Abstract
The relationship between changes in functional magnetic resonance imaging and neuronal activity remains controversial. Data collected during awake neurosurgical procedures for the treatment of epilepsy provided a rare opportunity to examine this relationship in human temporal association cortex. We obtained functional magnetic resonance imaging blood oxygen dependent signals, single neuronal activity and local field potentials from 8 to 300 Hz at 13 temporal cortical sites, from nine subjects, during paired associate learning and control measures. The relation between the functional magnetic resonance imaging signal and the electrophysiologic parameters was assessed in two ways: colocalization between significant changes in these signals on the same paired associate-control comparisons and multiple linear regressions of the electrophysiologic measures on the functional magnetic resonance imaging signal, across all tasks. Significant colocalization was present between increased functional magnetic resonance imaging signals and increased local field potentials power in the 50–250 Hz range. Local field potentials power greater than 100 Hz was also a significant regressor for the functional magnetic resonance imaging signal, establishing this local field potentials frequency range as a neuronal correlate of the functional magnetic resonance imaging signal. There was a trend for a relation between power in some low frequency local field potentials frequencies and the functional magnetic resonance imaging signal, for 8–15 Hz increases in the colocalization analysis and 16–23 Hz in the multiple linear regression analysis. Neither analysis provided evidence for an independent relation to frequency of single neuron activity.
Keywords: fMRI, single neuron activity, local field potentials, paired-associative learning, temporal cortex
Introduction
Functional magnetic resonance imaging (fMRI) with blood oxygen level dependent (BOLD) signal has become a major tool to investigate human brain function. The goal of many of these studies is to establish the location and nature of the neuronal events that generate human cognitive processes. However, the BOLD signal that is imaged does not measure neuronal events, but rather metabolic changes, in level of oxygenated haemoglobin and blood flow. The relation of these metabolic changes to neuronal activity has been the subject of much recent investigation (Raichle and Mintun, 2006). Simultaneous imaging and neuronal recording in visual cortex of anaesthetized monkey suggested that BOLD signal changes were most closely related to changes in local field potentials (LFPs) usually considered to reflect the afferent input to neurons, rather than the action potentials of neurons, which reflect their output (Logothetis et al., 2001; Goense and Logothetis, 2008). Similar findings have been reported in other animal studies (Harel et al., 2002; Lauritzen and Gold, 2003; Niessing et al., 2005). Studies in humans, comparing fMRI changes in one set of subjects with electrophysiologic changes in different subjects (or to those in nonhuman primates) during similar tasks, have conflicting findings. A relation between fMRI and the electrocorticogram (ECoG) has been reported (Puce et al., 1997; Schlosser et al., 1999) though not confirmed by others (McCarthy, 1999; Huettel et al., 2004). Nearly all of these studies were conducted in primary sensory cortices. Other studies concluded that fMRI changes reflect neuronal firing (Rees et al., 2000), including a study where human single neuronal activity in auditory cortex was directly recorded (Mukamel et al., 2005). A similar finding was reported by Ritter et al. (2008) for changes in neuronal activity in somatosensory cortex inferred from scalp EEG recordings obtained simultaneously with fMRI in the same subjects. Recent studies, however, comparing electrophysiologic measures and fMRI in the same subject during more complex cognitive processes suggest that this relationship may be different in different areas of cortex. Lachaux et al. (2007) reported that during a semantic task, association cortex sites, mostly in superior temporal or inferior frontal gyri, which had increased 40–150 Hz ECoG power during the task, were frequently within 15 mm of fMRI activation sites. In contrast, Ekstrom et al. (2009) in recordings from parahippocampal gyrus, found positive correlations between BOLD signal intensity and 4–8 Hz LFP power but not higher frequency LFP power or single neuron activity.
Utilizing a unique clinical opportunity, to record neuronal activity during awake neurosurgery for the treatment of epilepsy, we report here the relation between fMRI, neuronal firing and LFPs recorded from the same sites in human temporal association cortex, in the same subjects, during the same behavioural measure, a word pair association (PA) learning paradigm. The PA task was selected for this study based on our previous finding that it changed activity in a large proportion of human temporal cortical neurons (Weber and Ojemann, 1995; Ojemann and Schoenfield-McNeill, 1998). We investigated the relationships in two ways. First, we addressed the essential empirical issue in interpreting neuronal correlates of a significant fMRI change: are there any significant changes in neuronal activity that colocalize to the same sites during the same behavioural comparisons? Our second analytic approach used multiple linear regressions to identify significant independent electrophysiologic predictors of the magnitude of the BOLD signal. Both approaches identified statistically significant relationships between high frequency (>100 Hz) LFP power and BOLD signals. With the colocalization approach, we found the relationship to include LFP power between 50–250 Hz, but only for positive LFP power and BOLD signal changes. Both approaches also provide suggestive evidence for a relation to selected low frequency LFP power ranges, 8–15 Hz with colocalization, 16–23 Hz with multiple linear regressions. Neither approach identified any independent relation to frequency of single neuronal activity.
Methods
Overall experimental design
This study was performed on patients undergoing temporal resections for medically refractory epilepsy with a technique where they were awake under local anaesthesia for a portion of the operation so that physiologic information unperturbed by general anaesthesia could be obtained to plan the resection (Ojemann, 1995). Once this information was obtained and prior to any resection, microelectrode recording of single neuronal activity and LFPs was obtained. One or more days prior to the operation (mean 7 days, range 1–22), each subject participated in a fMRI protocol performing a paired-associate word learning task (two blocks) and an interposed word-identification (ID) task (one block), each with a fixation target control. These same tasks were repeated at the time of intraoperative recording of single neuronal activity and LFPs. The location of the intraoperative recording sites was recorded photographically in relation to cortical surface landmarks, sulci and veins. Those locations were then reconstructed on merged structural magnetic resonance image and magnetic resonance imaging venogram, using those landmarks, and this was coregistered with the fMRI data from that subject (Modayur et al., 1997). The coordinates of these sites were used to construct single voxel regions of interest (ROIs), 3.5 × 3.5 × 7 mm3 for each intraoperative recording site on a patient-to-patient basis. Whole brain data were collected during the fMRI protocol. fMRI changes on these tasks extended well beyond the ROIs, into temporal, middle frontal and tempro-occipital regions of both hemispheres. However, the intraoperative recording sites (and thus the ROIs) were limited by clinical considerations to those portions of one temporal lobe that was subsequently resected to treat the subject's seizure disorder. With this experimental design, single neuron activity, LFP power and fMRI activity were assessed for the same tasks, from each subject's recording site(s). The relation between BOLD and electrophysiologic changes was assessed in two ways, the extent of colocalization of significant changes and multiple linear regressions. Colocalization between BOLD changes and electrophysiology was assessed for significant changes on three comparisons between PA task and control conditions: PA compared with its fixation control, PA compared with ID and the PA fixation difference compared with the ID fixation difference. These comparisons were selected to include PA task-‘baseline’ comparisons commonly utilized in fMRI studies (PA-fixation) and comparisons that separate associative word learning behaviour from simple ID (PA-ID) and (PA-fixation) − (ID-fixation), a separation that we have previously shown changed activity in a large proportion of human lateral temporal cortical neurons (Weber and Ojemann, 1995; Ojemann and Schoenfield-McNeill, 1998). The multiple linear regression evaluated the relation between the magnitude of the BOLD signal and the different electrophysiologic measures, across all tasks.
