Abstract
Pasteurella multocida serogroup D causes progressive atrophic rhinitis in pigs and produces a potent, intracellular, mitogenic toxin known as P. multocida toxin (PMT), which is encoded by the toxA gene. Highly toxic to cells, PMT is a poor antigen and becomes more immunogenic after its native structure has been destroyed. Previously, we found that the N-terminal fragment of PMT (N-PMT) can induce a strong immune response that is protective against wild-type challenge. Here, an attenuated P. multocida mutant expressing only N-PMT was developed and its protective effect was evaluated. The mutant provides protective immune responses against bacterial and toxin challenges, and so is a good live vaccine candidate.
Résumé
Pasteurella multocida sérogroupe D est responsable de la rhinite atrophiante progressive du porc et produit une toxine intracellulaire puissante et mitogène dénommée toxine de P. multocida (PMT) qui est codée par le gène toxA. PMT est fortement toxique pour les cellules mais est un pauvre antigène et devient immunogène après que sa structure primaire ait été détruite. Précédemment, nous avons trouvé que le fragment N-terminal de PMT (N-PMT) peut induire une forte réponse immune qui est protectrice contre une infection défi avec une souche sauvage. Ici, un mutant atténué de P. multocida exprimant uniquement N-PMT a été développé et son effet protecteur évalué. Le mutant induit des réponses immunes contre une infection défi avec la bactérie et l’administration de toxine, et serait ainsi un bon candidat comme vaccin vivant.
(Traduit par Docteur Serge Messier)
Introduction
Pasteurella multocida-induced pneumonia and progressive atrophic rhinitis (PAR) are widespread diseases that cause growth retardation and a reduction in the efficiency of feed utilization among grower-finisher pigs (1–3). Pasteurella multocida toxin (PMT), a monomeric 146 kDa protein encoded by the toxA gene, is produced by some P. multocida serotype A and D strains (4). A poor antigen, PMT becomes more immunogenic after its native structure has been destroyed (5). Partially truncated proteins have been predicted to be good antigens (6). In a previous study, vaccination with a mixture of 3 recombinant fragments of PMT with/without inclusion of intact P. multocida resulted in high levels of neutralizing antibody (Ab) and protection against PMT challenge (6).
Attenuation of P. multocida can be achieved by the abrogation of the appropriate metabolic gene. In a previous study, an aroA mutant of P. multocida successfully protected calves against challenge with the pathogenic wild type (7). However, PMT was not involved since the toxin is expressed only in serotypes A and D in pigs (4). Previously, we demonstrated that none of the mice vaccinated with a toxA knock-out mutant that does not produce PMT were capable of surviving challenge with the wild type (8) indicating that mouse Abs against outer structural and/or inner cytosolic proteins of P. multocida are not protective. So, it is clear that the targeting of the protection against P. multocida serotypes A and/or D should be focused on PMT.
We have previously shown that the N-terminal fragment of PMT (N-PMT, amino acids 1-390) is the most immunogenic portion of the protein, and that N-PMT is partially protective for mice against wild type challenge (9). To clarify whether N-PMT expressed in vivo can induce protective immunity against bacterial and toxin challenge, a P. multocida mutant capable of expressing only N-PMT instead of the intact toxin was developed and its protective effect was evaluated.
Materials and methods
Escherichia coli and plasmids
Escherichia coli JM109 (Invitrogen, Carlsbad, California, USA) was used to propagate the plasmid construct. The pGEM®-T easy vector (Promega, Madison, Wisconsin, USA) was used for cloning procedures. Escherichia coli manipulations were performed according to the manufacturer’s instructions. Standard DNA and protein manipulations were carried out as previously described (10,11). Red helper plasmid pKD46 (12), which expresses λ Red recombinase, was used to allow the homologous recombination of linear DNA in strain JM109. pKD13 (12) was used as a template for the generation of kanamycin resistance gene (kanR). pKD46 is a temperature sensitive replicon, thus allowing its easy elimination at 43°C.
