Abstract
In this study, an unencapsulated Streptococcus suis mutant was used to investigate the pleiotropic effects resulting from capsule loss. The capsule deficient mutant of S. suis acquired a biofilm-positive phenotype, which was associated with significantly increased cell surface hydrophobicity. Cell-associated fibrinogen-binding and chymotrypsin-like activities were decreased in the unencapsulated mutant. The mutant did not differ significantly from the encapsulated parent strain for minimal inhibitory concentrations to penicillin G, ampicillin, and tetracycline. However, while the encapsulated strain was highly resistant to the bactericidal action of penicillin G and ampicillin, the unencapsulated mutant was approximately 60-fold more sensitive. Compared with the parent strain, the unencapsulated mutant induced a much higher inflammatory response in monocyte-derived macrophages resulting in an increased secretion of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8. The capsule appears to hinder important adhesins or hydrophobic molecules that mediate biofilm formation, as well as cell wall components capable of stimulating immune cells.
Résumé
Dans la présente étude, un mutant non-capsulé de Streptococcus suis a été utilisé afin d’examiner les effets pléiotropiques résultant de la perte de la capsule. Le mutant déficient de S. suis a acquis un phénotype positif pour le biofilm, qui était associé avec une augmentation significative de l’hydrophobicité de surface. La liaison du fibrinogène et l’activité apparentée à celle de la chymotrypsine étaient diminuées chez le mutant non-capsulé. Il n’y avait pas de différence significative entre le mutant et le parent capsulé en ce qui a trait aux concentrations minimales inhibitrices de la pénicilline G, de l’ampicilline et de la tétracycline. Toutefois, alors que la souche capsulée était très résistante à l’action bactéricide de la pénicilline G et de l’ampicilline, le mutant non-capsulé était approximativement 60 fois plus sensible. Comparativement à la souche parentale, le mutant non-capsulé a induit une réponse inflammatoire beaucoup plus marquée sur les macrophages dérivés des monocytes, ce qui a entraîné une sécrétion augmentée du facteur nécrosant des tumeurs (TNF)-α, d’interleukine (IL)-1β, IL-6 et IL-8. La capsule semble interférer avec des adhésines importantes ou des molécules hydrophobes qui sont impliquées dans la formation de biofilm, ainsi que des composantes de la paroi cellulaire capables de stimuler les cellules du système immunitaire.
(Traduit par Docteur Serge Messier)
Streptococcus suis is an important swine pathogen worldwide that causes meningitis, septicemia, arthritis, and endocarditis (1). This bacterium can also affect humans who have had close contact with sick or carrier pigs or with their derived-products (2). Although 35 serotypes are known, serotype 2 is most commonly associated with infections in swine and humans (1). In recent years, various potential virulence factors produced by S. suis have been described, including muramidase-released protein, extracellular protein factor, suilysin, adhesins, and capsule (3). Among the potential virulence determinants identified, the polysaccharide capsule appears to be critical for the pathogenicity of S. suis. Indeed, unencapsulated mutants were shown to be avirulent in mice and in 2 different swine models of infection (4,5). Charland et al (6) reported that an unen-capsulated mutant of S. suis was more susceptible to phagocytosis by macrophages compared with the parent strain. The capsular material of S. suis serotype 2 contains 5 different sugars: rhamnose, galactose, glucose, N-acetylglucosamine, and N-acetyl neuraminic acid (sialic acid), the latter being the 3rd most important in amounts (7). Sialic acid is well known as an antiphagocytic factor for many bacterial species through inhibition of the activation of the alternative complement pathway (8). For a bacterium, the presence of a capsule may provide particular cell surface properties while interfering physically with other cell surface components. The aim of this study was to investigate the pleiotropic effects associated with capsule loss, on selected biological properties of S. suis.