Subjects
The nine subjects and 13 recording sites represent all subjects and sites with complete data sets for fMRI and microelectrode recording. One subject did not have any neuronal activity recorded from one (of two) sites; that site was excluded from analysis. Data were thus available from one recording site in five subjects and two sites in four subjects. Six of the included subjects were female. All were right handed and left brain dominant for language, based on intracarotid amobarbital perfusion testing (Wada and Rasmussen, 1960). Mean age was 40 (range 25–60). All but one subject had medically refractory seizures, with a mean seizure onset at age 17 years (range 3–40, three subjects had onsets after 20 years). All subjects with seizures had mesial temporal foci; four had mesial temporal sclerosis, and four gliosis. The one subject without seizures had a medial temporal benign lesion. Verbal IQ was available for eight subjects. Mean verbal IQ was 86.5 (range 72–101). Six operations were on left brain, three on right. These studies and the procedures for obtaining informed consent were approved annually by the University of Washington Institutional Review Board.
Behavioural tasks
Three behavioural tasks were used in these studies; PA learning, ID and passive fixation. During the PA task, subjects were instructed to learn a set of 15 semantically unrelated visually presented word pairs. During the ID task, subjects were instructed to read single words. During passive fixation, subjects viewed a fixation cross in the centre of the screen. All tasks were done silently. Each fMRI and intraoperative recording included two blocks of PA task and an interspersed ID block, except for one subject where only one PA block could be administered intraoperatively.
Each PA block consisted of cycles where the same 15 word pairs (randomly re-ordered), presented over 21 s, alternated with a period of passive fixation of equal duration. Twelve cycles were presented for each block during fMRI recording, eight during intraoperative recording. In each cycle, each of the 15 word pairs was presented sequentially for 1 s with a 0.43 s interstimulus interval. Each PA block was followed by a two-choice recognition task, during which no fMRI data were collected.
The ID block had identical parameters as the PA trials, except that the subject was instructed to read the word silently as they were presented on the screen but was not instructed to remember them. Additionally, all of the words presented during this ID block were shown only once. Novel words were used for PA and ID condition, and for the intraoperative and fMRI studies.
These tasks were designed with E-Prime software (PST, Inc.) and presented to the subjects on a computer-controlled screen.
fMRI methods
All magnetic resonance data were acquired on a GE Sigma Horizon 1.5 T machine using gradient echo EPI sequence, with repetition time (TR) 3000/echo time (TE) 30 ms, Flip 90, matrix 64 × 64, 21 slices per scan, slice thickness 6 mm, skp 1, 168 volumes (8.24 s). Structural scans were also obtained, with 3-dimensional spoiled gradient (SPGR) with TR 29 ms/TE 7 ms, Flip angle 45, matrix 256 × 192, slice thickness 1.2 mm.
fMRI analysis
fMRI data analysis was carried out using statistical parametric map (SPM)2. Spatial processing included realignment, and spatial smoothing (8 mm fwhm). Statistical analysis used an adaptation of the General Linear Model, modelling separate effects of conditions (fixation, PAs and ID) and subject movement. Parameter estimates for the general linear model were obtained for each ROI, using SPM and Marsbar. Combinations of these parameter estimates yield contrast values for the three comparisons, PA versus fixation, PA versus ID, and the PA fixation difference versus the ID fixation difference. No corrections for multiple comparisons were applied as the tested hypotheses apply to regions of interest that were anatomically specified independent of fMRI findings (Friston, 1997).
Intraoperative recording
The microelectrode study was performed with the subject fully awake under local anaesthesia, having awakened from the propofol intravenous anaesthesia used for placement of the local anaesthetic field block and craniotomy at least an hour earlier. The sites of recording were in cortex that was free of interictal epileptiform discharges but that was to be subsequently resected as part of the surgical therapy of the subject's epilepsy. Two commercial tungsten microelectrodes were back loaded through a translucent 1 cm diameter footplate into each of two hydraulic microdrives. The footplate was used to dampen cortical pulsations. Care was taken to avoid blanching pial vessels. Two microdrives placed in lateral temporal cortex were used in all subjects although usable recordings from both drives were obtained in only four subjects. The sites of these recordings were identified by numbered tags and their location recorded photographically. Once stable neuronal activity free from evidence of injury or epileptiform burst activity (Calvin et al., 1973) was identified, the behavioural task was initiated. Activity from each microelectrode was divided into that from 1 to 1000 Hz and 100 to 5000 Hz. These channels were digitized at a frequency of 10 KHz (seven subjects) or 4 KHz (two subjects), along with separate channels for markers indicating when test items were presented. Each of these recordings included simultaneously recorded action potentials from a small number of well-isolated single neurons on the 100–5000 Hz channel, and LFPs on the 1–1000 Hz channel.
Single neuronal activity analysis
All analysis was performed off-line. Activity of each microelectrode channel was divided into that of individual neurons with an amplitude-width window discriminator and visual separation of the resulting amplitude-frequency histograms (Chart Software, AD Instruments 1994–2006). We have previously published examples of this separation (Schwartz et al., 1996). Commonly, activity of 1–4 nearby single neurons is recorded by each functioning microelectrode (Ojemann et al., 2000). Activity from all neurons isolated from a single electrode was then pooled together, and, if more than one electrode were in the same region of interest, the activity of all neurons from both electrodes pooled together, to assess the change in average neuronal activity in a region of interest. The frequency of this activity during the 1.3 s of each item of each trial of the tasks was determined, dividing the fixation period into periods of the same duration as the individual items of the PA and ID trials. Statistical analysis of frequency differences on each of the three comparisons obtained for each recording site (PA–PA fixation, PA–ID and PA–PA fixation compared with ID–ID fixation) used Mann–Whitney U-test.
LFP analysis
All analysis of the LFP data was performed using custom software written in Matlab (The Mathworks, Inc. 1984–2004). The recordings for each channel were visually inspected and sections with artefacts were removed prior to analysis. Each recording was segmented into individual 1.3 s of each item of each trial. The power spectrum of each trial was calculated as the squared magnitude of the Fourier transform. Peaks in these spectra caused by unrelated electrical noise in the recording were observed, including at 60 Hz, 75 Hz and their harmonics. These peaks were removed from the power spectra and the removed power values were replaced by a value interpolated from the average power of the surrounding frequencies. Power in a specific frequency range of interest was calculated as the integral of the power spectrum over those frequencies. When two electrodes were present in the same region of interest, the calculated power for each frequency range for each electrode was averaged. Analysis of the two PA blocks was performed for each block separately as well as for the two blocks combined. For the combined analysis, the power values for the two PA blocks were pooled before the statistical analysis. The same comparisons were analysed as for fMRI and single neuronal activity. All comparisons of statistical significance for power between conditions for the different frequency ranges were computed using the Wilcoxon sum ranks test.
