects of macrolides
| Antibiotic | Cellular parameters | References |
|---|---|---|
| Effects on pro-inflammatory mediators | ||
| • erythromycin, clarithromycin, roxithromycin | Reduced gene expression and production of ICAM-1 | 60,100,101 |
| • erythromycin, roxithromycin, clarithromycin, azithromycin | Reduced production of IL-6, IL-8, IL-1β, TNFα | 60,68,93,102,103,104 |
| • erythromycin | Reduction of epithelial-cell derived neutrophil attractant 78 (ENA-78) | 104 |
| • erythromycin, clarithromycin | Suppression of endothelin-1 mRNA and production | 40,94 |
| • erythromycin, roxithromycin | Suppression of GMCSF production | 102 |
| Effects on neutrophil function | ||
| • erythromycin, roxithromycin, azithromycin | Inhibition of neutrophil chemotaxis | 60,105 |
| • erythromycin | Inhibition of neutrophil elastolytic activity | 105 |
| • erythromycin, roxithromycin, azithromycin, dirithromycin | Inhibition of neutrophil oxidative burst (at high concentrations)a | 93,94,105 |
| • erythromycin | Inhibition of β2-integrin expression (CD11b/CD18) | 93 |
| • roxithromycin | Inhibition of neutrophil adhesion | 60 |
| Induction of neutrophil apoptosis | ||
| tilmicosin, erythromycin, clarithromycin, azithromycin, tulathromycin | 69,70,71,74,82,83,84 | |
Clarithromycin induces apoptosis in cancer cells (62,63,68). Other findings suggest that erythromycin and roxithromycin activate neutrophil apoptosis in vitro (69,70). A number of studies, in vitro and in vivo, have established that tilmicosin promotes neutrophil apoptotic clearance (see the following text). Recent observations indicate that tulathromycin also may induce apoptosis in bovine neutrophils in vitro (71). The possibility that these phenomena may at least in part account for the anti-inflammatory effects of these macrolides is intriguing. As is the case for tilmicosin (72), the human 15-ring azalide azithromycin has a high affinity for uptake in neutrophils (73). Azithromycin also induces apoptosis in human circulating neutrophils, in experimental settings where penicillin, erythromycin, or dexamethasone had no pro-apoptotic effects (74). Intriguingly, Streptococcus pneumoniae seems to abolish the pro-apoptotic effects of azithromycin (74). Future research will determine whether this effect may contribute to the difficulties in treating such infections, which would offer further support to the hypothesis that antibiotic-induced neutrophil apoptosis offers significant clinical benefits. Indeed, other findings have suggested that macrolides known to induce neutrophil apotptosis may significantly reduce the inflammatory injury associated with Haemophilus influenzae infections of the lower respiratory tract via unexplained mechanisms (75). However, the physiological significance of antibiotic-induced neutrophil apoptosis remains incompletely understood, and little published clinical evidence is available to support that this effect occurs during treatment in vivo. Much remains to be learned about the mechanisms responsible for this property. Also, the dose-dependent expression of some of these anti-inflammatory effects may at times confuse their clinical significance. To date, the most compelling body of evidence suggesting that antibiotic-induced neutrophil apoptosis confers anti-inflammatory benefits to an antibiotic comes from research carried out on tilmicosin.
Neutrophil apoptosis and anti-inflammatory benefits: Effects of tilmicosin
The veterinary drug tilmicosin is a 16-ring macrolide antibiotic with antimicrobial activity against gram-positive and gram-negative bacteria [including Pasteurella sp. (Mannheimia sp.), Actinobacillus sp., and Mycoplasma sp.]. Tilmicosin is used as a subcutaneous formulation to treat respiratory infections in cattle, or as a feed formulation to control bacterial pneumonia in swine (76,77). The treatment success of tilmicosin was initially attributed to its pharmacodynamic concentration in appropriate tissues and low inhibitory concentrations (78–81). Tilmicosin has a very high affinity for uptake within neutrophils, in which intracellular concentrations 40× greater than those achievable in the serum have been reported (72). In addition, there is now increasing evidence to suggest that tilmicosin also generates anti-inflammatory effects, via mechanisms that have yet to be fully characterized (79,82–84).
