Abstract
The objective of this study was to determine the effect of body position, chest wrap, and sedation on functional residual capacity (FRC) in 6 healthy dogs. Functional residual capacity was determined by helium dilution (re-breathing) whilst in different clinically relevant conditions. These conditions included the standing (sternal) and lateral positions in unsedated dogs and then again both standing and lateral following chest bandaging, and sedation with acepromazine, IV and butorphanol, IV. The mean FRC at each measurement point was determined, as was the change in FRC (delta FRC) from one measurement point to another. Analysis of variance (ANOVA) with repeated measures with Fisher’s LSD post hoc test was used to evaluate the effect of interventions. The differences in delta FRC were evaluated using a t-test or Wilcoxon rank-sum test. P < 0.05 was considered significant. The mean FRC at baseline, defined as standing, unsedated and unwrapped, was 75.3 ± 23.8 mL/kg. Body position or sedation had the most profound effect on FRC with right lateral recumbency lowering FRC by a median of 20.4 mL/kg and sedation lowering FRC by a median of 19.8 mL/kg. Common clinical procedures and positioning result in lowered FRC in healthy deep-chested dogs. In critically ill or injured dogs, the iatrogenic loss of FRC through chest bandaging, sedation, or body position may be clinically relevant.
Résumé
L’objectif de la présente étude était de déterminer l’effet de la position corporelle, d’un bandage thoracique et de la sédation sur la capacité résiduelle fonctionnelle (FRC) chez 6 chiens en santé. La capacité fonctionnelle résiduelle a été déterminée par dilution de l’hélium (ré-inhalation) lors de différentes conditions cliniques pertinentes. Ces conditions incluaient les positions debout (sternale) et latérale chez des chiens non sous-sédation et par la suite dans les mêmes positions mais suivant un bandage thoracique et sédation avec acépromazine, IV et butorphanol, IV. La FRC moyenne à chaque point de mesure était déterminée, tout comme le changement de FRC (delta FRC) entre un point de mesure à un autre. Une analyse de variance (ANOVA) avec mesures répétées à l’aide d’un test de Fisher post hoc des moindres carrés a été utilisée afin d’évaluer l’effet des interventions. Les différences dans les delta FRC ont été évaluées à l’aide d’un test de t ou le test de la somme des rangs de Wilcoxon. Une valeur P < 0,05 était considérée comme le seuil significatif. La FRC moyenne de base, définie chez l’animal en position debout, non sous-sédation et sans bandage était de 75,3 ± 23,8 mL/kg. La position corporelle ou la sédation avait l’effet le plus marquée sur la FRC avec le décubitus latéral droit réduisant la FRC par une valeur médiane de 20,4 mL/kg et la sédation réduisant la FRC par une valeur médiane de 19,8 mL/kg. Les procédures cliniques courantes et la position résultent en une FRC réduite chez les chiens à thorax profond en santé. Chez les chiens blessés ou atteint d’une maladie critique, la perte iatrogénique de FRC suite à un bandage thoracique, une sédation ou la position corporelle pourrait être cliniquement significative.
(Traduit par Docteur Serge Messier)
Introduction
Normal lung function is vital to adequate oxygenation and ventilation. Lung function may be affected through disease or trauma, or may be disturbed by medical or surgical procedures, in particular, by open thoracotomy. Thoracotomy is commonly performed in dogs for a variety of indications, including trauma, infection, and neoplasia. Both general anesthesia and thoracic surgery are widely known to reduce pulmonary function in dogs as well as people intra-operatively as well as post-operatively (1–4). Post-operative complications involving the respiratory system may be significant and may include hypoxemia and hypoventilation (5,6). Separate from primary pulmonary pathology, these complications may result from atelectasis due to collapse of a lobe or lobes, loss of normal thoracic cavity recoil pressures, residual pleural space disease, pulmonary edema associated with sudden re-expansion of lung after collapse, and hypoventilation associated with pain or excessive sedation. Barotrauma from over-zealous mechanical ventilation may contribute to lung injury and potentially oxygen toxicity might lead to the development of airway reactivity. Additionally, changes in chest wall mechanics resulting from the incision at the surgical site may develop. However, atelectasis is perhaps the most common clinical problem and may develop from compression of the dependent lung, or absorption atelectasis may occur in oxygen supplemented patients. Atelectasis may lead to hypoxemia by altering ventilation-perfusion (V-Q) relationships.
