Abstract
Little is known about the processing of non-verbal sounds in the primary progressive aphasias. Here, we investigated the processing of complex non-verbal sounds in detail, in a consecutive series of 20 patients with primary progressive aphasia [12 with progressive non-fluent aphasia; eight with semantic dementia]. We designed a novel experimental neuropsychological battery to probe complex sound processing at early perceptual, apperceptive and semantic levels, using within-modality response procedures that minimized other cognitive demands and matching tests in the visual modality. Patients with primary progressive aphasia had deficits of non-verbal sound analysis compared with healthy age-matched individuals. Deficits of auditory early perceptual analysis were more common in progressive non-fluent aphasia, deficits of apperceptive processing occurred in both progressive non-fluent aphasia and semantic dementia, and deficits of semantic processing also occurred in both syndromes, but were relatively modality specific in progressive non-fluent aphasia and part of a more severe generic semantic deficit in semantic dementia. Patients with progressive non-fluent aphasia were more likely to show severe auditory than visual deficits as compared to patients with semantic dementia. These findings argue for the existence of core disorders of complex non-verbal sound perception and recognition in primary progressive aphasia and specific disorders at perceptual and semantic levels of cortical auditory processing in progressive non-fluent aphasia and semantic dementia, respectively.
Keywords: auditory perception, non-verbal sound, agnosia, dementia, environmental sounds
Introduction
Since the key descriptions of Mesulam (1982) and Warrington (1975) the primary progressive aphasias (PPA) have attracted substantial clinical and neurobiological interest. These disorders together constitute a paradigm for understanding the neurodegenerative pathologies that produce discrete neuropsychological syndromes associated with focal cortical atrophy. Within the frontotemporal lobar degeneration spectrum, two canonical PPA syndromes are recognized: progressive non-fluent aphasia (PNFA), associated with speech production breakdown and agrammatism, and atrophy predominantly affecting left inferior frontal and peri-Sylvian cortex (Mesulam, 1982; Nestor et al., 2003; Rohrer et al., 2008a, 2009); and semantic dementia, associated with impaired single word comprehension and additional non-verbal semantic deficits, and atrophy predominantly affecting the anterior temporal lobes with a left-sided emphasis (Warrington, 1975; Lambon Ralph et al., 2001; Hodges and Patterson, 2007; Rohrer et al., 2008a, b, 2009). The study of these disorders has focused on language deficits; however, spoken language (speech) is a highly specialized signal in acoustic, cognitive and evolutionary terms, representing a particularly significant species of complex sound (Griffiths et al., 1999). An accumulating body of convergent evidence suggests that disorders in the PPA spectrum are clinically, neuroanatomically and pathologically distinct, and further, that PNFA and semantic dementia are likely to have fundamentally different pathophysiological mechanisms (Nestor et al., 2003; Gorno-Tempini et al., 2004; Hodges and Patterson, 2007; Rohrer et al., 2008a). An important issue concerns the true language specificity of disorders in the PPA spectrum. These disorders might represent more general derangements of cortical signal processing and in particular, generic disorders of complex sound processing arising from more fundamental pathophysiological mechanisms in different PPA subtypes. However, the processing of non-verbal sounds has not been assessed systematically in PNFA or semantic dementia. There are clinical and neuroanatomical grounds to anticipate that PNFA and semantic dementia should lead to distinct deficits in the analysis and understanding of complex non-verbal sounds, and that these disorders of complex sound processing may provide insights complementary to the study of language processing in these disorders.
Clinically, patients with PNFA often report altered perception of sound, and non-verbal perceptual and expressive deficits sometimes dominate the clinical presentation (Confavreux et al., 1992; Otsuki et al., 1998; Uttner et al., 2006; Iizuka et al., 2007; Jörgens et al., 2008). Failure to correctly identify and respond to environmental noises not uncommonly accompanies semantic dementia, and a deficit in recognition of meaningful environmental sounds has been documented (Bozeat et al., 2000). Impaired recognition of familiar voices often accompanies the development of prosopagnosia as evidence of a more general defect of person knowledge in semantic dementia (Gainotti et al., 2003). Anatomically, the brunt of the pathological process in these diseases (Mesulam, 2003) generally falls on cortical regions that overlap with non-primary and association auditory cortical areas implicated in aspects of complex sound processing both in functional brain imaging studies in healthy subjects (Griffiths and Warren, 2002; Warren et al., 2005b) and in patients with focal brain lesions (Griffiths et al., 1999). More specifically, distinct neuroanatomical profiles, potentially relevant to the development of specific disorders of complex sound analysis, are associated with PPA: in PNFA, damage variably involves widespread peri-Sylvian areas (Nestor et al., 2003; Gorno-Tempini et al., 2004; Rohrer et al., 2008a, 2009), while in semantic dementia, damage is more stereotyped and typically anterior and inferior (predominantly left-sided) temporal lobe areas are most strikingly affected (Hodges and Patterson, 2007; Rohrer et al., 2008b, 2009).