Statistical analysis
Colocalization across all sites of significant changes in the three comparisons between fMRI BOLD signal and each electrophysiologic parameter were evaluated statistically with Fisher's exact test. The quantitative relationship between changes in BOLD signal, and changes in single neuronal firing and LFP power in different ranges at the same sites, was analysed by constructing a general linear model. The difference in the average magnitude of the BOLD signal between a certain behavioural condition (PA, ID, PA fixation, ID fixation) and all behavioural conditions taken together was taken as the dependent variable (outcome). A number of independent variables were considered as possible regressors: subject, site, hemisphere (left or right), radial distance from temporal tip, behavioural condition and differences in average firing rates and LFP power in the different frequency ranges from 8 to 300 Hz between the behavioural condition and all behavioural conditions taken together, along with interaction terms between single neuronal activity and LFP power, and between different LFP power ranges. This general linear model comprised site-specific electrophysiological variables, which means we tested for a uniform relationship between electrophysiology and BOLD over sites (i.e. a linear relationship that was estimated with a single regression coefficient or parameter of our multiple linear regression model). Of the continuous independent variables none deviated significantly from the normal distribution, all had, in general, similar variances (when normalized by their means) and all were reasonably linearly related to the outcome. Changes in power in the LFP frequency ranges 100–150 Hz and 150–300 Hz were highly correlated, and only the first range was used in the model. Independent variables were then entered into the model by means of forward selection: variables with strongest bivariate associations were entered first, and were included when they predicted the outcome better than chance in the multiple linear regression models. This analysis was performed with Statistical Package for the Social Sciences (SPSS) 10.0 for Windows (SPSS Inc, Chicago, IL).
Quantification of ripples
The frequency of occurrence of ripples, short episodes of high-amplitude and high-frequency oscillations was determined in the LFP recordings using an automated ripple detector similar to that used by Grenier et al. (2001). We examined separately ripple episodes in the high-frequency (150–200 Hz) and in the very high-frequency (250–500 Hz) range. Raw LFP signal was band-pass filtered in the corresponding range using a fourth order elliptical Butterworth digital filter. We identified the ripple centres by isolating threshold crossings of the negative deflection of the filtered signal. We used a threshold of 4 SDs above the baseline noise level, which was calculated across the entire recording epoch. Ripple amplitude was defined as the absolute value of the negative deflection at the ripple centre. Ripple duration was defined as the time period between the two 2 SD threshold crossings of the envelope of the filtered signal immediately before and after the ripple centre. Only ripples of duration >40 ms were considered for the analysis.
Removal of spike waveforms
The LFP channel, initially sampled at 4 kHz, was filtered using third order narrow band-cut, two pass elliptic filters at 120, 180 and 240 Hz to remove harmonics of 60 Hz. In order to remove spike remnants on the LFP channel, spikes were removed using the spike timing and the mean spike waveform. The removal was performed by scaling the mean spike waveform with a scaling factor computed by the inner product of the corresponding part of the LFP to the spike time and the mean spike waveform, as shown mathematically below.
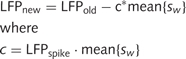 |
Subsequently, the signal was filtered using a third order two-pass elliptic low-pass filter at 500 Hz for the purpose of down-sampling at 1 kHz. The signal was then down-sampled at 1 kHz
Results
Behaviour
To determine if the subjects were performing the silent PA task used during both intraoperative recording and fMRI, a two-item forced choice recognition task was administered after each block of the PA task. In response to the first word in a pair, subjects correctly recognized the correct second word on an average of 87% of the PA items presented during the fMRI and 79% with intraoperative testing; performance for all subjects on both tests differed from chance at P < 0.05.
fMRI changes
The locations of the 13 recording sites from the nine subjects were superimposed on a standard brain, illustrated in Fig. 1. fMRI changes on these tasks extended well beyond these sites, into temporal, middle frontal and tempro-occipital regions of both hemispheres. However, the intraoperative recording sites were limited by clinical considerations to those portions of one temporal lobe that were subsequently resected to treat the subject's seizure disorder. Significant (P < 0.05, two-tailed) fMRI changes in at least one of the three comparisons between PA and control conditions (PA versus fixation, PA versus ID and PA-fixation difference versus ID-fixation difference) were present in all 13 ROIs, one at each recording site. Six of these (six subjects) had only significant fMRI increases with PA on one or more comparisons. Six others (five subjects) had only significant decreases and one site had a significant increase on one comparison and decrease on another. The location of these changes is also indicated in Fig. 1 and their distribution across comparisons in Table 1. Significant changes were present for 20 of the 39 comparisons between PA and control conditions (51%), equally divided between increases or decreases with PA compared with control conditions (Table 2).
Figure 1.
Location of recording sites, superimposed on standard brain. Orange indicates sites with only significant increases in fMRI BOLD signal on one or more comparisons in the region of interest at that site. Purple indicates sites with only significant decreases in that signal on one or more comparisons. Uncoloured site had a significant fMRI increase on one comparison and a significant decrease on another comparison. Numbered sites are from Subject 506 whose data are presented in Figs 2–5. White squares indicate approximate location of the cortical exposure provided by the operations where the recordings were obtained.
Table 1.
Significant (P < 0.05 two-tailed) changes in fMRI and electrophysiologic measures (frequency of single neuron activity, LFP power in 8–150 Hz and 150–300 Hz ranges) for each subject, site and comparison
| Comparison | PA-Fix |
PA–ID |
[(PA-fix)–(ID-fix)] |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject/site | Side | fMRI | Single neuron | LFP 8–150 | LFP 150–300 | fMRI | Single neuron | LFP 8–150 | LFP 150–300 | fMRI | Single Neuron | LFP 8–150 | LFP 150–300 |
| 414 | L | P | N | N | N | ||||||||
| 415 | L | P | P | P | P | P | |||||||
| 418 | L | P | P | P | P | P | P | P | |||||
| 420 | R | P | P | P | P | P | |||||||
| 506 21a | R | P | P | P | P | P | P | P | |||||
| 508 20 | L | P | N | P | P | P | P | P | |||||
| 408 21 | L | N | N | P | P | P | P | P | |||||
| 408 20 | L | N | N | P | P | ||||||||
| 413 | R | N | P | N | P | P | N | P | |||||
| 416 20 | L | N | P | N | N | P | P | ||||||
| 416 21 | L | N | N | N | P | N | |||||||
| 506 20a | R | N | N | N | N | N | N | N | |||||
| 508 21 | L | N | N | N | N | N | |||||||
P = increase; N = decrease; PA = PA task; ID = identification task; Fix = fixation for that task.
a Illustrative case: location of sites identified in Fig. 1, single neuron changes in Figs 2 and 3, LFP changes in Figs 4 and 5. Six sites with only fMRI BOLD increases at top. Six sites with only decreases at bottom.
Table 2.
Summary of the data across Table 1, indicating the proportion of the 39 comparisons (one at each of the 13 sites for PA versus fixation, PA versus ID and PA fixation versus ID fixation) that had significant changes (P < 0.05) for fMRI and each electrophysiologic measure, the proportion of those with greater activity for the PA task, the number of sites with significant changes for each measure, and the consistency of the changes at those sites across the three comparisons
| FMRI | Single neuron | LFP 8–150 | LFP 150–300 | |
|---|---|---|---|---|
| Number of 39 comparisons with significant changes | 20 (51%) | 21 (54%) | 18 (46%) | 17 (44%) |
| Number of these that had greater activity for behaviour | 10 (50%) | 10 (48%) | 12 (67%) | 14 (82%) |
| Number of 13 sites with significant changes | 13 | 12 | 11 | 12 |
| Sites with significant changes on: 2/3 comparisons | 4 | 5 | 2 | 5 |
| 3/3 comparisons | 1 | 1 | 1 | 0 |
Single neuron activity
As illustrated in Fig. 2 for the two recording sites in one subject, well-isolated single neuron activity with stable waveforms throughout the various blocks was recorded. The frequency of single neuron activity simultaneously recorded from two sites (20, 21) in the illustrative subject during the two blocks of PA and interposed ID block is also indicated. The location of those two recording sites is shown in Fig. 1. The changes in average activity for the two comparisons that had significant changes in fMRI signal at both sites, decreased fMRI activity at site 20 and increased at site 21, are also illustrated.