Cloning of toxA and kanR fragments
Pasteurella multocida type D was originally obtained from the National Veterinary Research & Quarantine Service, Korea. The N-terminal (amino acids 1–390) and C-terminal (amino acids 921–1285) regions of toxA were amplified using P. multocida genomic DNA as a template. For the selection of knock-out colonies, kanR was used for transformant selection, which was amplified by polymerase chain reaction (PCR) using pKD13 as a template. Six PCR primers (P1–P6) were designed using the Gene Runner software program (Hastings Software, Hastings, New York, USA) from the nucleotide sequences in the GenBank database (Table I). The amplified DNA products were electrophoresed on a 1.2% (w/v) agarose gel, purified using a PCR purification kit (Qiagen, Valencia, California, USA) according to the manufacturer’s instructions, and cloned into a pGEM®-T easy vector (Qiagen) to generate pGEM-toxA-N, pGEM-toxA-C, and pGEM-kanR (Figure 1). The construct was transformed into chemically competent E. coli JM109. The transformants were selected and the mini-scale isolation of the plasmid DNA was used to prepare the recombinant plasmid for sequencing on the plasmid DNA QIAprepSpin Mini Kit (Qiagen). EcoRI restriction analysis and DNA sequencing confirmed the presence and orientation of each gene or gene segment. DNA sequencing reactions were performed using an automated DNA sequencer (ABI PRISM 3100 Genetic Analyzer; Applied Biosystems, Foster City, California, USA).
Table I.
Nucleotide sequences of the primers used in this study (GenBank reference numbers: AF240778 for toxA gene in P. multocida and AY048744 for kanR gene in pKD13)
| Primers | Nucleotide sequence (5′– 3′) | |
|---|---|---|
| P1 | toxA-N-F | GCGC CTCGAG* ATGAAAACAAAACATTTTTT |
| P2 | toxA-N-R | GCGC AGATCT* GAGTAATGAAGAGCATAGT |
| P3 | toxA-C-F | GCGC GGTACC* ATTGACTTTTTCCTAAATAA |
| P4 | toxA-C-R | AATT GGATCC* TTATAGTGCTCTTGTTAAGC |
| P5 | kanR-F | GGCC AGATCT* ATGATTGAACAAGATGGAT |
| P6 | kanR-R | AATT GGTACC* TCAGAAGAACTCGTCAAGA |
Underlined: enzyme sites.
Figure 1.
Diagram of cloning strategy. After cloning, an insert was prepared by digesting pGEM-toxA-kanR with XhoI and BamHI restriction enzymes.
Cloning strategy
Two constructs (pGEM-toxA-N and pGEM-kanR) were digested with BglII and SacI, and the kanR gene was inserted into the BglII+SacI site of pGEM-toxA-N, generating pGEM-toxA-N-kanR. pGEM-toxA-C was digested with KpnI and SacI and inserted into KpnI+SacI site of pGEM-toxA-N-kanR, generating pGEM-toxA-kanR. A linear 2.97 kb DNA fragment (toxA-N-kanR-toxA-C) produced by digestion with XhoI and BamHI was used as an insert (100 ng/μL) for homologous recombination. The cloning strategy is depicted in Figure 1.
Gene disruption by homologous recombination
A 5-mL volume of a fresh overnight P. multocida culture was inoculated into 500 mL Brain-Heart Infusion (BHI). Cells were grown to an optical density at 600 nm (OD600) of approximately 0.5, chilled on ice for 20 min, and centrifuged at 4000 μg for 15 min at 4°C. The supernatant was removed and the pellet was concentrated 100-fold and washed 3 times with ice-cold 10% glycerol. The final preparation represented the competent cells.
For the induction of λ Red recombinase, competent cells were transformed with pKD46 using a Gene Pulser Xcell electroporation system (Bio-Rad, Hercules, California, USA) according to the manufacturer’s instructions. Transformants carrying pKD46 were grown in 5 mL SOB containing 10 mM each of ampicillin and L-arabinose (final) at 30°C to an OD600 of approximately 0.5, and then made electro-competent by concentrating 100-fold and washing 3 times with ice-cold 10% glycerol. Electro-competent cells (40 μL) were transformed with 1 μL (100 ng) of insert DNA (toxA-N-kanR-toxA-C) by electroporation according to the manufacturer’s instructions. Knock-out mutants were selected on LB plates containing 50 μg/mL kanamycin.