Streptococcus suis S735, a reference virulent serotype 2 strain of European phenotype, and the unencapsulated mutant BD101 were routinely grown in Todd Hewitt broth (THB; BBL Microbiology Systems, Cockeysville, Massachusetts, USA) at 37°C under aerobiosis. The mutant BD101 was constructed in a previous study and was impaired in capsule production as a result of the deletion of the cognate promoter of the aro operon, this in turn resulted in the abolishment of aroA, aroK, pheA, and orf10 expression (9). Mutant BD101 was devoid of capsular sialic acid, the absence of capsule was confirmed by electron microscopy (9). Although the exact explanation for the absence of capsule in the aro deficient mutant is still speculative, it has been proposed that it may be related to abolishment of the expression of orf10. This gene is predicted to belong to the LytR-cpsA-psr family of transcriptional regulators; members of this family have been shown to act as positive regulators of capsule expression in other species of streptococci (10).
The culture broth medium used to investigate biofilm formation by S. suis S735 and the unencapsulated mutant BD101 contained 0.5% glucose, 2% peptone (Proteose Peptone No. 3; Difco, Detroit, Michigan, USA), 0.3% K2HPO4, 0.2% KH2PO4, 0.01% MgSO4 • 7H2O, 0.002% MnSO4 • 6H2O, and 0.5% NaCl. This medium contained sufficient aromatic amino acids required for growth of mutant BD101. Biofilm formation was measured in polystyrene microtiter plates and crystal violet staining, as previously described (11). Assays were run in triplicate and the means ± standard deviations (s) of 2 independent experiments were calculated. The structural architecture of the S. suis (S735, mutant BD101) biofilm was examined by scanning electron microscopy. Streptococcus suis was inoculated in 35-mm dishes (Nunc, Denmark) containing a 10.5 × 22 mm plastic coverslip (Thermanox; Nunc). After 24 h of incubation, medium and free-floating bacteria were removed. The biofilms formed on each coverslip were incubated overnight in fixation buffer (4% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2), washed with 0.1 M cacodylate buffer (3 × 20 min), and post-fixed for 90 min at room temperature in 1% osmic acid containing 2 mM potassium ferrocyanide and 6% sucrose in cacodylate buffer. Samples were dehydrated through a graded series of ethanol (50%, 70%, 95%, and 100%), critical point dried, gold sputtered, and examined using a scanning electron microscope (JEOL JSM6360LV; JEOL, Tokyo, Japan) operating at 30 kV.
The relative cell surface hydrophobicity of S. suis S735 and the unencapsulated mutant BD101 was determined by measuring their absorption to n-hexadecane according to the procedure described by Rosenberg et al (12). To compare lectin-binding activity, 7 fluorescein isothiocyanate (FITC)-labeled lectins (E-Y Laboratories, San Mateo, California, USA) were used: Conavalia ensiformis agglutinin (Con A — specific for mannose and glucose residues), Dolichos biflorus agglutinin (DBA — specific for N-acetyl galactosamine residues), Maclura pomifera agglutinin (MPA — specific for N-acetyl galactosamine residues), peanut agglutinin (PNA) from Arachis hypogaea (specific for galactose residues), soybean agglutinin (SBA) from Glycine max (specific for N-acetyl galactosamine and galactose residues), Ulex europaeus agglutinin (UEA I — specific for fucose residues), and wheat germ agglutinin (WGA) (Triticum vulgaris, specific for N-acetyl glucosamine and sialic acid residues). Powdered lectins were dissolved, to a concentration of 1 mg/mL, in phosphate buffered saline (PBS) solution and stored at −20°C until used. Bacterial cells were harvested from THB agar plates, washed in PBS and suspended in diluted lectin solutions (40 μg/mL) at an optical density (OD) of 0.5 at 660 nm. Following incubation at room temperature under darkness for 60 min, bacteria were washed 3 times in PBS and suspended in the initial volume. Cell-bound lectins were quantified using a fluorometer with an excitation wavelength of 490 nm and an emission wavelength of 520 nm. These assays were run in triplicate and the means ± s of 2 independent experiments were calculated.