Studies in vivo demonstrate that tilmicosin induces apoptosis in pulmonary neutrophils of calves experimentally infected with M. haemolytica, and that this effect is associated with a reduction of pro-inflammatory Leukotriene B4 (LTB4) in bronchoalveolar lavages (82). Indeed, significantly higher levels of apoptosis are detected in pure bronchoalveolar neutrophil populations of tilmicosin-treated calves versus untreated infected animals 3 h after the inoculation of live bacteria. In the untreated infected lungs, pro-inflammatory LTB4 accumulated as the inflammatory reaction worsened, while tilmicosin treatment was associated with an inhibition of LTB4 synthesis (82). Subsequent studies using circulating bovine neutrophils in vitro showed that this effect could be observed regardless of the presence or absence of live bacteria, clearly demonstrating that tilmicosin had direct pro-apoptotic properties, independently of variable bacterial numbers in the lungs (83,84). This effect is also associated with direct inhibition of LTB4 in bovine neutrophils in vitro (84). Consistent with this immuno-modulating effect, recent data indicate that tilmicosin reduces the synthesis, by activated bovine alveolar macrophages in vitro, of another potent lipid mediator, prostaglandin E2 (85). In these experimental settings, other antibiotics, including penicillin, ceftiofur, and oxytetracycline, or the corticosteroid dexamethasone, did not induce neutrophil apoptosis at similar concentrations (83). Moreover, exposure of bovine neutrophils to tilmicosin enhances their subsequent phagocytic uptake by monocyte-derived macrophages (Figure 2), which bind to translocated phosphatidylserine on apoptotic neutrophils (83), further supporting the view that this effect may generate anti-inflammatory benefits (44–47,55). A recent field study demonstrated that treatment with tilmicosin, but not with ceftiofur, significantly increases the level of apoptosis in bronchoalveolar neutrophils of calves with subacute or chronic airway diseases (86). In contrast, another study was unable to detect significant levels of apoptosis in circulating neutrophils of tilmicosin-treated calves experimentally infected with M. haemolytica (87). However, the significance of these results remains difficult to interpret as apoptosis was only measured in circulating cells rather than in bronchoalveolar cells in that study (87). Indeed, the authors also failed to detect apoptosis in circulating neutrophils of animals given camptothecin, a known pro-apoptotic agent (87). Additional findings further confirmed the pro-apoptotic and anti-inflammatory properties of tilmicosin in pigs infected with Actinobacillus pleuro-pneumoniae (88). In summary, the induction of neutrophil apoptosis by tilmicosin has been observed in purified bovine cells as well as in live infected calves and in infected pigs, in the presence or absence of live bacteria, and the effect is at least in part drug-specific. Importantly, these effects occur without altering the antimicrobial properties of neutrophils, including cell chemotaxis, chemokinesis, oxidative burst, and phagocytic activity (82,88). Taken together, these observations indicate that tilmicosin directly induces apoptosis in bovine neutrophils, and that this effect is associated with anti-inflammatory benefits in the infected lung via mechanisms that have not yet been fully elucidated (Figure 3). Moreover, tilmicosin directly reduces gene expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in macrophages activated by bacterial lipopolysaccharide (89). Cyclooxygenase-2 activation is a powerful mediator of inflammation (12). Adding to these direct anti-inflammatory effects, findings from the same study also found that tilmicosin significantly decreased the production by these macro-phages of pro-inflammatory eicosanoids and cytokines, 6-keto-PGF1α, PGE2, TNFα, IL-1β, and IL-6, respectively (89). Conversely, tilmicosin increased the synthesis of macrophage IL-10, a cytokine with well-established anti-inflammatory properties (89). Clearly, additional evidence is needed to unequivocally distinguish what components of the antibiotic’s anti-inflammatory effects are directly due to the induction of neutrophil apoptosis, and which components reflect the anti-microbial properties of the drug. Studies using models of inflammation devoid of a microbial stimulus will help answer this question. Finally, recent research found that tilmicosin’s immunomodulatry capacity may positively interact with dietary energy intake in cattle (90). Therefore, studies using macrolides such as tilmicosin may offer powerful model systems to investigate the mechanisms whereby promotion of neutrophil apoptosis and other processes may confer anti-inflammatory benefits to an antibiotic.
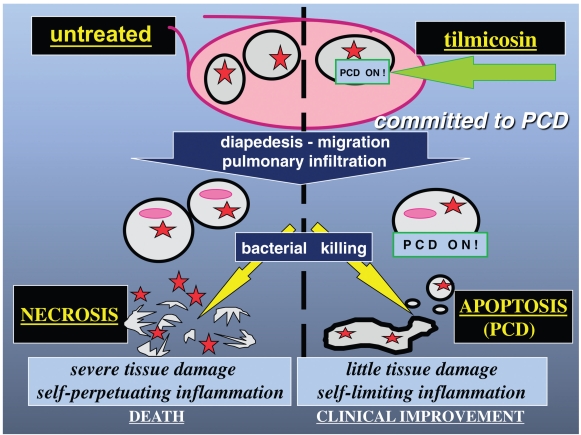
Figure 3.