Figure 2.
Single neuron activity recorded simultaneously from sites 20 and 21 of Subject 506. (A) Frequency of single neuron activity recorded from each site during the two blocks each with PA and associated fixation, and the interspersed block of ID and associated fixation. Inset in each block's data is the average waveform of the action potentials recorded during that block. Bar in that insert is 2 ms. The activity illustrated for each block is the average of the eight cycles of fixation and behaviour in 100 ms bins, with the red line dividing the equal 21 s periods of fixation or behaviour. Bar below each block is the 1.3 s duration of an individual item in behavioural portion, and equivalent segment of fixation, expanded into individual item peristimulus time histograms in Figure 3. (B) Average frequency of single neuronal firing for each condition. Site 20 blue, 21 green. The fMRI had significantly increased activity for two comparisons, PA fixation and [(PA–PA fixation) – (ID–ID fixation)] at site 21, and significantly reduced activity for all three comparisons at site 20. The change in average single neuron firing rates at both sites is shown for the two comparisons with significant fMRI changes at both sites, increases at site 21 and decreases at site 20. (C) Difference in average firing rates between PA compared with PA fixation. Site 20 blue, 21 green. Asterisk above bar, significant change at P < 0.025. Asterisk below pair, significant difference between sites at P < 0.025. (D) Difference in average firing rate for [(PA–PA fixation) − (ID–ID fixation)] comparison, presented as in C. As indicated by the consistent action potential waveforms at each site, stable single neuron activity was recorded throughout the blocks. In this patient, there is increased average activity during ID at both sites, compared with ID fixation. However, activity during PA shows a differential response: greater activity for PA compared with PA fixation at site 21, less activity with PA at site 20. The increase in neuronal firing at site 21 is significant for only one of the two comparisons that had significant fMRI signal increases at this site, while significantly decreased neuron firing occurred at site 20 on both comparisons, both of which also had significant fMRI signal decrease at this site.
As indicated in Table 2, significant changes in frequency of single neuron activity were present for over half of the comparisons, with changes at all but one site, in all subjects. These significant effects were present in a high proportion of the single neuron sample despite the small number of randomly recorded neurons included in the sample at each site, an average of 2.5 neurons (range 1–4). The high proportion of neurons changing activity with PA learning was also present in our earlier studies of temporal cortical neuronal activity associated with learning of PA pairs (Weber and Ojemann, 1995; Ojemann and Schoenfield-McNeill, 1998). The distribution of these changes across comparisons is also indicated in Table 1. Consistent changes in more than one comparison were present for half of the sites (Table 2). Figure 3 illustrates the average peristimulus time histograms for individual items included in the different blocks of Fig. 2. The change in activity during PA is tonic, sustained throughout the 1s the item is presented with a small reduction during the interstimulus interval. The presence of tonic sustained changes in single neuron activity distinguished learned from unlearned items in a previous study (Ojemann and Schoenfield-McNeill, 1998).
Figure 3.
Average peristimulus time histograms in 50 ms bins for individual items that make up the blocks of Figure 2, for each condition for each site. Item activity during the two blocks of PA has been averaged together. For both PA and ID, average activity is tonic, with little modulation during the 1-s period of item presentation. Such sustained, tonic activity was a feature of human temporal lobe neuronal activity associated with learning of a word-pair association (Ojemann and Schoenfield-McNeill, 1998).
LFP changes
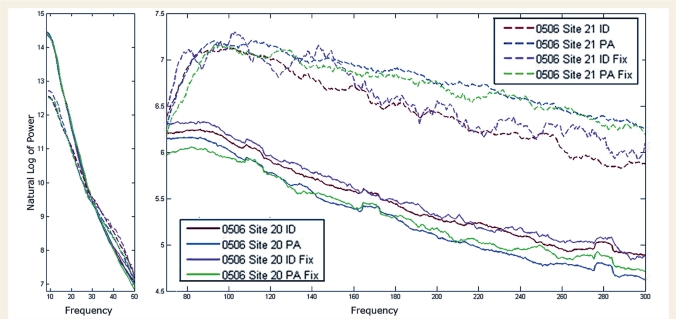
Microelectrodes have relatively high impedances that attenuate LFP values in the very low frequencies. For this reason, LFPs below 8 Hz were not analysed statistically. Figure 4 plots the LFP power for the 8–300 Hz frequency range simultaneously recorded at the two sites in the illustrative subject during the various conditions of this study. The changes in this activity during the two comparisons with significant fMRI changes at those two sites are shown in Fig. 5. For overall statistical analysis, LFPs were divided into the 8–150 Hz and 150–300 Hz ranges. As indicated in Table 2, changes in power were present for slightly less than half of the comparisons, at all but one site for each frequency range, for 8–150 Hz in all subjects, for 150–300 Hz in all but one subject. The distribution of these changes across comparisons is indicated in Table 1. LFP power changes were not as consistent as the changes in single neuron activity (Table 2).
Figure 4.
LFP power simultaneously recorded from sites 20 and 21 of Subject 506, averaged for each condition of this study. Log LFP power, smoothed with a moving average shown in 8–50 Hz and 70–300 Hz blocks. Site 21(dashed values) had significant increases in fMRI BOLD signal with two comparisons, PA fixation and [(PA fixation)-(ID fixation)]. Site 20 (solid lines) had significant decreases in fMRI signal on all three comparisons. The 50-70 Hz segment with filter artefact omitted. Greater LFP power in the 70–300 Hz range is evident at site 21, the site with significant fMRI increases, with the greatest increases for the PA task.
Figure 5.
Changes in LFP power for the two comparisons, PA fixation and [(PA fixation) − (ID-fixation)], that had significant increased fMRI signal at site 21 (green), and significant decrease at site 20 (blue) in the illustrative Case 506. Median power difference is shown for each of the frequency ranges of Table 3. Asterisks above each bar identify significant changes at P < 0.025. Asterisks below each pair of bars identify significant differences (P < 0.025) between sites 20 and 21. (A) [(PA-PAfixation) – (ID-IDfixation)] comparison. Significant increased power at site 21, with a significant difference from site 20 are present in the 8–15 and 50–300 Hz ranges. (B) PA fixation comparison. Power at site 21 is significantly increased in the 50–100 Hz range, a significant difference from site 20. Site 20 had significantly decreased LFP power in the same frequency range.