By analogy with other categories of sensory information, the cortical processing of complex sounds is likely to be broadly hierarchically organized with more or less distinct stages of early perceptual analysis, representation of the structural features of auditory objects (apperceptive level) and attribution of meaning to those objects (semantic level) (Griffiths and Warren, 2002, 2004; Warren et al., 2005b) However, several issues complicate the assessment of complex sound processing in patients with cognitive impairment (Griffiths et al., 1999). In contrast to the visual agnosias, analogous disorders of complex sound processing have proved relatively difficult to define and clinically relevant models of auditory processing are needed. Furthermore, established neuropsychological instruments and normative data to assess these disorders systematically are lacking. The available clinical evidence has mainly been obtained either for visual without parallel auditory assessments, or via cross-modal response procedures (Bozeat et al., 2000; Garrard and Carroll, 2006, Crutch and Warrington, 2008). Within the auditory modality, instruments to specifically assess different levels of processing and potentially relevant interactions between processing stages (Clarke et al., 1996; Rogers et al., 2004; Kveraga et al., 2007) have not been widely applied. Finally, neuropsychological tests that rely on sustained attention, naming or other cross-modal response procedures may be contaminated by other cognitive deficits, making interpretation of any primary complex sound deficit more difficult.
Here, we set out to assess the processing of complex non-verbal sounds in detail, in a consecutive series of patients with the canonical PNFA and semantic dementia subtypes of PPA. We designed a novel experimental neuropsychological battery to probe complex sound processing at perceptual, apperceptive and semantic levels of processing, using within-modality response procedures that minimized other cognitive (in particular, linguistic) demands. In order to assess the modality specificity of any auditory disorder identified, we designed matching tests in the visual modality. Our hypotheses were three-fold: that complex sound processing is disordered in PPA; that specific disorders of complex sound processing accompany and distinguish the PNFA and semantic dementia subtypes of PPA; and that the characteristics of the cortical auditory syndromes reflect the core pathophysiological processes underpinning these PPA subtypes.
Methods
Subjects
Twenty consecutive patients (12 males) who met current consensus criteria (Neary et al., 1998) for a diagnosis of PNFA (n = 12) or semantic dementia (n = 8) were recruited from a tertiary cognitive disorders clinic. Twelve healthy control subjects with no history of neurological or psychiatric illness also participated. Demographic data for all subjects are summarized in Table 1. Patient and control groups were well-matched for educational background, and the patient groups were well-matched for disease duration. Males were under-represented in the control group relative to the patient sample. The mean age of the patients with semantic dementia was younger (Mann–Whitney P < 0.01) than either the PNFA group or the healthy control group. Age and gender were accordingly incorporated as covariates in all subsequent analyses.
Table 1.
General demographic data for all subjects
|
N |
Age (years) | Education (years) | Disease duration (years) | ||
|---|---|---|---|---|---|
| Total | Female | Mean (SD) | Mean (SD) | Mean (SD) | |
| PNFA | 12 | 4 | 73.1 (6.1) | 13.4 (2.6) | 6.4 (2.5) |
| Semantic dementia | 8 | 4 | 61.5 (4.9) | 13.1 (2.3) | 6.3 (1.4) |
| Control | 12 | 8 | 71.3 (4.9) | 12.0 (2.3) | N/A |
SD = standard deviation
Brain image acquisition
Brain MRI scans were acquired in all subjects on a 1.5 T GE Signa scanner (General Electric, Milwaukee, WI). T1-weighted volumetric images were obtained using a spoiled fast gradient recalled acquisition in steady state (GRASS) sequence technique with a 24 cm field of view and 256 × 256 matrix to provide 124 contiguous 1.5 mm thick slices in the coronal plane. The scan acquisition parameters were as follows: repetition time = 15 ms; echo time = 5.4 ms; flip angle = 15°; inversion time = 650 ms.
Assessment of subcortical auditory function
In the majority of patients (14/20), peripheral hearing was assessed using pure tone audiometry (PTA), tympanometry and transient otoacoustic emissions. In the remaining patients and all healthy control subjects a brief PTA screening assessment was used. Auditory brainstem responses were also recorded in a subset of patients (10/20). These procedures are summarized in Appendix A, available as supplementary material online. For each subject, pure tone thresholds at 0.5, 1 and 2 KHz at each ear were averaged to give a ‘3 Frequency Average’ (3FA), and thresholds at 4, 6 and 8 KHz were averaged to give a ‘High Frequency Average’ (HFA). 3FA and HFA were then compared with age-corrected norms (Davis, 1995) and categorized as normal or abnormal. Lastly, for each subject, categorizations were collapsed across ears to give a single measure for each subject within each hearing range (3FA-S, HFA-S), which was considered abnormal only if both ears were abnormal.