Schematic illustration demonstrating how tilmicosin, by promoting neutrophil apoptosis, also called programmed cell death (PCD), may generate clinical benefits. By inducing neutrophil apoptosis, antibiotics such as tilmicosin prevent severe tissue damage secondary to leukocyte necrosis. Induction of neutrophil apoptosis confers anti-inflammatory properties to the antibiotic, without interfering with cell diapedesis, migration, and pulmonary infiltration.
Conclusion
Ample evidence demonstrates that infection-induced inflammatory reactions directly and indirectly affect growth, feed intake, milk production, reproduction and metabolic health. Therefore, compounds that generate both antibacterial as well as anti-inflammatory effects are likely to be most effective at treating bacteria-induced inflammatory diseases. The role of neutrophil apoptosis in the final outcome of an inflammatory reaction is the topic of ardent research. It has been well established that neutrophil apoptosis is an injury-limiting disposal mechanism which, during severe inflammation, protects tissues against the potentially harmful contents of necrotic neutrophils. These effects are time and dose-dependent. For example, studies have found that macrolides may induce apoptosis in neutrophils without affecting their antimicrobial properties, including oxidative burst, pulmonary infiltration rates, and phagocytic activity (74,82,91,92). Other studies, mostly carried out in vitro and which used higher concentrations and/or longer experimental exposure times to the macrolides, indicate that these same parameters of neutrophil function may be altered upon treatment (75,93,94). Regardless, macrolides are well known for their immuno-modulating properties in addition to their antimicrobial effects. A number of reports suggest that these drugs inhibit pro-inflammatory cytokine production. Recent observations indicate that clinical concentrations of tilmicosin and other macrolides may induce neutrophil apoptosis, and that this effect exhibits at least some degree of drug specificity. Extensive studies in vitro and in vivo, using tilmicosin as a model, have demonstrated that antibiotic-induced neutrophil apoptosis provides anti-inflammatory benefits. We postulate that macrolide/azalide-induced neutrophil apoptosis is responsible, at least in part, for their anti-inflammatory benefits, and that this phenomenon contributes to their clinical efficacy. It is too early to speculate on the molecular events responsible for the pro-apoptotic effects of macrolides, but one area of interest may be the characterization of how the antibiotics may modulate NF KappaB, a central transcription factor during inflammation, and one that was previously found to modulate programmed cell death. Elucidation of specific mechanisms whereby these phenomena occur may help design novel anti-infective compounds. Recent observations indicate that tilmicosin may offer a powerful model system to study these mechanisms at the cellular level as well as in their true clinical setting.
Acknowledgments
Parts of the experimental findings discussed in this chapter have been generated with the financial support of the Natural Sciences and Engineering Research Council of Canada, the Alberta Agricultural Research Institute, the Margaret Gunn Endowment for Animal Health Research, and Provel Division Eli Lilly Canada Inc.
References
- 1.Husband AJ. The immune system and integrated homeostasis. Immunol Cell Biol. 1995;73:377–382. doi: 10.1038/icb.1995.58. [DOI] [PubMed] [Google Scholar]
- 2.Kenison DC, Elsasser TH, Fayer R. Tumor necrosis factor as a potential mediator of acute metabolic and hormonal responses to endotoxemia in calves. Am J Vet Res. 1991;52:1320–1326. [PubMed] [Google Scholar]