Colocalization of changes in fMRI signal and changes in single neuron activity and LFP power
Colocalization as used here means that a reliable change in fMRI at a particular location is associated with a non-random change in an electrophysiologic measure, which is in the same direction at the same location when assessed on the same comparison. As indicated earlier, these comparisons were selected to provide behavioural controls for the PA task that reflect common fMRI practice (to fixation), were previously shown to change activity in a large proportion of neurons (to ID) and the combination (PA fixation compared with ID fixation). Although these comparisons of condition specific differences are not statistically orthogonal, they reflect these sufficiently different ways of assessing changes in neuronal responses with the PA behaviour to be interesting. With the data in Table 1, the degree of colocalization of significant fMRI changes and significant changes in single neuron activity and LFP power for the same comparisons, at the same sites in the same subjects can be evaluated. In addition to addressing the practical question of what if any electrophysiologic change might reasonably be expected when a significant fMRI change was observed, this type of analysis has the additional advantage that it does not require any assumptions about the timing of any link between the rapid electrophysiologic events (measured in ms) and the slow fMRI haemodynamic response measured in seconds. Most fMRI studies are directed at determining regions where the haemodynamic response increases during behaviour compared with some ‘control’ condition. Thus our initial analysis was directed at establishing any relation between increases in the fMRI haemodynamic response, frequency of single neuron activity and LFP power. Table 2 indicates the proportion of the comparisons with significant changes that represented increased activity for the PA task, about half of the fMRI and single neuron changes and a larger proportion of the LFP changes (67%–82%).
The electrophysiologic changes at the six sites with only increased fMRI BOLD activity for PA on one or more comparisons are shown at the top of Table 1. Independent of the specific comparison, increases in the electrophysiologic measures were significantly more likely at these sites compared with decreases (22 of 26 significant changes at the fMRI positive sites were positive, P < 0.001, sign test) and to activity at the remaining sites, where 14 of 30 significant changes were positive (P < 0.005). A general increase in electrophysiologic activity is present at sites with increased fMRI BOLD signal. Table 3 indicates the probability of that increased electrophysiologic activity occurring on the same comparisons as the fMRI increased BOLD signal. Only increased LFP power in the 150–300 Hz range has significant colocalization to the same sites and comparisons (P < 0.01 one-tailed). In the lower portion of Table 3, LFP power is subdivided into narrower frequency ranges. Significant colocalization is present for the 50–100 and 150–250 Hz ranges, with trend for 8–15, 100–150 and 250–300 Hz ranges.
Table 3.
Colocalization of increased fMRI signal and increased activity in single neurons and power in LFP in various frequency ranges, based on the 39 comparisons of this study
| Physiologic measure | Number of the 10 comparisons with significant increases in fMRI signal that also had significant increases in that physiologic measure | Number of comparisons with significant increases in that physiologic measure that did not have significant increases in fMRI signal | Probability of the relation between significant fMRI signal increase and an increase in the physiologic measure (Fisher's exact test, one-tailed) |
|---|---|---|---|
| Single neuron firing frequency | 4 | 6 | 0.21 |
| LFP 8–150 Hz | 5 | 7 | 0.13 |
| LFP 150–300 Hz | 7 | 7 | 0.01 |
| LFP power in narrower frequency ranges | |||
| 8–15 | 5 | 6 | 0.09 |
| 16–23 | 3 | 6 | 0.42 |
| 24–34 | 4 | 5 | 0.15 |
| 35–50 | 4 | 5 | 0.15 |
| 50–100 | 7 | 7 | 0.01 |
| 100–150 | 7 | 10 | 0.06 |
| 150–200 | 7 | 9 | 0.04 |
| 200–250 | 7 | 9 | 0.04 |
| 250–300 | 6 | 8 | 0.07 |
Although most fMRI studies have concentrated on increases in the BOLD signal with behaviour, there has been recent interest in the reverse, relative decreases with behaviour, compared with the control (Gusnard and Raichle, 2001). These decreases have been modelled as reflecting spontaneous neuronal activity in the absence of an explicit input, a ‘default’ network separate from networks for task specific responses reflected in the fMRI increases (Fox and Raichle, 2007; Buckner et al., 2008). As illustrated in Fig. 1, decreases were the only fMRI change at six sites (five subjects). These sites are in the mid portion of middle temporal gyrus, approximating the lateral temporal cortical region that Gusnard and Raichle (2001) included as part of the default network. This is also a region where we identified relative decreases as a characteristic of single neuron activity in an earlier study investigating changes during recent memory encoding in a different series of subjects (Ojemann et al., 2009). However, sites with consistent fMRI decreases did not have a general decrease in electrophysiologic activity, as only 15 of 25 significant changes in electrophysiologic measures were decreased (P > 0.2), and there was no significant colocalization of comparisons with significant fMRI decreases and decreases in single neuron activity or LFP power in any frequency band (data not shown, all P-values >0.05). Despite this lack of colocalization when all comparisons in all subjects are considered, such decreases on fMRI and one or more electrophysiologic signal were evident in some individual comparisons such as those illustrated for site 20 in Figs 2 and 5.
In order to understand better the high frequency LFP power that our data relate to increased fMRI signal, we undertook two additional analyses. It has been suggested that high frequency LFPs may include power from single neuron action potentials, though action potential power is usually thought to make little contribution to LFP power at frequencies below 500 Hz (Greiner et al., 2001). Nevertheless, we reanalysed a sample of our LFP data (recorded at 1–1000 Hz) after removing segments associated temporally with the action potentials we had recorded on the channel filtered at 100–4000 Hz. No differences in power were identified between the original data and that with identifiable action potential segments removed. High frequency LFP activity has also been investigated in the context of ‘ripples’ (<250 Hz) and ‘fast ripples’ (250–500 Hz); brief (25–75 ms) bursts of this high frequency activity. ‘Ripples’ have been demonstrated in ‘normal’ somatosensory and neocortex in a variety of species. Intracellular recordings during ‘ripples’ in rat and cat somatosensory cortex indicate that they reflect synaptic input, and excitatory drive modulated by inhibition perhaps mediated by interneurons (Jones et al., 2000; Greiner et al., 2001). Fast ripples in medial temporal lobe have been related to epileptic activity in animal models and humans (Staba et al., 2002). Using an automated ripple detection analysis, we found no significant relation between increased ripple amplitude or duration in the 150–200 Hz frequency range and increased fMRI signal (P > 0.66), suggesting that the increased power in this frequency range that is associated with increased fMRI signal is not organized in ripples. Moreover, fast ripples in the 250–500 Hz range, the type related to epileptic activity, had a significant ‘negative’ relation to increased fMRI signal (P = 0.02, two-tailed). This finding indicates that the high frequency activity we relate to the fMRI is not organized in ripples and not a reflection of the subjects’ underlying epilepsy.
Effect of comparing PA results with different control conditions
As indicated in Table 1, although nine sites had significant changes in fMRI with the PA fixation comparison (four increases), single neuron and especially LFP power changes were infrequent. To adjust for this discrepancy between the PA fixation comparison and the other two comparisons, the relation between significant increases in fMRI and the electrophysiologic measures was reanalysed for only the PA–ID and [(PA-fixation) − (ID-fixation)] comparisons. The results were similar to the original analysis. There was a significant relation between increases in fMRI and LFP 150–300 Hz power at P < 0.007, and now a trend for LFP 8–150 Hz power (P = 0.08), but not single neuron activity (P = 0.13). When the narrower LFP power frequency ranges of Table 1 were reanalysed in this way, significant effects were present for the 150–200 and 200–250 Hz bands at P = 0.01 and the 8–15, 50–100 and 100–150 Hz bands at P < 0.05.