During anesthetic recovery following a thoracotomy, dogs are often positioned in lateral recumbency and administered analgesics to prevent post-operative pain. Additionally, thoracic bandages are frequently placed to either protect the incision or to protect an indwelling thoracostomy tube. All these iatrogenic factors are hypothesized to combine to worsen lung function by reducing lung volume and therefore effective ventilation.
In dogs, more than in other species, there is a variation in the chest wall conformation. Dogs are considered “deep-chested,” if their chest height is much greater than their chest width, “normal-chested” if their chest height and width are approximately equal, and “barrel-chested” if their chest is wider than it is tall. Ventilation and perfusion are affected to some extent by gravity and body position, with dependent lung lobes being better ventilated.
Functional residual capacity is the volume of gas remaining in the lung at the end of a normal (not forced) expiration. Decreases in FRC will often parallel losses in pulmonary compliance, and represent loss of active lung volume. Peripheral airway collapse often occurs with decreases in FRC, and there may be resultant hypoxemia. A lower FRC in a given patient could be expected to accompany hypoxemia, as healthy lung has undergone atelectasis. Similarly, if routine procedures affect FRC in healthy dogs, simple inexpensive management strategies could improve outcome, such as shifting a dog’s body position or removing an excessively tight chest wrap. Although FRC is considered a static lung volume determined by lung versus chest wall compliance, it is in fact alterable by endogenous or exogenous factors. We hypothesized that procedures routinely employed during and after general anesthesia and thoracotomy in dogs would be sufficient to reduce FRC in the absence of surgery in healthy dogs; the magnitude of this decrease may be critical in some patients. The goal of this study was to determine the impact of routine management practices, including body position, sedation, and chest wrapping on functional residual capacity (FRC) in healthy non-operated deep-chested dogs.
Materials and methods
Dogs
Healthy, deep-chested dogs were recruited from the students and staff of the Tufts Cummings School of Veterinary Medicine. Three German shepherds, 2 Irish setters and 1 redbone coonhound were recruited. The mean weight [± standard deviation (s)] of the dogs was 31.5 ± 6.9 kg and the mean age was 5.5 ± 3.3 y. There were 3 spayed females, 2 castrated males, and 1 intact male. All dogs were assessed as healthy based upon a complete physical examination with no evidence of any cardiopulmonary disorder. Additionally, all dogs had a hematocrit, total protein, venous blood gas, and electrolyte panel evaluated, which were found to be within normal limits. All dogs were receiving heartworm prophylaxis. Dogs with a poor temperament or those that were over or underweight (Purina body condition score ≤ 3 or ≥ 6 on a scale of 1 to 9) were excluded. The study was approved by the Cummings School of Veterinary Medicine at Tufts University’s Institutional Animal Care and Use Committee.
Measurement of functional residual capacity
Functional residual capacity (FRC) was determined using helium dilution as previously described (7,8). Briefly, a known volume (4 × estimated tidal volume) and concentration of test gas [10% helium (He), 21% oxygen (O2), balance nitrogen (N)] was used to fill a reservoir bag connected to a 3-way stopcock and a tightly fitting low dead space face mask. Following expiration, the 3-way stopcock was turned to permit ventilation only between the dog and reservoir bag. Re-breathing occurred for 60 s and then following expiration, the 3-way stopcock was turned off to the reservoir bag and the patient returned to breathing room air. The final helium concentration was then determined. Additionally, the final carbon dioxide (CO2) content of the reservoir bag was determined. The ambient temperature, humidity and barometric pressure of the room were recorded. The initial and final concentrations of He and the were determined using specified analyzers final concentration of CO2 (Helium analyzer — PK Morgan, Chatham, Kent, United Kingdom; CO2 analyzer CD-3A — Amtek, Pittsburgh, Pennsylvania, USA). The dilution of He (assumed to be a non-exchangeable gas) gave a measure of FRC according to the following equation:
where: Hei represents the initial concentration and Hef concentration of He, and DSins the instrument dead space (7).