- 3.Axelrod J, Reisine TD. Stress hormones: Their interactions and regulation. Science. 1984;224:452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- 4.Buret AG. How stress induces intestinal hypersensitivity. Am J Pathol. 2006;168:3–5. doi: 10.2353/ajpath.2006.050958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth JA, Perino LJ. Immunology and prevention of infection in feedlot cattle. Vet Clin N Am Food Anim Pract. 1998;14:233–256. doi: 10.1016/S0749-0720(15)30252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fair JM, Hansen ES, Ricklefs RE. Growth development stability and immune response in juvenile Japanese quails. Proc R Soc Lond B. 1999;266:1735–1742. doi: 10.1098/rspb.1999.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen SA, Hasselquist D, Folstad I, Eridstad KE. Costs of immunity: Immune responsiveness reduces survival of a vertebrate. Proc R Soc Lond B Biol Sci. 2004;271:925–930. doi: 10.1098/rspb.2004.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norris K, Evans MR. Ecological immunology: Life history trade-offs and immune defense in birds. Behav Ecol. 2000;11:19–26. [Google Scholar]
- 9.Pilorz V, Jaeckel M, Knudsen K, Trillich F. The cost of specific immune response in young guinea pigs. Physiol Behav. 2005;85:205–211. doi: 10.1016/j.physbeh.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Horak P, Ots I, Tegelmann L, Moller AP. Health impact of phytoaemagglutinin-induced immune challenge on great tit nestlings. Can J Zool. 2000;78:905–910. [Google Scholar]
- 11.Nockels CF. Mineral alterations associated with stress, trauma, and infection, and the effects on immunity. The Compendium. 1990;12:1133–1139. [Google Scholar]
- 12.Hurley BP, McCormick BA. Multiple roles of phospholipase A2 during lung infection and inflammation. Infect Immun. 2008;76(6):2259–2272. doi: 10.1128/IAI.00059-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korver DR, Klasing KC. Dietary fish oil alters specific and inflammatory immune responses in chicks. J Nutr. 1997;127:2039–2046. doi: 10.1093/jn/127.10.2039. [DOI] [PubMed] [Google Scholar]
- 14.Liu YL, Li DF, Gong LM, Yi GF, Gaines AM, Carroll JA. Effects of fish oil supplementation on the performance and the immunological, adrenal, and somatotropic responses of weaned pigs after and Escherichia coli lipopolysaccharide challenge. J Anim Sci. 2003;81:2758–2765. doi: 10.2527/2003.81112758x. [DOI] [PubMed] [Google Scholar]
- 15.Klasing KC. Protecting animal health and well-being: Nutrition and immune function. In: Garnsworthy RC, Wiseman J, editors. Recent advances in animal nutrition. Nottingham Univer Pr; 2005. pp. 13–20. [Google Scholar]
- 16.Tracey KL, Wei H, Manogue K, et al. Cachetin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988;167:1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boisclair YR, Wang J, Shi J, Hurst KR, Oi GT. Role of the suppressor of cytokine signaling-3 in mediating the inhibitory effects of interleukin-1 beta on the growth hormone-dependent transcription of the acid-labile subunit gene in liver cells. J Biol Chem. 2000;275:3841–3847. doi: 10.1074/jbc.275.6.3841. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RW. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J Anim Sci. 1997;75:1244–1255. doi: 10.2527/1997.7551244x. [DOI] [PubMed] [Google Scholar]
- 19.Gabler NK, Spurlock E. Intergrating the immune system with the regulation of growth and efficiency. J Anim Sci. 2008;86:E64–74. doi: 10.2527/jas.2007-0466. [DOI] [PubMed] [Google Scholar]
- 20.Klasing KC, Johnstone BJ. Monokines in growth and development. Poult Sci. 1991;70:1781–1789. doi: 10.3382/ps.0701781. [DOI] [PubMed] [Google Scholar]
- 21.Waldhorn MR, Overton TR. Effects of inflammation on nutrition: Is sickness causing weakness in your diet?. 21st Ann SW Nutr and Manag Conf; Tempe, Arizona, USA. 2006. pp. 164–173. [Google Scholar]
- 22.Burciaga-Robles LO, Step DL, Holland BP, et al. Effects of an intratracheal Mannheimia haemolytica challenge on intake and nitrogen balance in fed and fasted steers. 2006. [Last accessed 23 July, 2009]. [page on Internet]. Available from http://www.ansi.okstate.edu/research/
- 23.Walker RD, Hopkins FM, Schultz TW, McCracken MD, Moore RN. Changes in leukocyte populations in pulmonary lavage fluids of calves after inhalation of Pasteurella haemolytica. Am J Vet Res. 1985;46:2429–2433. [PubMed] [Google Scholar]
- 24.Yoo HS, Maheswaran SK, Lin G, Townsend EL, Ames TR. Induction of inflammatory cytokines in bovine alveolar macrophages following stimulation with Pasteurella haemolytica lipopolysaccharide. Infect Immun. 1995;63:381–388. doi: 10.1128/iai.63.2.381-388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai KS, Grayson MH. Pulmonary defense mechanisms against pneumonia and sepsis. Curr Op Pulm Med. 2008;14:260–265. doi: 10.1097/MCP.0b013e3282f76457. [DOI] [PubMed] [Google Scholar]