Colocalization of changes in fMRI, single neuron activity and LFP power in individual cases with recording from two separate sites
In addition to the group relationship, the relation between changes in fMRI signal and LFP power was also evident in individual subjects. Four subjects had recordings from two separate sites in temporal cortex. Three of these subjects had one site with only increased fMRI signal on one or more comparisons and another site with only decreased signal. This provides an opportunity to assess the regional specificity within an individual subject's temporal association cortex of any relationship between changes in fMRI and the electrophysiologic measures. The location of the recording sites for one of these subjects is shown in Fig. 1, the changes in single neuron activity in Fig. 2 and LFP power in Fig. 5. Concordant significant increases in fMRI signal and 150–300 Hz power, at the same one of the two sites on at least one of the same comparison were present for all three subjects. Concordant increases in single neuron activity and 8–150 Hz LFP power were present for two subjects, including the illustrative case. Concordant decrease in fMRI signal, single neuron activity and 150–300 Hz LFP power on at least one comparison was present for the other site in two of these subjects, but only one had concordant decrease in 8–150 Hz LFP power. The fourth subject had decrease in fMRI signal at one site, with concordant decrease in 8–150 Hz LFP power, and both fMRI increases and decreases on different comparisons at the other site, with concordant increase in 8–300 Hz power for the comparison with increased fMRI signal.
Quantitative relations between magnitude of the fMRI haemodynamic response and electrophysiologic changes
Our colocalization approach to establishing a relationship between fMRI and electrophysiologic changes parallels the type of analysis commonly used in behavioural fMRI studies, providing a basis for inferring the presence of a non-random change in an electrophysiologic measure when a significant fMRI BOLD change is identified. However, this type of analysis does not necessarily provide evidence for a relation between the magnitude of changes in the BOLD signal and the electrophysiologic measures. To evaluate the relationship between BOLD signal, single neuronal firing and LFPs, a general linear model was constructed as described under Methods. The following site-specific independent variables were found to be significant and independent predictors of the changes in the BOLD signal over conditions and sites, and were included in the final multiple linear regression model (Table 4): subject, change in LFP power in the 16–23 Hz range and in the 100–150 Hz range, and the interaction between change in single neuronal firing and change in LFP power in the 16–23 Hz range. Power in the 16–23 Hz range of the LFP was marginally not significant, but was nonetheless included in the model, as its inclusion significantly changed the coefficients of the rest of the regressors.
Table 4.
Independent variables included in the final multiple linear regression model and their relationship to the BOLD signal
| Variable | ta | SEa | Significancea |
|---|---|---|---|
| LFP power, 16–23 Hz | −0.576 | 0.067 | 0.071 |
| LFP power, 100–150 Hz | −0.361 | 0.034 | 0.036 |
| Single neuron firing × LFP power, 16–23 Hz | 2.665 | 0.003 | 0.045 |
Adjusted R2 value for the model: 0.19 (P = 0.029). t: standardized coefficient; SE: standard error.
a All values adjusted for subject effect.
Discussion
This study indicates that a site in temporal association cortex with significantly increased activity for a paired associate learning behaviour on fMRI has a non-random chance of also having significantly increased activity in LFP power in the 50–250 Hz frequency range. We have established this relationship based on the stringent criterion of colocalization of significant fMRI and LFP power increases to the same region of interest during the same task-control comparison in the same subject. The relation was identified for at least one comparison at one site in seven of the nine subjects. We did not find colocalization of fMRI increases to increased power in more conventional gamma frequencies <50 Hz, nor any evidence for colocalization between decreases in fMRI and LFP activity. Our quantitative analysis supports this finding, with 100–150 Hz LFP power as a significant regressor. Both analytic approaches also provide weaker evidence for a relation between the fMRI and low frequency LFP power, with a trend to colocalization in the 8–15 Hz range and in the multiple linear regression analysis, 16–23 Hz range alone as a marginally significant regressor and the interaction of single neuron firing and 16–23 Hz power as a significant regressor.
These findings are in line with the growing body of evidence relating fMRI BOLD to LFP changes in various frequency ranges. Nonhuman animal studies in sensory cortices have related fMRI activations to LFP activation most often in low and middle gamma frequencies (<50 Hz) (Logothetis et al., 2001; Harel et al., 2002; Lauritzen and Gold, 2003; Kayser et al., 2004; Niessing et al., 2005; Goense and Logothetis, 2008). Findings in humans have been variable. Mukamel et al. (2005) reported a relation to LFP frequencies up to 130 Hz in microelectrode recordings from auditory cortex and Lachaux et al. (2007) 40–150 Hz ECoG increases within 15 mm of predominately temporal association cortex sites with fMRI activation. On the other hand, Ekstrom et al. (2009) reported a positive correlation with only 4–8 Hz in recordings from parahippocampal gyrus, and no correlation between BOLD signal and LFP activity in hippocampus. Whether the differences between these studies and with ours reflect the different brain regions investigated, or the different behavioural assessments is unknown, although a link between high frequency gamma and low frequency (θ 5–7 Hz) human ECoG activity has been described (Canolty et al., 2006).
Although the presence of a significant positive change in fMRI signal is likely to colocalize with a significant increase in the LFP high frequency power, this relationship is not absolute. Only 7 out of 10 comparisons with increased fMRI haemodynamic response changes had increased high frequency LFP power, and this represented about half (7 out of 15) of the comparisons with increased LFP power in this frequency range. This is similar to the relation between fMRI and high frequency ECoG increases described by Lachaux et al. (2007). Several aspects of our study may contribute to the failure to find an even more robust relationship. The electrophysiologic measures were averaged across the 1.3 s presentation time of each item, as it was our hypothesis that changes in average activity during behaviour would be more likely to be related to changes in the fMRI haemodynamic response that also lasts seconds. Our findings are in line with this hypothesis. However, with this approach we may miss brief phasic changes in the electrophysiologic measures which potentially could induce a haemodynamic change. In the Lachaux et al. (2007) study, such brief phasic increases in 40–150 Hz ECoG were identified, but at sites not close to fMRI increases. The choice of behavioural comparisons also clearly influenced the likelihood of finding electrophysiologic correlates of fMRI. The standard fMRI comparison, to visual fixation, was associated with very few increases in any of the electrophysiologic measures, though fMRI increases were evident. While there was one example of high frequency LFP increase at a site with fMRI increases on that comparison, it was only with comparisons of the PA task to ID that we could reliably show the association. The Lachaux et al. (2007) study also used comparison control conditions involving behaviours more complex than simple visual fixation.
The role of this high frequency graded response activity in human cortical function has been the subject of considerable research interest over the last decade (Jensen et al., 2007 for review). In human motor cortex, movement-induced increases in ECoG power in the 76–100 Hz range have the same pattern as the motor homunculus, a pattern not evident at lower ECoG frequencies (Crone et al., 1998; Miller et al., 2007). Power of ECoG frequencies in the 40–200 Hz range recorded from fusiform and lateral occipital cortex was modulated by a face detection task (Lachaux et al., 2003). Increased ECoG power in this range has been associated with recent ‘working’ memory, in auditory cortex (Howard et al., 2003), temporal cortex (Raghavachari et al., 2001) and also Broca and Rolandic cortex (Mainy et al., 2007). In this last study the changes were related specifically to the encoding phase of working memory. Increased power in the 60–250 Hz range of the ECoG was observed with deviant auditory stimuli (Edwards et al., 2005). Transient suppression of ECoG activity in the 20–150 Hz range in left ventral lateral frontal cortex has been associated with attending to words or letters during a reading task (Lachaux et al., 2008). Increased activity in these ‘gamma’ frequencies during human cognitive processes has also been observed in the EEG and magnetoencephalogram (Tallon-Baudry et al., 1998; Lutzenberger et al., 2002; Kaiser et al., 2003; Jokisch and Jensen 2007). The present study extends the relation of increased high frequency graded response activity to LFP power and to an associative learning task in human temporal association cortex.