Measurements of FRC were made in duplicate; if greater than a 5% difference existed or if the dog did not complete the test due to sudden movement, a 3rd measurement was obtained. In addition, FRC was corrected for body weight (FRC/kg).
Experimental design
In order to mimic routine post-operative management, dogs were measured both in the standing body position [Time point 1], in right lateral recumbency (restrained for 10 min and then measured while still in right lateral recumbency), [Time Point 2] following application of a thoracic bandage by a single investigator (ER) to a standard intra-bandage pressure of 5 cm H2O in standing [Time Point 3] and in right lateral recumbency [Time Point 4]. The intra-bandage pressure was measured by placing an esophageal balloon connected to a water manometer within the bandage and then loosening or tightening the bandage as needed to reach 5 cm H2O. Next, the dogs were sedated with acepromazine [0.03 mg/kg (4 × 10−7 oz/lb)] IV and butorphanol [0.1 mg/kg (1.6 × 10−6 oz/lb )] IV and with the bandage still in place, measured in sternal recumbency [Time Point 5] and in right lateral recumbency [Time Point 6], and finally, the bandage was removed and the dogs were again measured in right lateral recumbency [Time Point 7] and finally in sternal recumbency [Time Point 8].
Statistical analysis
Data from each time point is shown as mean ± s or median ± range. The data from each time point was examined for normalcy with a one-sample Kolmogorov-Smirnov Test. Data were compared using analysis of variance (ANOVA) with repeated measures with a Fisher’s LSD post-hoc test to evaluate for differences between time points. As sternal and standing are not considered interchangeable, statistical analysis was not used to attempt to compare the time points, but rather data simply presented. A P-value of < 0.05 was considered significant. The average change in FRC (mL/kg) termed delta FRC, was calculated for each individual dog at each time point by determining the difference in FRC in mL/kg at the individual time points. The delta FRC/kg for each time point was determined for the entire group of dogs and compared using a Wilcoxon rank-sum test or t-test. Commercially available software was used for analysis (SPSS Version 13; SPSS, Chicago, Illinois, USA).
Results
All dogs tolerated the procedure well. The testing of each dog took approximately 2 h, to permit ample time for washout between measurements. All intra-time point measurements were at least 10 min apart. Dogs were measured standing for the unsedated time points and in sternal recumbency for the sedated time points. While sternal and standing are not interchangeable, the dogs were unwilling to stand while sedated, thus were measured in sternal recumbency. In one case, a triplicate measurement was made, as one of the setters (Dog 4), at the first measurement, struggled at the end of the recording, due to the dog’s unfamiliarity with the test equipment. The other samples from this dog, and the other dogs were obtained uneventfully.
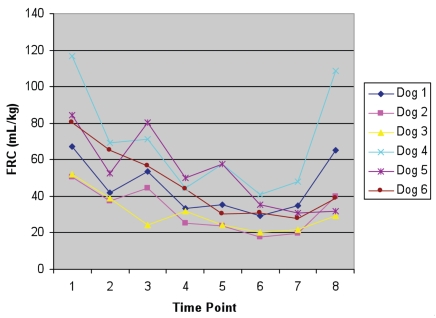
The results confirmed a significant difference between time points, with a repeated measures ANOVA P-value of 0.02. The mean FRC/kg for each time point is shown in Table I, while the mean FRC results from each individual dog at each time point is shown in Figure 1. The time points 4, 5, 6, and 7, corresponding respectively to lateral bandage no sedation, sternal bandage sedation, lateral bandage sedation and lateral no bandage sedation, were all significantly decreased from baseline (standing, no bandage, no sedation).