- 26.Paape MJ, Bannerman DD, Zhao X, Lee JW. The bovine neutrophil: Structure and function in blood and milk. Vet Res. 2003;34:597–627. doi: 10.1051/vetres:2003024. [DOI] [PubMed] [Google Scholar]
- 27.Squier MKT, Shenert AJ, Cohen JJ. Apoptosis in leukocytes. J Leukoc Biol. 1995;57:2–10. doi: 10.1002/jlb.57.1.2. [DOI] [PubMed] [Google Scholar]
- 28.Henson PM, Johnston RB. Tissue injury in inflammation: Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987;79:669–678. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox GJ, Crossely J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995;12:232–237. doi: 10.1165/ajrcmb.12.2.7865221. [DOI] [PubMed] [Google Scholar]
- 31.Lechtenberg KR, Shryock TR, Moore GM. Characterization of an Actinobacillus pleuropneumoniae seeder pig challenge-exposure model. Am J Vet Res. 1994;55:1703–1709. [PubMed] [Google Scholar]
- 32.Kerr JFR, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatydilserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 34.Morris RG, Hargreaves AD, Duvall E, Wyllie AH. Hormone-induced cell death. II Surface changes in thymocytes undergoing apoptosis. Am J Pathol. 1984;115:426–430. [PMC free article] [PubMed] [Google Scholar]
- 35.Dransfield I, Buckle AM, Savill JS, McDowall A, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 expression. J Immunol. 1994;153:1254–1260. [PubMed] [Google Scholar]
- 36.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 37.Hughes J, Liu Y, VanDamme J, Savill J. Human glomerular mesangial cell phagocytosis of apoptotic neutrophils: Mediation by a novel CD36-independent vitronectin receptor/ thrombospondin recognition mechanism that is uncoupled from chemokine secretion. J Immunol. 1997;158:4389. [PubMed] [Google Scholar]
- 38.Hart SP, Ross JA, Ross K, Haslett C, Dransfield I. Molecular characterization of the surface of apoptotic neutrophils: Implications for functional deregulation and recognition by phagocytes. Cell Death Differ. 2000;7:493–499. doi: 10.1038/sj.cdd.4400680. [DOI] [PubMed] [Google Scholar]
- 39.Takizawa F, Tsuji S, Nagasawa S. Enhancement of macrophage phagocytosis upon iC3b deposition on apoptotic cells. FEBS Lett. 1996;397:269–275. doi: 10.1016/s0014-5793(96)01197-0. [DOI] [PubMed] [Google Scholar]
- 40.Mevorach D, Mascarenhas JO, Gershow D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren Y, Stuart L, Lindberg FP, et al. Nonphlogistic clearance of late apoptotic neutrophils by macrophages: Efficient phagocytosis independent of β2 integrins. J Immunol. 2001;166:4743–4750. doi: 10.4049/jimmunol.166.7.4743. [DOI] [PubMed] [Google Scholar]
- 42.Schagat TL, Wofford JA, Wright JR. Surfactant Protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J Immunol. 2001;166:2727–2733. doi: 10.4049/jimmunol.166.4.2727. [DOI] [PubMed] [Google Scholar]
- 43.Savill J. Apoptosis in resolution of inflammation. J Leukocyte Biol. 2000;61:375. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 44.Savill J, Fadok VA. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 45.Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J Leukocyte Biol. 1992;52:269–276. [PubMed] [Google Scholar]
- 46.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–899. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: Lipoxins rapidly stimulate nonphlogisitc phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 48.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: Role of proteases. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 49.Gilroy DW. Inflammatory resolution: New opportunities for drug discovery. Nature Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 50.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma, and lipoxin A4 to promote the resolution of allergic airway inflammation. Nature Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serhan CN, Brain SD, Buckley CD, et al. Resolution of inflammation: State of the art, definitions and terms. FASEB Journal. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossi AG, Hallett JM, Sawatzky DA, Teixeira MM, Haslett C. Modulation of granulocyte apoptosis can influence the resolution of inflammation. Biochem Soc Trans. 2007;35:288–291. doi: 10.1042/BST0350288. [DOI] [PubMed] [Google Scholar]