The fibrinogen-binding activity of S. suis S735 and the unencapsulated mutant BD101 were quantified using an enzyme-linked immunosorbent assay (ELISA) according to the procedure described by Bonifait et al (13). Cell-associated chymotrypsin-like activitiy was determined, as previously described, using N-succinyl-Ala-Ala-Pro-Phe-pNa as the substrate (14). Assays for fibrinogen-binding and chymotrypsin-like activities were run in triplicate and the means ± s of 2 independent experiments were calculated.
The minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations (MBC) of planktonic cultures of S. suis S735 and the capsule deficient mutant BD101 to penicillin G, ampicillin, and tetracycline were determined using a microdilution procedure. Briefly, 2-fold serial dilutions (5000 ng/mL to 0.6 ng/mL) of antibiotics were prepared in THB. The wells of a microtiter plate, each containing 100 μL of medium, were inoculated with 100 μL of an overnight culture of S. suis diluted in fresh THB to obtain an OD660 of 0.2 (equivalent CFUs for S735 and BD101). The plate was then incubated at 37°C for 24 h. The MICs were the lowest concentrations of antibiotic, for which no significant increase in OD660 was noted following inoculation and incubation. To determine the MBCs, 10 μL of culture was recovered from wells showing no visible growth and spread on THB agar plates. The MBCs were the lowest concentrations of antibiotic at which no colonies grew on THB plates. Three independent experiments were performed.
U937 cells (ATCC CRL-1593.2), a monoblastic leukemia cell line, were cultivated at 37°C in a 5% CO2 atmosphere in RPMI-1640 medium (HyClone Laboratories, Logan, Utah, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (RPMI-FBS) and 100 μg/mL of penicillin-streptomycin. Monocytes (2 × 105 cells/mL) were incubated in RPMI-FBS containing 10 ng/mL of phorbol myristic acid (PMA) for 48 h to induce differentiation into adherent macrophage-like cells. Following the PMA treatment, the medium was replaced with fresh medium and the differentiated cells were incubated for an additional 24 h prior to use. Adherent monocyte-derived macrophages were washed and suspended in RPMI with 1% heat-inactivated FBS without antibiotics at a density of 1 × 106 cells/ mL and incubated in 6-well plates (2 × 106 cells/well in 2 mL) at 37°C in a 5% CO2 atmosphere for 2 h prior stimulation. Streptococcus suis cells (S735 and mutant BD101) were harvested by centrifugation at 11 000 × g for 10 min and suspended in RPMI medium to a concentration of 1 × 109 bacteria/mL, as determined using a Petroff-Hausser counting chamber. The bacterial suspensions were added to the monocyte-derived macrophages to obtain a multiplicity of infection (MOI) of 1, 10, 50, and 100. After 24 h of incubation, the culture medium supernatants were collected and stored at −20°C until used. Monocyte-derived macrophages were also stimulated with a cell wall fraction (10, 25, and 50 μg/mL) of S. suis, prepared as previously described (15). Commercial ELISA kits (R&D Systems, Minneapolis, Minnesota, USA) were used to quantify interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-8 (IL-8) concentrations in the cell-free culture supernatants according to the manufacturer’s protocols. The absorbance at 450 nm (A450) was read using a microplate reader with the wavelength correction set at 550 nm. The rated sensitivities of the commercial ELISA kits were 3.9 pg/mL for IL-1β, 15.6 pg/mL for TNF-α, 9.3 pg/mL for IL-6, and 31.2 pg/mL for IL-8. All stimulation assays were run in triplicate and the means ± s were calculated.