Table I.
The mean ± standard deviation (s) FRC/kg of 6 dogs. P-values are for differences in comparison with time point 1. The time points are in brackets in the left hand column and the P-values in brackets in the right hand column
| Position (time point) | FRC (mL/kg) [P =] |
|---|---|
| Standing, no bandage, no sedation [1] | 75.3 ± 23.8 |
| Lateral, no bandage, no sedation [2] | 50.8 ± 13.2 [0.10] |
| Standing, bandage, no sedation [3] | 55.2 ± 19.3 [0.21] |
| Lateral, bandage, no sedation [4] | 38.2 ± 9.1 [0.009]* |
| Sternal, bandage, sedation [5] | 38.2 ± 15.2 [0.009]* |
| Lateral, bandage, sedation [6] | 29.1 ± 8.6 [0.001]* |
| Lateral, no bandage, sedation [7] | 30.4 ± 10 [0.002]* |
| Sternal, no bandage, sedation [8] | 52 ± 29.5 [0.10] |
P < 0.05.
Figure 1.
The individual values for FRC (mL/kg) for each dog. The time points are 1 — standing, no bandage, no sedation; 2 — lateral, no bandage, no sedation; 3 — standing, bandage, no sedation; 4 — lateral, bandage, no sedation; 5 — sternal, bandage, sedation; 6 — lateral, bandage, sedation; 7 — lateral, no bandage, sedation; and 8 — sternal, no bandage, sedation.
Body position
Lateral recumbency lowered FRC in awake, unwrapped dogs by a median of 20.4 mL/kg (range: 13 to 47.0 mL/kg) although this was not significant. In awake dogs with chest wraps, lateral recumbency significantly lowered (P < 0.001) the FRC by a median of 19.9 mL/kg (range: −7.4 to 38 mL/kg), while in sedated dogs with chest wraps, the FRC/kg also decreased significantly (P = 0.003) with a median decrease in FRC in moving from sternal to lateral recumbency of 6.8 mL/kg (range: 1.7 to 20.8 mL/kg). Finally, in sedated unwrapped dogs, sternal recumbency resulted in a significant increase (P = 0.004) in FRC of 15.5 mL/kg (range 0.8 to 60.7 mL/kg).
Sedation
Sedation resulted in a lower FRC in dogs with and without chest wraps. For dogs with chest wraps, sedation results in an apparent decline in FRC when measured in the standing or sternal positions. In this case, the dogs were initially measured in a standing position and then became sternal after the sedation. Thus in the standing (unsedated/chest wrap) to sternal (sedated/chest wrap) dogs, there was median delta FRC of 19.8 mL/kg (range: 0.2 to 26.4 mL/kg). As inherent differences exist between sternal and standing, statistical analysis was not used to compare these results. For dogs in lateral recumbency with chest wraps, the addition of sedation resulted in a nonsignificant decrease in FRC (P = 0.06) with a median delta FRC of 9.6 mL/kg (range: 3.5 to 14 mL/kg).
In dogs without chest wraps, sedation alone resulted in a significant decrease in both sternal (P = 0.003) and lateral recumbency (P < 0.001). In sternal recumbency, the median delta FRC was 16.8 mL/kg (range: 2.0 to 52.6 mL/kg), while in lateral recumbency the median delta FRC was 19.2 mL/kg (range: 6.8 to 37.3 mL/kg).
Chest wrap
The addition of a chest wrap to a standing unsedated dog did not significantly lower FRC (P = 0.07). In right lateral recumbency, the application of a bandage to an unsedated dog resulted in a significant decrease in FRC (P < 0.001). The median decrease in FRC in standing dogs following the addition of a chest wrap was 18.6 mL/kg (range: 3.8 to 45.9 mL/kg) while in lateral dogs, the median decrease was 10.3 mL/kg (range: 2.5 to 24.3 mL/kg). In a sedated dog, the addition of a chest wrap did not significantly lower FRC in sternal (P = 0.079) or lateral recumbency (P = 0.382).