- 54.Hallett JM, Leitch AE, Riley NA, Duffin R, Haslett C, Rossi AG. Novel pharmacological strategies for driving inflammatory cell apoptosis and enhancing the resolution of inflammation. Trends Pharmacol Sci. 2008;29:250–257. doi: 10.1016/j.tips.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Rossi AG, Sawatzky DA, Walker A, et al. Cyclin-dependent kinases inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nature Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 56.Labro MT. Anti-inflammatory activity of macrolides: A new therapeutic potential? J Antimicrob Chemother. 1998;41(Suppl.B):37–46. doi: 10.1093/jac/41.suppl_2.37. [DOI] [PubMed] [Google Scholar]
- 57.Wales D, Woodhead M. The anti-inflammatory effects of macrolides: Erythromycin and clarithromycin attenuate cytokine-induced endothelin-1 expression in human bronchial epithelial cells. Thorax. 1999;54(Suppl.2):S58–S62. doi: 10.1136/thx.54.2008.s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ianaro A, Ialenti A, Maffia P, et al. Anti-inflammaotry activity of macrolide antibiotics. J Pharmacol Exp Therapeut. 2000;292:156–163. [PubMed] [Google Scholar]
- 59.Rubin BK, Tamaoki J. Macrolide antibiotics as biological response modifiers. Curr Op Invest Drugs. 2000;1:169–172. [PubMed] [Google Scholar]
- 60.Cervin A. The anti-inflammatory effects of erythromycin and its derivatives, with special reference to nasal polyposis and chronic sinusitis. Acta Otolaryngol. 2001;121:83–92. doi: 10.1080/000164801300006326. [DOI] [PubMed] [Google Scholar]
- 61.Azuma A. Roles of macrolides in treatment of lung injury. In: Parnham MJ, Rubin BK, Tamaoki J, editors. Antibiotics as Anti-inflammatory and Immunomodulatry Agents. Basel: Birkhaeuser (Basel, Springer); 2005. pp. 219–226. [Google Scholar]
- 62.Peeters TL. Erythromycin and other macrolides as prokinetic agents. Gastroenterology. 1993;105:1886–1899. doi: 10.1016/0016-5085(93)91089-z. [DOI] [PubMed] [Google Scholar]
- 63.Mikasa K, Sawaki M, Kita E, et al. Significant survival benefits to patients with advanced non-small-cell lung cancer from treatment with clarithromycin. Chemotherapy. 1997;43:288–296. doi: 10.1159/000239580. [DOI] [PubMed] [Google Scholar]
- 64.Yatsunami J, Fukuno Y, Nagat M, et al. Antiangiogenic and antitumor effects of 14-membered macrolides on mouse B16 melanoma cells. Clin Exp Metastasis. 1999;17:361–367. doi: 10.1023/a:1006605725619. [DOI] [PubMed] [Google Scholar]
- 65.Rubin BK, Druce H, Ramirez OE, Palmer R. Effect of clarithromycin on nasal mucus properties in healthy subjects and in patients with purulent rhinitis. Am J Respir Crit Care Med. 1997;155:2018–2023. doi: 10.1164/ajrccm.155.6.9196110. [DOI] [PubMed] [Google Scholar]
- 66.Tamaoki J, Takemyama K, Yamawaki I, Kondo M, Konno K. Lipopolysaccharide-induced goblet cell hypersecretion in the guinea pig trachea: Inhibition by macrolides. Am J Physiol. 1997;272:L15–L19. doi: 10.1152/ajplung.1997.272.1.L15. [DOI] [PubMed] [Google Scholar]
- 67.Woo PC, Tsoi HW, Wong LP, Leung HC, Yuen KY. Antibiotics modulate vaccine-induced humoral immune response. Clin Diagn Lab Immunol. 1999;6:832–837. doi: 10.1128/cdli.6.6.832-837.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sassa K, Mizushima Y, Kobayashi M. Differential modulatory effects of clarithromycin on the production of cytokines by a tumor. Antimicrob Agents Chemother. 1999;43:2787–2789. doi: 10.1128/aac.43.11.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aoshiba K, Nagai A, Konno K. Erythromycin shortens neutrophil survival by accelerating apoptosis. Antimicrob Ag Chemother. 1995;39:872–877. doi: 10.1128/aac.39.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inamura K, Ohta N, Fukaze S, Kasajima N, Aoyagi M. The effects of erythromycin on human peripheral neutrophil apoptosis. Rhinology. 2000;38:124–129. [PubMed] [Google Scholar]
- 71.Fisher CD, Zvaigzne C, Morck DW, Lucas MJ, Robb EJ, Buret AG. Tulathromycin inhibits NF-kB signaling and induces apoptosis in bovine neutrophils in caspase-3-dependent fashion. FASEB J. 2008;22:lb591. [Google Scholar]
- 72.Scorneaux B, Shryock TR. Intracellular accumulation, subcellular distribution, and efflux of tilmicosin in bovine mammary, blood, and lung cells. J Dairy Sc. 1999;82:1202–1212. doi: 10.3168/jds.S0022-0302(99)75343-9. [DOI] [PubMed] [Google Scholar]