What this high frequency ECoG-LFP activity represents is not entirely clear. It has been recorded in experimental animals during behavioural manipulations, particularly rat hippocampus during exploratory behaviour (Bragin et al., 1995). The mechanism responsible for that activity has been extensively studied (Bartos et al., 2007; Mann and Paulsen, 2007 for reviews). It is generally assumed that this activity represents synchronous oscillations of summed postsynaptic inputs and that GABAergic interneuron activity has an important role in this synchronization. For this model, the importance of interneuron inhibition, interneuron electrical coupling, phasic excitation and the spatial extent of the synchronized activity remain controversial. The role of interneuron inhibition has been particularly explored in the context of ‘ripples’ (Jones et al., 2000; Greiner et al., 2001). However, the high frequency LFP activity we relate to increased fMRI signal does not seem to be organized in ripples. Nor is it related to ‘fast ripples’ that have been related to epileptic activity in animal models and humans (Staba et al., 2002) indicating that it does not represent an epileptic epiphenomenon. There is an alternative model for high frequency ECoG-LFP activity, that there is no particular temporal synchronous oscillating structure, but rather that the pattern of power at high frequencies is a statistical property of summing random variations of fast ionic currents, including GABA-A, excitatory AMPA-glutamate, and perhaps action potentials (Motokawa, 1943; Edwards, 2007). Experimental animal studies have not resolved these alternative models. High gamma activity was much more sensitive to increases in neuronal synchrony than firing rate in a recent report (Ray et al., 2008), while other work emphasized LFP components unrelated to synaptic events (Buzsaki and Chrobak, 1995; Kandel and Buzsaki, 1997). Our finding that removal of the brief segments of the LFP associated with the action potentials identified in recordings with the appropriate filter setting for single neuron activity did not change findings for a sample of our high frequency LFP recordings suggests that at least the action potentials from nearby larger neurons contribute little to LFP power up to 300 Hz.
The relation of fMRI changes to single neuronal activity remains controversial (Raichle and Mintun, 2006). Mukamel et al. (2005), based on recording single neuronal activity from human auditory cortex in one groups of subjects, and fMRI in another, claimed that ‘fMRI signals can provide a reliable measure of the firing rate of human cortical neurons’. However, in their study, changes in single neuronal activity were confounded with changes in LFP power in the 40–130 Hz range. Ritter et al. (2008) simultaneously recorded fMRI and very high frequency (600 Hz) scalp EEG responses evoked from human somatosensory cortex by median nerve stimulation. They inferred that a component of the 600 Hz evoked response reflected mass synchronous neuronal action potentials (Baker et al., 2003) and found that component to colocalize with an fMRI BOLD signal in somatosensory cortex, with variations in the amplitude of that component occurring with different rates of median nerve stimulation reflected in that fMRI signal (Ritter et al., 2008). We did not find significant colocalization between non-random changes (increases or decreases) in single neuron firing and fMRI signal in recordings from the same comparisons in the same subjects at the same sites, in human temporal association cortex, nor was single neuronal firing by itself a significant regressor in the multiple linear regression models. That our findings may reflect recording from association cortex rather than primary sensory cortices is supported by the report of Ekstrom et al. (2009) who also found no relation between BOLD signal changes and single neuronal firing in recordings from parahippocampal gyrus and hippocampus. However, their study found a relation between changes in BOLD activity and low frequency (4–8 Hz) LFP power in parahippocampal gyrus recordings suggesting that the relation to LFP power also may vary across association cortex. Our findings could also reflect the small sample of neurons we recorded, or the predilection of extracellular microelectrodes to record activity from a subpopulation of very nearby larger pyramidal neurons. However, our study was adequate to identify colocalization of significant increases in LFP power and fMRI signal, perhaps because LFP power reflects the wider range of neuronal signals (action potentials from interneurons as well as pyramidal neurons and presynaptic potentials) from a greater volume of tissue in the LFP compared with single neuron activity. Moreover, the failure to identify significant colocalization between fMRI and single neuron activity increases was not due to a failure to identify significant increases in single neuron activity, for these were present in 26% (10 out of 39) of our comparisons, but on different comparisons from different sites than the 26% of comparisons with significant increases in fMRI, as indicated in Table 1. Although not evident in that group data, a relation was evident in at least one comparison in each of four subjects. However, these cases do not establish a relationship, as the same subjects had four comparisons with fMRI but not single neuron activity increases and three comparisons with single neuron activity but not fMRI increases. In human temporal association cortex fMRI increases do not seem to provide a reliable indicator of increased single neuronal activity. We have previously shown that LFP activity in the 75–150 Hz range is partially predictive of firing of neurons recorded through the same electrode (Zanos et al., 2006), suggesting that any apparent relation of fMRI to single neuronal activity probably reflects a common relationship to high frequency LFPs. Several nonhuman animal studies report findings similar to ours. Kayser et al. (2004) compared fMRI in anaesthetized cats with LFP and single neuron recordings in visual cortex of awake cats, using the same visual stimuli, with a match between BOLD and LFP 20–50 Hz activity but not single neuron firing. Similarly in simultaneous fMRI, LFP and single neuron recordings from visual cortex of awake monkey, Goesner and Logothetis (2008) concluded that LFPs were a better and more reliable predictor of fMRI signal than the single neuron activity, and that any correlation of neuron firing to fMRI BOLD signal was by virtue of correlations to LFP.
Human fMRI behavioural studies commonly ascribe findings to changes in ‘neuronal activity’ when what is actually reported are positive changes in the BOLD metabolic signal. Based on the findings reported here, it now seems reasonable to infer that those positive BOLD findings reflect at least one change in actual neuronal activity, increased power in high frequency LFP, when the positive finding is in temporal association cortex. Remaining to be determined is the applicability of this finding to other regions of cortex and the mechanisms that may link high frequency LFP and regulation of blood flow or oxygen extraction that is responsible for the fMRI signal.
Funding
National Institutes of Health grants EB 2663, DC 2310 and DC 3099 and a grant from Shirley and Herb Bridge.