PCR verification
Three PCR procedures were used to show that mutants had the correct genomic structure. Genomic DNA from a mutant colony was prepared (50 ng/μL) and used as a template. The expected sizes and targets of the 3 PCR approaches are represented in Figure 2. P1 and P6 were used for PCR 1, P5 and P4 for PCR 2, and P1 and P4 for PCR 3.
Figure 2.
Diagram of PCR verification for genomic structure. The expected sizes and targets of three PCR approaches (1–3) were obtained.
In vitro expression of N-PMT
The expression of N-PMT in vitro was confirmed by a Western blot assay. Briefly, freshly prepared lysate of the PMT mutant was subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a 0.45-μm nitrocellulose membrane (Bio-Rad). For immunodetection, the membrane was blocked in phosphate buffered saline (PBS) + 0.05% Tween-20 (pH 7.4) (PBST) containing 1% skim milk for 1 h. The membrane was washed 3 times with PBST before being incubated with the primary and secondary Abs for 1 h at 37°C. Anti-N-PMT mouse polyclonal Ab was produced by immunization of mice with recombinant N-PMT produced in E. coli (9), and used as a primary Ab. Goat anti-mouse IgG horseradish peroxidase (HRP)-conjugated Ab (Serotec, Oxford, United Kingdom) was used as the secondary Ab. The membrane was developed in a diaminobenzidine (DAB) substrate buffer containing a DAB concentrate (Serotec) until brownish bands were observed. Color development was quenched by thorough washing in PBS.
Vaccination with PMT mutant
Pasteurella multocida toxin mutant bacterial cultures were prepared in BHI broth at a concentration of 1 × 108 cells/mL. Forty-eight 6-week-old ICR mice of both sexes (Charles River, Yokohama, Japan) were divided into 4 groups (Table II). Experimental groups were inoculated with 100 μL of freshly prepared culture (1 × 107 bacteria/ mouse) and controls received 100 μL PBS. This was followed by 3 more booster injections with bacteria or PBS at 2-week intervals.
Table II.
Experimental design for toxigenicity test and protection study. Mice in groups 1 and 2 were vaccinated with live PMT mutant P. multocida bacterial culture, while controls (groups 3 and 4) with PBS. For challenge study, group 2 and 4 were challenged with W/T P. multocida, while group 1 and 3 with W/T bacterial lysate
| Group | Route | Dosage per mouse | Trial number | Challenge with |
|---|---|---|---|---|
| 1 | Intraperitoneal | PMT mutant (1 × 107 cells) | 12 | Bacterial lysate 500 μL |
| 2 | Intraperitoneal | PMT mutant (1 × 107 cells) | 12 | Bacterial culture (1 × 107 cells) |
| 3 | Intraperitoneal | PBS 100 μL | 12 | Bacterial lysate 500 μL |
| 4 | Intraperitoneal | PBS 100 μL | 12 | Bacterial culture (1 × 107 cells) |
Ab induction in vivo
To check if partial truncated N-PMT expressed by PMT mutant in vivo could induce Abs, mouse blood samples were collected from the tail vein 7 d after the final booster injection and were used for an enzyme-linked immunosorbant (ELISA) assay. Briefly, 96-well plates (Maxisorp; Nunc, Roskilde, Denmark) were coated with recombinant N-PMT (1 μg/well/90 μL) as previously described (9). The plates were blocked with PBS containing 1% skim milk and washed with PBST. The sera for IgG analysis were prepared at a 1:100 dilution (shown in preliminary experiments to be the optimal dilution). Ab in 90 μL PBST were incubated for 1 h at 37°C, emptied, and washed with PBST 3 times. The bound IgG was detected using goat anti-mouse HRP-conjugated IgG (Pierce, Rockford, Illinois, USA) diluted 1:500. The secondary Ab was incubated for 1 h at 37°C, emptied, and washed 3 times with PBST. A substrate solution consisting of 10 mL of 0.1 M citric acid buffer (pH 4.0), 250 μL of 3-ethylbenzthiazoline-6-sulfonic acid (ABTS) stock solution (ABTS 100 mg ABTS in 4.5 mL distilled water), and 50 μL hydrogen peroxide (H2O2) was used to detect the presence of any bound secondary Ab. The plate was developed in the dark at room temperature for 15 min. The absorbance at 405 nm was read using a Multiskan EX ELISA reader (Thermo LabSystems, Beverly, Massachusetts, USA). The results were expressed as the average ± standard deviation (s) of the end-point titers. A t-test was used to examine the differences in the mean Ab values using GraphPad Instat Software 3.05 (GraphPad software, La Jolla, California, USA).