- 73.Bonnet M, van der Auwera P. In vitro and in vivo intraleukocytic accumulation of azithromycin (CP-62,993) Antimicrob Ag Chemother. 1992;36:1302–1309. doi: 10.1128/aac.36.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koch CC, Esteban DJ, Chin AC, et al. Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: Effects of Streptococcus pneumoniae. J Antimicrob Chemother. 2000;46:19–26. doi: 10.1093/jac/46.1.19. [DOI] [PubMed] [Google Scholar]
- 75.Khair OA, Devalia JL, Abdelaziz MM. Effects of erythromycin on H. influenzae endotoxin-induced release of IL-6, IL-8, and sICAM-1 by cultured human bronchial epithelial cells. Eur Respir J. 1995;8:1451–1457. [PubMed] [Google Scholar]
- 76.Gorham PE, Carroll LH, McAskill JW. Tilmicosin as a single injection treatment for respiratory disease of feedlot cattle. Can J Vet Res. 1990;31:826–829. [PMC free article] [PubMed] [Google Scholar]
- 77.Moore GM, Mowrey DH, Tonkinson LV, Lechtenberg KF, Schneider JH. Efficacy dose determination study of tilmicosin phosphate in feed for control of pneumonia caused by Actinobacillus pleuropneumoniae in swine. Am J Vet Res. 1996;57:220–223. [PubMed] [Google Scholar]
- 78.Jordan WH, Byrd RA, Cochrane RL. A review of the toxicology of the antibiotic Micotil 300. Vet Hum Toxicol. 1993;35:151–158. [PubMed] [Google Scholar]
- 79.Morck DW, Merrill JK, Gard MS. Treatment of experimentally induced pneumonic pasteurellosis of young calves with tilmicosin. Can J Vet Res. 1997;61:187–192. [PMC free article] [PubMed] [Google Scholar]
- 80.Moore GM, Basson RP, Tonkinson LV. Clinical field trials with tilmicosin phosphate in feed for the control of naturally acquired pneumonia caused by Actinobacillus pleuropneumoniae and Pasteurella multocida in swine. Am J Vet Res. 1996;57:224–227. [PubMed] [Google Scholar]
- 81.Diarra MS, Malouin F, Jacques M. Postantibiotic and physiological effects of tilmicosin, tylosin, and apramycin at sub-minimal and suprainhibitory concentrations on some swine and bovine respiratory tract pathogens. Int J Antimicrob Ag. 1999;12:229–237. doi: 10.1016/s0924-8579(99)00069-2. [DOI] [PubMed] [Google Scholar]
- 82.Chin A, Morck DW, Merrill JK, et al. Anti-inflammatory benefits of tilmicosin in calves with P. haemolytica-infected lungs. Am J Vet Res. 1998;59:765–771. [PubMed] [Google Scholar]
- 83.Chin A, Lee WD, Murrin KA, et al. Tilmicosin induces apoptosis in bovine neutrophils in the presence or in the absence of P. haemolytica and promotes neutrophil phagocytosis by macrophages. Antimicrob Ag Chemother. 2000;44:2465–2470. doi: 10.1128/aac.44.9.2465-2470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee WD, Flynn AN, LeBlanc JM, et al. Tilmicosin-induced bovine neutrophil apoptosis is cell-specific and downregulates spontaneous LTB4 synthesis without increasing Fas expression. Vet Res. 2004;35:213–224. doi: 10.1051/vetres:2004004. [DOI] [PubMed] [Google Scholar]
- 85.Lakritz J, Tyler JW, Marsh AE, Romesburg-Cockrell M, Smith K, Holle JM. Tilmicosin reduces lipopolysaccharide-stimulated bovine alveolar macrophage prostaglandin E(2) production via a mechanism involving phospholipases. Vet Ther. 2002;3:7–21. [PubMed] [Google Scholar]
- 86.Weinhold U, Hofmann W, Mulling C, Budras KD. Untersuc-hungen von Bronchialzellsedimenten lungenkranker Kaelber waehrend der Behandlung mit den Antibiotika Tilmicosin und Ceftiofur auf das Vorkommen der Apoptosis der neutrophilen Granulozyten, Tieraerztl. Unschau. 2003;58:191–199. [Google Scholar]
- 87.Fajt VR, Apley MD, Roth JA, et al. The effects of danofloxacin and tilmicosin on neutrophil function and lung consolidation in beef heifer calves with induced Pasteurella (Mannheimia) haemolytica pneumonia. J Vet Pharmacol Therap. 2003;26:173–179. doi: 10.1046/j.1365-2885.2003.00477.x. [DOI] [PubMed] [Google Scholar]
- 88.Nerland EM, Leblanc JM, Fedwick JP, et al. Oral Tilmicosin induces leukocyte apoptosis, reduces Leukotriene B4, and attenuates inflammation in the Actinobacillus pleuropneumoniae-Infected Porcine Lung. Am J Vet Res. 2005;66:100–107. doi: 10.2460/ajvr.2005.66.100. [DOI] [PubMed] [Google Scholar]