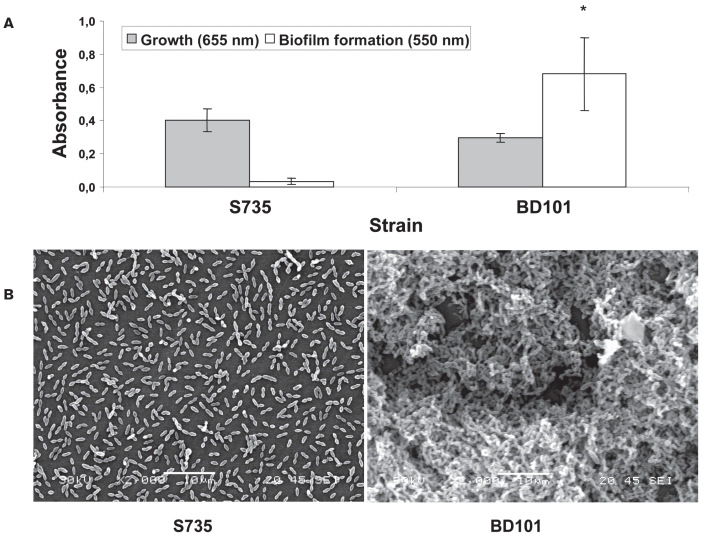
The effect of capsule loss on the capacity of S. suis to form biofilms was evaluated using a polystyrene microtiter plate assay. As shown in Figure 1A, the parent strain S735 was unable to form biofilm, whereas the unencapsulated mutant BD101 acquired the capacity to form a thick biofilm that stained with crystal violet. Biofilm formation was not related to a more pronounced growth of the capsule-deficient mutant since the growth of both strains was comparable, as evaluated by measuring the A660. An examination by scanning microscope confirmed the results obtained with the microtiter plate assay (Figure 1B). Individual bacteria and short chains of S. suis S735 were observed attached to the polystyrene surface but were rarely bound to each other. On the other hand, aggregates and microcolonies of the capsule deficient mutant (BD101) almost completely covered the surface of the polystyrene support. The capacity of the unencapsulated mutant BD101 to form a biofilm is in agreement with previous studies reporting that capsule inhibited the adherence of S. suis to epithelial (16) and endothelial cells (17). Interestingly, capsules produced by Neisseria meningitidis (18) and Escherichia coli (19) have also been reported to prevent biofilm formation. The inhibition of bacterial attachment to mammalian cells and of biofilm formation by the capsule of S. suis is likely related to the masking of adhesins and other structures, making them non-functional. Infections by such pathogens would thus require modulation of capsule production allowing transitions from highly encapsulated states where bacteria could resist host immune system to lower unencapsulated states where bacteria could form biofilm and adhere to host cells.
Figure 1.
Biofilm formation by Streptococcus suis S735 and its unencapsulated mutant BD101. (A) Crystal violet-stained biofilm following growth in polystyrene microplate. (B) Scanning electron micrographs of S. suis biofilms formed after 24 h of growth. * indicates a significant difference (P < 0.01) with the Student’s t-test between S. suis S735 and its unencapsulated mutant BD101.
The polysaccharide capsule of S. suis likely provides particular properties to bacterial cells. First, capsule loss resulted in a significant modification of the cell surface hydrophobicity. The hydrophobicity of the unencapsulated mutant was 90.7% ± 1.6, whereas it was 11.2% ± 3.2 for the parent strain (Table I). This suggests that hydrophilic capsule hinders more-hydrophobic structures or components important for biofilm formation by S. suis. The possibility that hydrophobic interactions may be important for biofilm formation by S. suis is in agreement with the study by Yi et al (18), who reported a direct correlation between biofilm formation by Neisseria meningitidis and cell surface hydrophobicity.
Table I.
Comparative analyses of Streptococcus suis S735 and its unencapsulated mutant BD101 for lectin-binding, fibrinogen-binding, and chymotrypsin-like activities
| Activity |
S. suis |
% change (parent versus mutant) |
|
|---|---|---|---|
| S735 | BD101 | ||
| Lectin-binding activity (RFU) | |||
| WGA | 1859 ± 54 | 431 ± 46 | −77a |
| SBA | 743 ± 40 | 658 ± 45 | −11 |
| MPA | 613 ± 19 | 572 ± 23 | −7 |
| Fibrinogen-binding activity (ng bound/well) | 193 ± 10 | 136 ± 7 | −30a |
| Chymotrypsin-like activity (A405) | 1.25 ± 0.07 | 0.86 ± 0.04 | −31a |
RFU — Relative fluorescence units; WGA — wheat germ agglutinin from Triticum vulgaris, specific for N-acetyl glucosamine and sialic acid residues; SBA — soybean agglutinin from Glycine max, specific for N-acetyl galactosamine and galactose residues; MPA — Maclura pomifera agglutinin, specific for N-acetyl galactosamine residues.