Discussion
The results of this study demonstrate the substantial impact of body position, chest wrapping, and sedation on FRC as measured by helium dilution in healthy deep-chested dogs. Functional residual capacity is a lung volume that reflects an “equilibrium volume,” meaning that the chest wall muscles are relaxed and the elastic recoil forces from both the lung parenchyma and chest wall are equal. The FRC (mL/kg) in the dogs in this study was substantially higher than the values that have been previously reported in beagles, and likely reflects breed-related conformational differences, as beagles are normal-chested (9).
Functional residual capacity was chosen as a sensitive marker of atelectasis and the loss of exchangeable lung volume. More clinically applicable tests, such as pulse oximetry could also have been used to evaluate lung function; however, pulse oximetry is relatively insensitive to slight changes in oxygenation. Functional residual capacity is dynamic, there is not a “normal value” for all patients, and the value that becomes important is the relationship of the FRC to that while the patient is normal (eupnic, in a normal body position). The consequences of a low FRC include a decrease in the static compliance of the lung, gas trapping, increased intrapulmonary shunt, and hypoxemia (10). A correlation has been identified in decreases in the FRC and increases in the Alveolar-arterial (A-a) gradient (11). Lower airway flow rates will also increase airway resistance (12). Conversely, in diseases accompanied by airway obstruction (such as, human asthmatics) there is an increase in FRC brought about by air-trapping and a lower FRC accompanies clinical improvement (13).
A critique of our methods reveals 2 assumptions. First, we assumed that FRC is determined by physical factors (lung versus chest wall compliance) and is not dynamically altered; it is equivalent to relaxation volume. In fact, FRC is a dynamic value and changes may occur over seconds to minutes, which usually reverse quickly. Thus variation in the measurement of FRC, whatever technique is used, is a biological feature of breathing. Hence, the variation in FRC measurements within and between patients and between treatments may reflect subtle changes in FRC controlled involuntarily by the dogs. The use of duplicate measures was an attempt to minimize the impact of such natural variations, but these may be important in conscious measures of FRC. Second, the technique of helium dilution is precise in subjects for whom inspired helium rapidly equilibrates between the conducting airways and alveoli. In the absence of airway obstruction or emphysema, therefore, helium dilution is an accurate measurement of FRC. We assumed for the purpose of this study, that chest wrapping would induce changes in lung volume due to restriction of the chest wall rather than airway closure, and past studies support this assumption (14–16). Alternative methods to measure FRC include computed tomography (17) and body plethysmography (18) but these are not practical in un-anesthetized dogs.
In this study, interventions used commonly following thoracotomy to support the surgical chest wound, resulted in reductions in FRC. Body position in particular had a significant effect on FRC. The dogs in this study were deep-chested (greater height versus width), and it is possible that the effect would be lessened in normal (for example, Labrador retriever) or barrel-chested (for example, Pug) dogs. In fact, one study of mixed breed dogs anesthetized with thiopental sodium showed no effect of body position (19). However in horses, lateral recumbency was associated with a significant decline in FRC (20). Body position studies in awake large animals are uncommon due to the inherent challenges in subject positioning. This study was compromised by the inability to measure sedated dogs in a standing position. Biomechanics of standing and sternal body position are not equivalent, and as such, results obtained in one position may not be directed compared with the other.
Body position has previously been evaluated in healthy people, with the highest FRC identified in a sitting (standing) position and the lowest in a supine (face up) position (21). Body position has been examined with increased enthusiasm in critically ill people with acute lung injury in recent years. In patients with acute lung injury, the rotation of the patient from the supine to the prone (face down) positions, has resulted in significant improvement in ventilatory variables (22,23). The mechanism for the improvement in ventilatory variable in acute lung injury patient reflects the recruitment of de-recruited lung and as well as decreased intra-pulmonary shunt; increased FRC will accompany this increase.