Bacterial and toxin challenges
For the preparation of bacterial toxin as previously described (8), 50 mL of wild type P. multocida overnight cultures (1 × 108 cells/mL) were washed twice with 10 mL PBS, sonicated, and the supernatants filtered through a 0.2-μm membrane filter. Fifteen days after vaccination, groups 1 and 3 were challenged with 500 μL of freshly prepared bacterial lysate (bacterial toxin, which corresponds to LD100), while groups 2 and 4 where challenged with 100 μL of freshly prepared wild type P. multocida culture (corresponds to LD100). The mortality rate was recorded for 10 d after the inoculation. A t-test was used to examine the differences in the protection rates.
Results
Disruption of toxA by homologous recombination
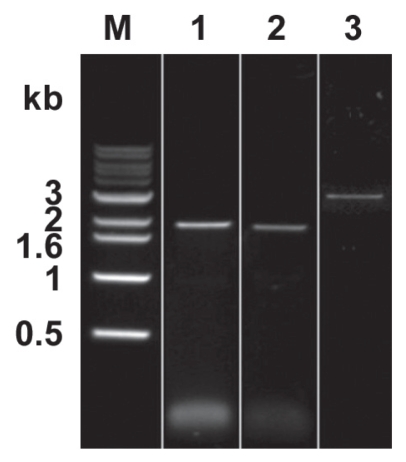
Figure 3 shows the results of the PCR verification. As expected, the 1.95 kb PCR1, 1.88 kb PCR2, and 2.97 kb PCR3 fragments were produced. The sequences of the PCR verification products were analyzed and confirmed by DNA sequencing (data not shown).
Figure 3.
Results of PCR verification. PCR products with expected sizes (1.95 kb for PCR1, 1.88 kb for PCR2, and 2.97 kb for PCR3) were produced. Lane 1: PCR1, lane 2: PCR2, lane 3: PCR3.
Expression of N-PMT in vitro
Anti-N-PMT mouse polyclonal Ab raised against recombinant N-PMT reacted with intact PMT from wild type P. multocida lysate and against a PMT mutant protein of approximately 47 kDa (Figure 4), confirming the in vitro production of a truncated N-PMT.
Figure 4.
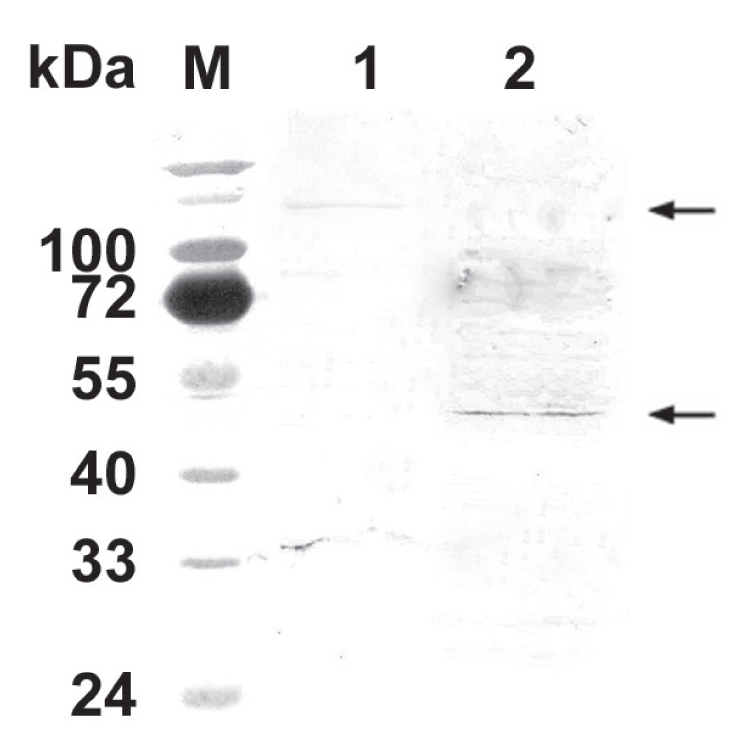
Antigenic recognition of N-PMT. Mouse polyclonal Ab raised against the recombinant N-PMT reacted with intact PMT (lane 1 upper arrow, approximately 146 kDa) and a bacterial protein of approximately 47 kDa (lane 2 lower arrow). M: molecular size marker.