Significant difference (P < 0.01) with the Student’s t-test between S. suis S735 and its unencapsulated mutant BD101.
The cell surface of both S. suis S735 and the mutant BD101 was further characterized by investigating the binding of fluorescein-labeled lectins. Among the 7 lectins tested, positive binding to S. suis S735 cell surface was observed with WGA and to a lesser extent with SBA and MPA (Table I). The unencapsulated mutant showed a significant reduction (77%) in binding of WGA, while it was only slightly affected for SBA and MPA binding. This decreased capacity to bind WGA, a lectin specific for N-acetyl glucosamine and sialic acid residues, is consistent with the fact that sialic acid is a major constituent of the S. suis capsule.
The effect of capsule loss on 2 cell-associated properties, namely fibrinogen-binding activity and chymotrypsin-like activity, was then investigated. Using a fibrinogen-binding microplate assay, both the parental strain and the capsule deficient mutant were found to bind human fibrinogen on their surface (Table I). The parent strain possessed significantly higher fibrinogen-binding activity than the mutant BD101. Regarding the cell-associated chymotrypsin-like activity, the parent strain also showed significantly higher activity compared with the unencapsulated mutant (Table I). This suggests that capsule expression may help stabilize these cell surface activities.
Table II shows the MICs and MBCs for S. suis S735 and the mutant BD101 to penicillin G, ampicillin, and tetracycline. Penicillin G and ampicillin were found to be much more effective than tetracycline on S. suis S735, as demonstrated by the low MICs. Compared with the parent strain, MICs for the unencapsulated mutant were either not modified (ampicillin) or one dilution lower (penicillin G and tetracycline). The MBCs for the capsule deficient mutant to all 3 antibiotics were found to be markedly decreased. More specifically, MBCs to penicillin G and ampicillin were approximately 60-fold lower for the mutant BD101 compared to the parent strain. This may be related to the fact that the internal concentration of antibiotics required to kill bacteria are more easily reached when the capsule is absent in S. suis. It is also possible that the capsule stabilizes the plasma membrane and protecting it from rupture and bacterial death.
Table II.
Minimal inhibitory and minimal bactericidal concentrations of Streptococcus suis S735 and its unencapsulated mutant BD101 to penicillin G, ampicillin, and tetracycline. Values are representative of 3 independent experiments
| Penicillin G (ng/mL) |
Ampicillin (ng/mL) |
Tetracycline (ng/mL) |
||||
|---|---|---|---|---|---|---|
| Strain | MIC | MBC | MIC | MBC | MIC | MBC |
| S. suis S735 | 20 | 1250 | 20 | 2500 | 312 | > 5000 |
| S. suis BD101 | 10 | 20 | 20 | 40 | 156 | 1250 |
MIC — minimal inhibitory concentrations; MBC — minimal bactericidal concentrations.