The profound effect on FRC of lateral recumbency is multifactorial, and includes a decrease in chest wall recoil, smaller lung volumes, atelectasis, and increased pulmonary recoil. Conformation and body weight are also important. Large animal species, particularly ruminants, develop hypoxemia when placed in lateral recumbency, even unsedated (24,25). The dogs in this study were evaluated in right lateral recumbency. This was chosen as the lung mass on the right side is slightly larger than on the left side in dogs; however, it is possible that a similar effect would have been seen had left lateral recumbency been chosen. Additionally, these pet dogs were not positioned in dorsal recumbency, so the effect of that body position remains unknown in this group of dogs.
Sedation also had a significant impact on the dogs in this study. General anesthesia is widely known to affect FRC (26). In humans, the FRC is reduced by approximately 0.5 L by general anesthesia (3). One previous study in experimental dogs, found no change in FRC associated with anesthesia induced by thiopental sodium while another study found a decrease in FRC of approximately 15% in sheep anesthetized with halothane and nitrous oxide (19,27). One study evaluating the effects of general anesthesia in dogs identified a significant decrease in static compliance. This decrease was prevented with periodic full lung inflations (sighs) and the compliance decrease was hypothesized to be secondary to atelectasis and decrease in FRC (28). In contrast, with conscious sedation, the effect on FRC appears dependent upon the agent used. A decrease of approximately 13% was found in ponies sedated with 0.04 mg/kg of acepromazine IM (26). In women given 10 mg of morphine, a significant decrease in FRC occurred, while in contrast, in children sedated with ketamine, no significant change in FRC was identified (10,29). Children, as well as other baby mammals such as puppies, have relatively compliant chest walls; this would have been expected to help maintain FRC. Additionally, ketamine is a dissociative anesthetic as well as bronchodilator, these factors may have also supported the maintenance of FRC. The mechanism of the impact on FRC by sedation and anesthesia is likely multifactorial. The largest contributor appears to be lower tidal volume and shallower breathing that accompanies anesthesia. Muscle relaxation within the thoracic wall will further reduce chest wall recoil. This effect is observed in ventilated patients as well as spontaneously breathing patients; thus, independent effects of the anesthetic agent or loss of recruitment maneuvers (sighs) may also be factors. Clearly, in dogs following a thoracotomy, analgesics are warranted. However, the type of agent(s) used should be considered carefully. One drawback of our study design was that the sedation was administered only once, and dogs may have had relatively less sedation by the end of the study than shortly after receiving the agent.
Finally, chest wrapping results in significant decreases in FRC in laterally recumbent unsedated dogs, although not in dogs in sternal or standing or with sedation. In humans, the application of thoracic wraps has been associated with lower FRC while in horses the application of a tight girth has been documented to affect pulmonary function (14–16). The mechanism of loss of FRC in a thoracic wrap is likely through decreased elastic recoil of the thoracic cavity, which then would permit increased static recoil within the lung parenchyma and increased collapse.
Importantly, FRC is normally a dynamic value, and decreases in FRC do not necessarily indicate abnormal lung function. However, excessive decrease in FRC may be associated with pulmonary dysfunction. For example, normal sleep is associated with a loss of FRC in both normal and asthmatic people, but the FRC loss in asthmatics is greater and may contribute to nocturnal dyspnea in some individuals (30).
A potential limitation in this study includes the lack of simultaneous spirometric measurements to exclude inadequate equilibration of helium as a source of measurement error. Helium dilution relies upon adequate ventilation in order for equilibration to occur. Dogs in this study appeared to be ventilating adequately and the values obtained showed a similar level of reproducibility. However, future studies could attempt to measure the minute ventilation or to evaluate the differing equilibration times. In this study, 60 seconds was used for equilibration, which is longer than reported in previous canine studies and longer than that recommended for humans (7).