Acknowledgements
The authors thank E. Lettich for assistance in the intraoperative recordings, C. Wendelken in fMRI analysis and S. Hakimian for advice on the study and manuscript
Glossary
Abbreviations
- BOLD
blood oxygen level dependent
- ECoG
electrocorticogram
- fMRI
functional magnetic resonance imaging
- ID
word identification
- LFP
local field potential
- PA
paired associate
- ROI
region of interest
References
- Baker SN, Curio G, Lemon RN. EEG oscillations at 600 Hz are macroscopic markers for cortical spike bursts. J Physiol. 2003;550:529–34. doi: 10.1113/jphysiol.2003.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–10. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Calvin WH, Ojemann GA, Ward AA., Jr Human cortical neurons in epileptogenic foci: comparison of inter-ictal firing patterns to those of "epileptic" neurons in animals. Electroencephalogr Clin Neurophysiol. 1973;34:337–51. doi: 10.1016/0013-4694(73)90086-2. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–8. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121(Pt 12):2301–15. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Edwards E. Eletrocortical activation and human brain mapping. PhD Thesis. Berkley: University of California; 2007. [Google Scholar]
- Edwards E, Soltani M, Deouell LY, Berger MS, Knight RT. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. J Neurophysiol. 2005;94:4269–80. doi: 10.1152/jn.00324.2005. [DOI] [PubMed] [Google Scholar]
- Ekstrom A, Suthana N, Millett D, Fried I, Bookheimer S. Correlation between BOLD fMRI and theta-band local field potentials in the human hippocampal area. J Neurophysiol. 2009;101:2668–78. doi: 10.1152/jn.91252.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Testing for anatomically specified regional effects. Hum Brain Mapp. 1997;5:133–6. doi: 10.1002/(sici)1097-0193(1997)5:2<133::aid-hbm7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18:631–40. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. Focal synchronization of ripples (80–200 Hz) in neocortex and their neuronal correlates. J Neurophysiol. 2001;86:1884–98. doi: 10.1152/jn.2001.86.4.1884. [DOI] [PubMed] [Google Scholar]
- Gusnard D, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–17. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–74. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Huettel SA, McKeown MJ, Song AW, Hart S, Spencer DD, Allison T, et al. Linking hemodynamic and electrophysiological measures of brain activity: evidence from functional MRI and intracranial field potentials. Cereb Cortex. 2004;14:165–73. doi: 10.1093/cercor/bhg115. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–24. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci. 2007;27:3244–51. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MS, MacDonald KD, Choi B, Dudek FE, Barth DS. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2000;84:1505–18. doi: 10.1152/jn.2000.84.3.1505. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Ripper B, Birbaumer N, Lutzenberger W. Dynamics of gamma-band activity in human magnetoencephalogram during auditory pattern working memory. Neuroimage. 2003;20:816–27. doi: 10.1016/S1053-8119(03)00350-1. [DOI] [PubMed] [Google Scholar]
- Kandel A, Buzsaki G. Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J Neurosci. 1997;17:6783–97. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Kim M, Ugurbil K, Kim DS, Konig P. A comparison of hemodynamic and neural responses in cat visual cortex using complex stimuli. Cereb Cortex. 2004;14:881–91. doi: 10.1093/cercor/bhh047. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, et al. Relationship between task-related gamma oscillations and BOLD signal: new insights from combined fMRI and intracranial EEG. Hum Brain Mapp. 2007;28:1368–75. doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, George N, Tallon-Baudry C, Martinerie J, Hugueville L, Minotti L, et al. The many faces of the gamma band response to complex visual stimuli. Neuroimage. 2005;25:491–501. doi: 10.1016/j.neuroimage.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Jung J, Mainy N, Dreher JC, Bertrand O, Baciu M, et al. Silence is golden: transient neural deactivation in the prefrontal cortex during attentive reading. Cereb Cortex. 2008;18:443–50. doi: 10.1093/cercor/bhm085. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Gold L. Brain function and neurophysiological correlates of signals used in functional neuroimaging. J Neurosci. 2003;23:3972–80. doi: 10.1523/JNEUROSCI.23-10-03972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–7. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lutzenberger W, Ripper B, Busse L, Birbaumer N, Kaiser J. Dynamics of gamma-band activity during an audiospatial working memory task in humans. J Neurosci. 2002;22:5630–8. doi: 10.1523/JNEUROSCI.22-13-05630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O, Lachaux JP. Neural correlates of consolidation in working memory. Hum Brain Mapp. 2007;28:183–93. doi: 10.1002/hbm.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–9. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- McCarthy G. Event-related potentials and functional MRI: a comparison of localization in sensory, perceptual and cognitive tasks. In: Berber C, Celesia G, Hashimoto I, Kagigi R, editors. Functional neuroscience: evoked potentials and magnetic fields. Amsterdam: Elsevier; 1999. pp. 3–12. [PubMed] [Google Scholar]
- Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, et al. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 2007;27:2424–32. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modayur B, Prothero J, Ojemann G, Maravilla K, Brinkley J. Visualization-based mapping of language function in the brain. Neuroimage. 1997;6:245–58. doi: 10.1006/nimg.1997.0301. [DOI] [PubMed] [Google Scholar]
- Motokawa K. Uber den Mechanismus der Entstehung und Hemmung der alpha-Wellen des Hirnpotentials. Tohoky J Experim Med. 1943;45:297–308. [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–4. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–51. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Ojemann GA. Awake operations with mapping in epilepsy. In: Schmidek H, Sweet W, editors. Operative neurosurgical techniques. Philadelphia: Saunders; 1995. pp. 1317–22. [Google Scholar]
- Ojemann GA, Schoenfield-McNeill J. Neurons in human temporal cortex active with verbal associative learning. Brain Lang. 1998;64:317–27. doi: 10.1006/brln.1998.1982. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Schoenfield-McNeill J, Corina D. The roles of human lateral temporal cortical neuronal activity in recent verbal memory encoding. Cereb Cortex. 2009;45:630–40. doi: 10.1093/cercor/bhn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA, Schoenfield-McNeill J, Corina DP. Anatomic subdivisions in human temporal cortical neuronal activity related to recent verbal memory. Nat Neurosci. 2002;5:64–71. doi: 10.1038/nn785. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Spencer SS, Spencer DD, McCarthy G. Comparison of cortical activation evoked by faces measured by intracranial field potentials and functional MRI: Two case studies. Human Brain Mapping. 1997;5:305. doi: 10.1002/(SICI)1097-0193(1997)5:4<298::AID-HBM16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, et al. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–83. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–76. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60-200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28:11526–36. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci. 2000;3:716–23. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Ritter P, Freyer F, Curio G, Villringer A. High-frequency (600 Hz) population spikes in human EEG delineate thalamic and cortical fMRI activation sites. Neuroimage. 2008;42:483–90. doi: 10.1016/j.neuroimage.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Schlosser MJ, Luby M, Spencer DD, Awad IA, McCarthy G. Comparative localization of auditory comprehension by using functional magnetic resonance imaging and cortical stimulation. J Neurosurg. 1999;91:626–35. doi: 10.3171/jns.1999.91.4.0626. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Ojemann GA, Haglund MM, Lettich E. Cerebral lateralization of neuronal activity during naming, reading and line-matching. Brain Res Cogn Brain Res. 1996;4:263–73. doi: 10.1016/s0926-6410(96)00062-6. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80-500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–52. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–54. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada J, Rasmussen T. Intracarotid injections of sodium amytal for the lateralization of cerebral speech dominance. J Neurosurg. 1960;17:266–82. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- Weber PB, Ojemann GA. Neuronal recordings in human lateral temporal lobe during verbal paired associate learning. Neuroreport. 1995;6:685–9. doi: 10.1097/00001756-199503000-00025. [DOI] [PubMed] [Google Scholar]
- Zanos T, Zanos S, Courellis S, Marmarelis V, Ojemann G. Nonlinear dynamic modeling of the relationship between local field potentials and neural discharges in human temporal cortex. Abstract: Soc Neurosci. 2006;748:18. [Google Scholar]