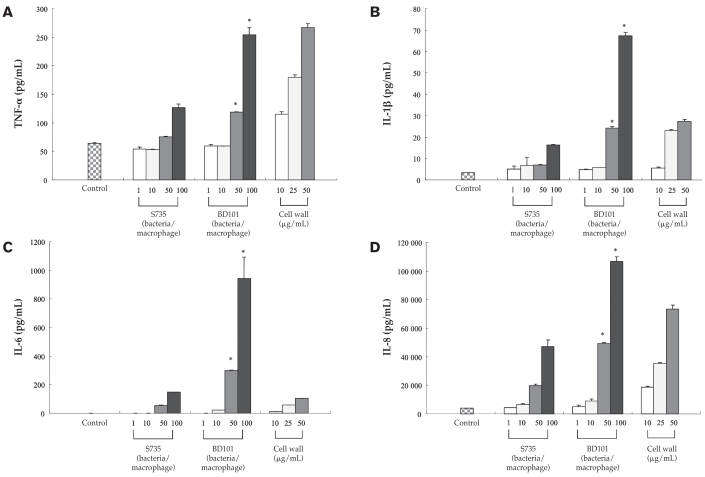
The secretion of TNF-α, IL-1β, IL-6, and IL-8 by monocyte-derived macrophages stimulated with whole cells of either S. suis S735 or the mutant BD101 is reported in Figure 2. For all 4 cytokines, stimulation of macrophages with the parental strain induced a dose-dependent increased secretion. In general, MOIs of 50 and 100 were necessary to induce a marked cytokine response. For all cytokines, the response induced by the mutant BD101 was significantly higher (P < 0.01) at MOIs of 50 and 100 compared with that induced by S. suis S735. More specifically, at an MOI of 100, the secretion of TNF-α, IL-1β, IL-6, and IL-8 by monocyte-derived macrophages was increased 2-, 4-, 5-, and 2-fold, respectively, compared with the parent strain. A cell wall preparation of S. suis was also found to induce dose-dependent TNF-α, IL-1β, IL-8, and, to a lesser extent, IL-6 responses in monocyte-derived macrophages (Figure 2). The above results suggest that the absence of capsule uncovers the components, namely the cell wall, that triggers the monocyte-derived macrophages. This is in agreement with the results found by Graveline et al (20), who showed that an unencapsulated mutant (B218) derived from a S. suis virulent field isolate induced significantly higher levels of TNF-α and IL-1β in the THP-1 human monocytic cell line. Although the biological significance of this observation is unclear, 2 consequences are possible. One, the capsule of S. suis may suppress the host’s immunological response, a critical step at the early step of infection to prevent the bacteria from growing following by severe septicemia and death. Two, the loss of capsule in S. suis may be associated with an exaggerated immunological response induced by cell wall components, leading to uncontrolled inflammatory reactions. This phenomenon can increase the permeability of the blood-brain barrier, of which microvascular endothelial cells are the main constituents. An increased permeability of the blood-brain barrier facilitates the migration of bacteria and leukocytes that may promote the development of an inflammatory exudate and a severe disease outcome.
Figure 2.
Secretion of tumor necrosis factor (TNF)-α (panel A), interleukin (IL)-1β (panel B), IL-6 (panel C), and IL-8 (panel D) by monocyte-derived macrophages infected with whole cells of Streptococcus suis S735 and its capsule deficient mutant BD101 at multiplicities of infection (MOI) of 1, 10, 50, and 100. Monocyte-derived macrophages were also stimulated with a cell wall preparation (10, 25, and 50 μg/mL) of S. suis. * indicates a significant difference (P < 0.01) with the Student’s t-test between S. suis S735 and its unencapsulated mutant BD101.
In conclusion, this study showed that loss of capsule in S. suis is associated with several pleiotropic effects. Capsule appears to hinder important adhesins or hydrophobic molecules that mediate biofilm formation, as well as cell wall components capable of stimulating immune cells. These observations suggest that future studies to better understand how the capsule expression is regulated in vivo will also contribute to a better knowledge of the pathogenesis of S. suis.
Acknowledgments
The authors thank Marie-Pier Levasseur for her technical assistance. This study was supported by Natural Sciences and Engineering Research Council of Canada (NSERC).