Not all dogs in the study responded with equivalent alterations in the measured FRC at different time points. Dog 4, the relatively non-compliant Irish setter, in particular, had more variable responses to different interventions, than dogs 2 and 3, which were calmer dogs. Relative “voluntary” contributions to FRC based on chest wall tone and recruitment maneuvers (voluntary signs) will also impact volumes, and may account for individual patient differences.
This study demonstrates that body position, sedation, and chest wrapping resulted in a significant decrease in FRC as measured by helium dilution in healthy dogs. The impact of these interventions in dogs with pulmonary disease or following a thoracotomy is unknown, but may be important and warrants further investigation. Future studies that define the alterations in gas exchange, spirometry, and regional effects on lung mechanics associated with these perturbations are also warranted.
References
- 1.Stobie D, Caywood DD, Rozanski EA, et al. Evaluation of pulmonary function and analgesia after intercostal thoracotomy and use of morphine administered intramuscularly or intrapleurally and bupivacaine administered intrapleurally. Am J Vet Res. 1995;56:1098–1109. [PubMed] [Google Scholar]
- 2.Berg RJ, Orton EC. Pulmonary function in dogs after intercostal thoracotomy: Comparison of morphine, oxymorphone, and selective intercostal nerve block. Am J Vet Res. 1986;47:471–474. [PubMed] [Google Scholar]
- 3.Hedenstierna G, Edmark L. The effects of anesthesia and muscle paralysis on the respiratory system. Intensive Care Med. 2005;31:1327–1335. doi: 10.1007/s00134-005-2761-7. [DOI] [PubMed] [Google Scholar]
- 4.Richardson J, Sabanathan S, Shah R. Post thoracotomy spirometric lung function: The effect of analgesia. A review. J Cardiovasc Surg (Torino) 1999;40:445–456. [PubMed] [Google Scholar]
- 5.Brainard BM, Alwood AJ, Kushner LI, et al. Postoperative pulmonary complications in dogs undergoing laparotomy: Anesthetic and perioperative factors. J Vet Emerg Crit Care. 2006;16:184–191. [Google Scholar]
- 6.Furrer M, Rechsteiner R, Eigenmann V, et al. Thoracotomy and thoracoscopy: Postoperative pulmonary function, pain and chest wall complaints. Eur J Cardiothorac Surg. 1997;12:82–87. doi: 10.1016/s1010-7940(97)00105-x. [DOI] [PubMed] [Google Scholar]
- 7.Amis TC, Jones HA. Measurement of functional residual capacity and pulmonary carbon monoxide uptake in conscious greyhounds. Am J Vet Res. 1984;45:1447–1450. [PubMed] [Google Scholar]
- 8.Bedenice D, Rozanski E, Bach J, et al. Canine awake head-out plethysmography (HOP): Characterization of external resistive loading and spontaneous laryngeal paralysis. Respir Physio Neurobiol. 2006;151:61–73. doi: 10.1016/j.resp.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Dubin S, Westcott RJ. Functional residual capacity of normal unanethetized beagle dogs. Am J Vet Res. 1969;30:2027–2030. [PubMed] [Google Scholar]
- 10.Shulman D, Beardsmore CS, Aronson HB, et al. The effect of ketamine on the functional residual capacity in young children. Anesthesiology. 1985;62:551–556. doi: 10.1097/00000542-198505000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hickey RF, Visick D, Fairley HB, et al. Effects of halothane anesthesia on functional residual capacity and alveolar-arterial oxygen difference. Anesthesiology. 1974;38:20–24. doi: 10.1097/00000542-197301000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Briscoe WA, Dubois AB. The relationship between airway resistance, airway conductance, and lung volumes in subjects of different ages and body size. J Clin Invest. 1958;37:1279–1285. doi: 10.1172/JCI103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121:1042–1050. doi: 10.1378/chest.121.4.1042. [DOI] [PubMed] [Google Scholar]