- 89.Cao XY, Dong M, Shen JZ, et al. Tilmicosin and tylosin have anti-inflammatory properties via modulation of COX-2 and iNOS gene expression and production of cytokines in LPS-induced macrophages and monocytes. Int J Antimicrob Ag. 2006;27:431–438. doi: 10.1016/j.ijantimicag.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 90.Reuter RR, Carroll JA, Dailey JW, Cook BJ, Galyean ML. Effects of dietary energy source and level and injection of tilmicosin phosphate on immune function in lipopolysaccharide- challenged beef steers. J Anim Sci. 2008 doi: 10.2527/jas.2007-0838. (April 11; epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 91.Levert H, Gressier B, Brunte C, et al. Time and concentration dependent influence of dirithromycin on neutrophils oxidative burst. J Antibiot. 1999;52:127–133. doi: 10.7164/antibiotics.52.127. [DOI] [PubMed] [Google Scholar]
- 92.Kanota JI, Iwashita T, Matsubara Y, et al. Inhibitory effect of erythromycin on superoxide anion production by human neutrophils primed with granulocyte-colony stimulating factor. Antimicrob Ag Chemother. 1998;42(7):1866–1867. doi: 10.1128/aac.42.7.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin HC, Wang CH, Liu CY, Yu CT, Kuo HP. Erythromycin inhibits β2-integrins (CD11b/CD18) expression, interleukin-8 release and intracellular oxidative metabolism in neutrophils. Respir Med. 2000;94:654–660. doi: 10.1053/rmed.1999.0781. [DOI] [PubMed] [Google Scholar]
- 94.Takisawa H, Desaki M, Ohtoshi T. Erythromycin and clarithromycin attenuate cytokine-induced endothelin-1 expression in human bronchial epithelial cells. Eur Respir J. 1998;12:57–63. doi: 10.1183/09031936.98.12010057. [DOI] [PubMed] [Google Scholar]
- 95.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide regulates pro-inflammatory cytokine expression in mouse myofibroblasts and skeletal muscle. Am J Physiol Integr Comp Physiol. 2002;283:R698–R709. doi: 10.1152/ajpregu.00039.2002. [DOI] [PubMed] [Google Scholar]
- 96.Schrick FN, Hockett ME, Saxton AM, Lewis MJ, Dowlen HH, Oliver SP. Influence of subclinical mastitis during early lactation on reproductive parameters. J Dairy Sci. 2001;84:1407–1412. doi: 10.3168/jds.S0022-0302(01)70172-5. [DOI] [PubMed] [Google Scholar]
- 97.Hockett ME, Hopkins FM, Lewis MJ, et al. Endocrine profiles of dairy cows following experimentally induced clinical mastitis during early lactation. Anim Reprod Sci. 2000;58:241–251. doi: 10.1016/s0378-4320(99)00089-5. [DOI] [PubMed] [Google Scholar]
- 98.Rajala-Schultz PJ, Grohn YT, McCulloch CE, Guard CL. Effects of clinical mastitis on milk yield in dairy cows. J Dairy Sci. 1999;82:1213–1220. doi: 10.3168/jds.S0022-0302(99)75344-0. [DOI] [PubMed] [Google Scholar]
- 99.Shuster DE, Kehrli ME, Baumrucker CR. Relationship of inflammatory cytokines, growth hormone, and insulin-like growth factor-1 to reduced performance during infectious disease. Proc Soc Exp Biol Med. 1995;210:140–149. doi: 10.3181/00379727-210-43933. [DOI] [PubMed] [Google Scholar]
- 100.Miyanohara T, Ushikai M, Matsune S, Ueno K, Katahira S, Kurono Y. Effects of clarithromycin on cultured human nasal epithelial cells and fibroblasts. Laryngoscope. 2000;110:126–131. doi: 10.1097/00005537-200001000-00023. [DOI] [PubMed] [Google Scholar]
- 101.Kawasaki S, Takisawa H, Ohtoshi T. Roxithromycin inhibits cytokine production by and neutrophil attachment to human bronchial epithelial cells in vitro. Antimicrob Ag Chemother. 1998;42:1499–1502. doi: 10.1128/aac.42.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fujita K, Shimizu T, Majima Y, Sakakura Y. Effects of macrolides on interleukin-8 secretion from human nasal epithelial cells. Eur Arch Otorhinolaryngol. 2000;257:199–204. doi: 10.1007/s004050050222. [DOI] [PubMed] [Google Scholar]
- 103.Schultz M, Speelman P, Zaat S. Erythromycin inhibits TNF-alpha and IL-6 production induced by heat-killed S. pneumoniae in whole blood. Antimicrob Ag Chemother. 1998;42:1605–1609. doi: 10.1128/aac.42.7.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schultz MJ, Speelman P, Hack CE, Buurman WA, van Deventer SJH, van der Poll T. Intravenous infusion of erythromycin inhibits CXC chemokine production, but augments neutrophil degranulation in whole blood stimulated with S. pneumoniae. J Antimicrob Ag Chemother. 2000;46:235–240. doi: 10.1093/jac/46.2.235. [DOI] [PubMed] [Google Scholar]
- 105.Ichikawa Y, Ninomiya H, Koga H. Erythromycin reduces neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am Rev Resp Dis. 1992;146:196–203. doi: 10.1164/ajrccm/146.1.196. [DOI] [PubMed] [Google Scholar]