References
- 1.Higgins R, Gottschalk M. Streptococcal Diseases. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of Swine. 9th ed. Ames, Iowa: Iowa Univ Pr; 2005. pp. 769–783. [Google Scholar]
- 2.Huang YT, Teng LJ, Ho SW, Hsueh PR. Streptococcus suis infection. J Microbiol Immunol Infect. 2005;38:306–313. [PubMed] [Google Scholar]
- 3.Gottschalk M, Segura M. The pathogenesis of the meningitis caused by Streptococcus suis: The unresolved questions. Vet Microbiol. 2000;76:259–272. doi: 10.1016/s0378-1135(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 4.Charland N, Harel J, Kobisch M, Lacasse S, Gottschalk M. Streptococcus suis serotype 2 mutants deficient in capsular expression. Microbiology. 1998;144:325–332. doi: 10.1099/00221287-144-2-325. [DOI] [PubMed] [Google Scholar]
- 5.Smith HE, Damman M, van der Velde J, et al. Identification and characterization of the cps locus of Streptococcus suis serotype 2: The capsule protects against phagocytosis and is an important virulence factor. Infect Immun. 1999;67:1750–1756. doi: 10.1128/iai.67.4.1750-1756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charland N, Kobisch M, Martineau-Doizé B, Jacques M, Gottschalk M. Role of capsular sialic acid in virulence and resistance to phagocytosis of Streptococcus suis capsular type 2. FEMS Immunol Med Microbiol. 1996;14:195–203. doi: 10.1111/j.1574-695X.1996.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 7.Elliot SD, Tai J. The type-specific polysaccharides of Streptococcus suis. J Exp Med. 1978;148:1699–1704. doi: 10.1084/jem.148.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards MS, Kasper DL, Jennings HJ, Baker CJ, Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982;128:1278–1283. [PubMed] [Google Scholar]
- 9.Fittipaldi N, Harel J, D’amours B, Lacouture S, Kobisch M, Gottschalk M. Potential use of an unencapsulated and aromatic amino acid-auxotrophic Streptococcus suis mutant as a live attenuated vaccine in swine. Vaccine. 2007;25:3524–3535. doi: 10.1016/j.vaccine.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 10.Cieslewicz MJ, Kasper DL, Wang Y, Wessels MR. Functional analysis in type Ia group B Streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of streptococci. J Biol Chem. 2001;276:139–146. doi: 10.1074/jbc.M005702200. [DOI] [PubMed] [Google Scholar]
- 11.Grenier D, Grignon L, Gottschalk M. Characterisation of bio-film formation by a Streptococcus suis meningitis isolate. Vet J. 2009;179:292–295. doi: 10.1016/j.tvjl.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. [Google Scholar]
- 13.Bonifait L, Grignon L, Grenier D. Fibrinogen induces biofilm formation by Streptococcus suis and enhances its antibiotic resistance. Appl Environ Microbiol. 2008;74:4969–4972. doi: 10.1128/AEM.00558-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jobin MC, Grenier D. Identification and characterization of four proteases produced by Streptococcus suis. FEMS Microbiol Lett. 2003;220:113–119. doi: 10.1016/S0378-1097(03)00088-0. [DOI] [PubMed] [Google Scholar]
- 15.Segura M, Stankova J, Gottschalk M. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis alpha and interleukin-6 production by murine macrophages. Infect Immun. 1999;67:4646–4654. doi: 10.1128/iai.67.9.4646-4654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benga L, Goethe R, Rohde M, Valentin-Weigand P. Non-encapsulated strains reveal novel insights in invasion and survival of Streptococcus suis in epithelial cells. Cell Microbiol. 2004;6:867–881. doi: 10.1111/j.1462-5822.2004.00409.x. [DOI] [PubMed] [Google Scholar]
- 17.Vanier G, Segura M, Friedl P, Lacouture S, Gottschalk M. Invasion of porcine brain microvascular endothelial cells by Streptococcus suis serotype 2. Infect Immun. 2004;72:1441–1449. doi: 10.1128/IAI.72.3.1441-1449.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi K, Rasmussen AW, Gudlavalleti SK, Stephens DS, Stojiljkovic I. Biofilm formation by Neisseria meningitidis. Infect Immun. 2004;72:6132–6138. doi: 10.1128/IAI.72.10.6132-6138.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schembri MA, Dalsgaard D, Klemm P. Capsule shields the function of short bacterial adhesins. J Bacteriol. 2004;186:1249–1257. doi: 10.1128/JB.186.5.1249-1257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graveline R, Segura M, Radzioch D, Gottschalk M. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int Immunol. 2007;19:375–389. doi: 10.1093/intimm/dxm003. [DOI] [PubMed] [Google Scholar]