- 14.De Troyer A. Mechanics of the chest wall during restrictive thoracic strapping. Respiration. 1980;39:241–250. doi: 10.1159/000194223. [DOI] [PubMed] [Google Scholar]
- 15.van Noord JA, Demedts M, Clement J, et al. Effect of rib cage and abdominal restriction on total respiratory resistance and reactance. J Appl Physiol. 1986;61:1736–1740. doi: 10.1152/jappl.1986.61.5.1736. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman AM, Swanson LG, Bruns SJ, et al. Effects of tension of the girth strap on respiratory system mechanics in horses at rest and during hyperpnea induced by administration of lobeline hydrochloride. Am J Vet Res. 2005;66:1167–1174. doi: 10.2460/ajvr.2005.66.1167. [DOI] [PubMed] [Google Scholar]
- 17.Krayer S, Rehder K, Beck KC, et al. Quantification of thoracic volumes by three-dimensional imaging. J Appl Physiol. 1987;62:591–598. doi: 10.1152/jappl.1987.62.2.591. [DOI] [PubMed] [Google Scholar]
- 18.Kendrick AH. Comparison of methods of measuring static lung volumes. Mondali Arch Chest Dis. 1996;51:431–439. [PubMed] [Google Scholar]
- 19.Lai YL, Rodarte JR, Hyatt RE. Respiratory mechanics in recumbent dogs anesthetized with thiopental sodium. J Appl Physiol: Respirat, Environ Exercise Physiol. 1979;46:716–720. doi: 10.1152/jappl.1979.46.4.716. [DOI] [PubMed] [Google Scholar]
- 20.Sorenson PR, Robinson NE. Postural effects on lung volumes and asynchronous ventilation in anesthetized horses. J Appl Physiol. 1980;48:97–103. doi: 10.1152/jappl.1980.48.1.97. [DOI] [PubMed] [Google Scholar]
- 21.Lumb AB, Nunn JF. Respiratory function and ribcage contribution to ventilation in body positions commonly used during anesthesia. Anesth Analg. 1991;73:422–426. doi: 10.1213/00000539-199110000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Voggenreiter G, Aufmkolk M, Stiletto RJ, et al. Prone positioning improves oxygenation in post-traumatic lung injury — A prospective randomized trial. J Trauma. 2005;59:333–343. doi: 10.1097/01.ta.0000179952.95921.49. [DOI] [PubMed] [Google Scholar]
- 23.Mancebo J, Fernandez R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173:1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 24.Wagner AE, Muir WM, Grospitch BJ. Cardiopulmonary effects of position in conscious cattle. Am J Vet Res. 1990;51:7–10. [PubMed] [Google Scholar]
- 25.Hall LW. Cardiovascular and pulmonary effects of recumbency in two conscious ponies. Equine Vet J. 1984;16:89–92. doi: 10.1111/j.2042-3306.1984.tb01866.x. [DOI] [PubMed] [Google Scholar]
- 26.McDonnell WN, Hall LW. Functional residual capacity in conscious and anesthetized horses. Br J Anaesth. 1974;46:802–803. [Google Scholar]
- 27.Dueck R, Rathbun M, Greenburg AG. Lung volume and VA/Q distribution response to intravenous versus inhalation anesthesia in sheep. Anesthesiology. 1984;61:55–65. [PubMed] [Google Scholar]
- 28.Corcoran BM, Abercromby RH. Effects of general anesthesia on static respiratory compliance in dogs. Vet Rec. 1989;125:450–453. doi: 10.1136/vr.125.18.450. [DOI] [PubMed] [Google Scholar]
- 29.Tantucci C, Paoletti F, Bruni B, et al. Acute respiratory effects of sublingual buprenorphine: Comparison with intramuscular morphine. Int J Clin Pharmacol Ther Toxicol. 1992;30:202–207. [PubMed] [Google Scholar]
- 30.Ballard RD, Irvin CG, Martin RJ, et al. Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physio. 1990;68:2034–2041. doi: 10.1152/jappl.1990.68.5.2034. [DOI] [PubMed] [Google Scholar]