Ab induction in vivo
Mouse Abs against N-PMT expressed in vivo were successfully induced, as shown by an ELISA. Analysis of the mouse IgG level against N-PMT demonstrated that the mice, intraperitoneally immunized with the mutant P. multocida, successfully produced IgG (mean OD value = 1.472, s = 0.195, P < 0.001). However, no significant increase in IgG level was shown in the control group (mean OD value = 0.267, s = 0.025).
Bacterial and toxin challenge
Most of the mice immunized with mutant P. multocida were protected upon challenge (Table III). In total, 21 of the mice (87.5%) survived in the PMT mutant-immunized group, while none survived in the PBS control group (P < 0.0001).
Table III.
Result of challenge studies using live bacterial culture or lysate
| Days after challenge |
||||||
|---|---|---|---|---|---|---|
| Group | 1 | 3 | 5 | 7 | 10 | Total |
| 1 | — | — | — | — | 2 | 2/12* |
| 2 | — | — | — | — | 1 | 1/12* |
| 3 | 2 | 10 | — | — | — | 12/12 |
| 4 | — | 6 | — | — | 6 | 12/12 |
Two-sided P-value is lower than 0.0001, which is extremely significant on Fisher’s exact test.
Discussion
Recently, a genetically modified PMT toxin produced by replacing the serine at position 1164 with alanine, and the cysteine at position 1165 with serine led to a complete loss of the toxic effects of PMT without impairing the ability to induce protective immunity in pigs (13). Also, vaccination of sows with a mixture of 3 recombinant fragments of the PMT fragments (N-terminal, middle, and C-terminal) with/without intact P. multocida produced high levels of neutralizing Ab and protection of offspring against a PMT challenge (13). These suggest that the induction of IgG and/or IgA protective immunity against pasteurellosis can be achieved when the appropriate immunogens and administration routes are used.
Currently, clinical PAR is usually controlled by combined vaccination with Bordetella bronchiseptica and P. multocida, which consists mainly of toxoid and/or somatic antigens of both bacteria. Using these vaccines, pathogenic lesions, excretion of bacteria, and the time to market under experimental and field conditions are decreased. Some vaccines also have been shown to protect pigs against the development of lung lesions when administrated properly. Although vaccination has unquestionable beneficial effects, current vaccines are not able to eliminate the bacteria (14). Also, in our previous study (8), none of the mice vaccinated with a PMT-eliminated mutant (toxA knock-out) survived after wild type challenge, indicating that mouse Abs against outer structural and/or inner cytosolic proteins of P. multocida are not protective. So, the toxigenicity of this bacterium seemed to be derived mainly from PMT and the protective immunity against wild type P. multocida can be acquired only when protection against PMT has been established (13). Also, animals vaccinated with recombinant N-PMT are successfully protected after wild type challenge (9), which prompted us to develop a mutant expressing only N-PMT.
Expression of N-PMT in vitro and in vivo was confirmed by western blot and ELISA indicating that the elimination of the middle and C-terminal fragments of PMT has no influence on protein expression. Mice immunized with mutant successfully produced anti-N-PMT Abs without any pathogenic effects of PMT, indicating this mutant has been well attenuated and N-terminal itself has little damage to animals. In the challenge experiments, only 3 out of 24 mice in groups 1 and 2 (12.5%) were killed, while all control mice succumbed. This indicates that protective Ab against PMT can be induced by purified (9) and/or bacterial (this study) recombinant N-PMT.
In this study, we constructed an attenuated P. multocida expressing only N-PMT to demonstrate the potential of this mutant to elicit a protective immune response in mice. Although further experiments with pigs will be performed, we conclude that this mutant represents a good candidate for the development of live bacterial vaccine.
Acknowledgment
The authors thank Ms. Myeonghwa Kim (College of Veterinary Medicine, Chonnam National University) for her excellent technical assistance.
References
- 1.de Jong MF. Progressive and nonprogressive atrophic rhinitis. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of Swine. Ames, Iowa: Iowa State Univer Pr; 1999. pp. 355–384. [Google Scholar]
- 2.Pijoan C. Pneumonic pasteurellosis. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of Swine. Ames, Iowa: Iowa State Univer Pr; 1999. 1999. pp. 511–520. [Google Scholar]
- 3.Hunt ML, Adler B, Townsend KM. The molecular biology of Pasteurella multocida. Vet Microbiol. 2000;72:3–25. doi: 10.1016/s0378-1135(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 4.Pullinger GD, Bevir T, Lax AJ. The Pasteurella multocida toxin is encoded within a lysogenic bacteriophage. Mol Microbiol. 2004;51:255–269. doi: 10.1046/j.1365-2958.2003.03829.x. [DOI] [PubMed] [Google Scholar]
- 5.van Diemen PM, de Vries Reilingh G, Parmentier HK. Immune response of piglets to Pasteurella multocidatoxin and toxoid. Vet Immunol Immunopathol. 1994;41:307–321. doi: 10.1016/0165-2427(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 6.Liao CM, Huang C, Hsuan SL, et al. Immunogenicity and efficacy of three recombinant subunit Pasteurella multocida toxin vaccines against progressive atrophic rhinitis in pigs. Vaccine. 2006;24:27–35. doi: 10.1016/j.vaccine.2005.07.079. [DOI] [PubMed] [Google Scholar]
- 7.Hodgson JC, Finucane A, Dagleish MP, et al. Efficacy of vaccination of calves against hemorrhagic septicemia with a live aroA derivative of Pasteurella multocida B:2 by two different routes of administration. Infect Immun. 2005;73:1475–1481. doi: 10.1128/IAI.73.3.1475-1481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim TJ, Lee JI, Lee BJ. Development of a toxA gene knock-out mutant of Pasteurella multocida and assessment of its protective effects. J Microbiol. 2006;44:320–326. [PubMed] [Google Scholar]
- 9.Seo JY, Pyo HJ, Lee SM, et al. Expression of 4 truncated fragments of Pasteurella multocida toxin and their immunogenicity. Can J Vet Res. 2009;73:184–189. [PMC free article] [PubMed] [Google Scholar]
- 10.Ausubel FM, Brent R, Kingston RE, et al. Short Protocols in Molecular Biology. New York, New York: John Wiley & Sons; 2002. [Google Scholar]
- 11.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Lab Pr; 2001. 2001. [Google Scholar]
- 12.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.To H, Someno S, Nagai S. Development of a genetically modified nontoxigenic Pasteurella multocida toxin as a candidate for use in vaccines against progressive atrophic rhinitis in pigs. Am J Vet Res. 2005;66:113–118. doi: 10.2460/ajvr.2005.66.113. [DOI] [PubMed] [Google Scholar]
- 14.Haesebrouck F, Pasmans F, Chiers K, Maes D, Ducatelle R, Decostere A. Efficacy of vaccines against bacterial diseases in swine: What can we expect? Vet Microbiol. 2004;100:255–268. doi: 10.1016/j.vetmic.2004.03.002. [DOI] [PubMed] [Google Scholar]