General neuropsychological assessment
General neuropsychological functions were assessed in patients using standard measures (summarized in Table 2), at the time of initial ascertainment and contemporaneous with the experimental assessment. Baseline tests provided a neuropsychological characterization of PPA subgroups: these included measures of non-verbal fluid intelligence and executive processing (Raven's matrices: Raven et al., 2003; Trail Making: Reitan, 1959), attention (Dual Number Cancellation: Mohs et al., 1997), object naming (novel test), spoken word repetition (McCarthy and Warrington, 1984), word comprehension (a shortened 30 item version of the British Picture Vocabulary Scale, Dunn et al., 1982), grammar processing (a shortened 20 item version of the Test of Reception of Grammar: Bishop, 1989), reading (novel test of irregular words) and face recognition (Warrington and James, 1967). Contemporaneous tests allowed correlation of general neuropsychological functions with experimental findings: these tests comprised measures of executive function (Non-Verbal Design Fluency, Delis et al., 2001) verbal semantic processing (Synonyms test, Warrington et al., 1998), visual (pictorial) recognition memory (the Camden Memory Tests, Warrington, 1996) and visual apperceptive processing (the Object Decision test, Warrington and James, 1991). All patients completed the Mini-Mental State Examination (MMSE) (Folstein et al., 1975), a general cognitive screening instrument, as an index of disease severity at the time of the experimental assessment.
Table 2.
Results of general neuropsychological assessment: raw scores and differences in group means adjusted for age and gender
| Raw scores Mean (SD) |
Differences in group means Mean difference (95% CI) |
||||||
|---|---|---|---|---|---|---|---|
| Max score | PNFA (N = 12) | Semantic dementia (N = 8, *N = 7) | Control (N = 40) | PNFA–semantic dementia | PNFA–Control | Semantic dementia–control | |
| Baseline Neuropsychology | |||||||
| Non-verbal intelligence (shortened Raven's matrices) | 12 | 5.2 (2.8) | *8.1 (2.7) | 7.4 (2.7) | −1.6 (−4.5 to 1.5) | −1.8 (−3.7 to 0.1) | −0.2 (−2.6 to 2.0) |
| Naming | 20 | 13.8 (5.7) | *4.1 (3.8) | 19.4 (1.1) | 10.4 (5.7 to 14.0) | −5.2 (−8.7 to −2.7) | −15.7 (−18.1 to −12.2) |
| Word-picture matching (shortened BPVS) | 20 | 19.3 (1.2) | *12.1 (5.4) | 19.9 (0.3) | 7.4 (3.7 to 11.2) | −0.4 (−1.5 to 0.1) | −7.8 (−11.7 to −4.2) |
| Famous face recognition | 12 | 10.5 (2.2) | *6.4 (5.3) | 11.6 (0.7) | 3.8 (0.3 to 7.9) | −1.2 (−2.7 to −0.1) | −5.0 (−8.9 to −1.6) |
| Famous face recall | 12 | 5.9 (3.6) | *1.0 (2.2) | 9.6 (1.6) | 4.4 (1.1 to 6.8) | −3.9 (−6.2 to −1.9) | −8.3 (−9.6 to −5.7) |
| Repetition | 30 | 24.5 (9.3) | *30.0 (0.0) | 29.9 (0.3) | −5.1 (−12.1 to −1.4) | −5.2 (−11.5 to −1.4) | −0.1 (−1.6 to 1.3) |
| Reading | 30 | 19.5 (8.1) | *14.9 (10.7) | 27.0 (2.9) | 5.0 (−4.5 to 14.1) | −7.3 (−12.8 to −3.2) | −12.4 (−20.2 to −4.7) |
| Grammar (shortened TROG) | 20 | 16.1 (2.6) | *15.6 (3.6) | 19.4 (0.7) | 0.6 (−2.3 to 4.1) | −3.3 (−4.9 to −1.8) | −3.9 (−6.9 to −1.7) |
| Dual number cancellation | 40 | 13.4 (5.0) | *22.7 (5.9) | 24.9 (5.3) | −5.7 (−11.0 to −0.7) | −10.1 (−13.1 to −6.5) | −4.4 (−8.9 to 0.2) |
| Trails Making—condition A (scaled) | – | 4.9 (3.4) | *8.4 (3.2) | 9.3 (2.2) | −3.5 (−6.3 to −0.4) | −4.3 (−6.1 to −2.1) | −0.8 (−3.2 to 1.5) |
| Trails Making—condition B (scaled) | – | 4.6 (2.9) | *9.0 (3.4) | 10.2 (2.7) | −4.4 (−7.6 to −1.8) | −5.6 (−7.5 to −3.8) | −1.2 (−3.3 to 1.7) |
| Contemporaneous Neuropsychology | |||||||
| MMSE | 30 | 20.9 (6.6) | 18.9 (6.8) | −0.6 (−9.9 to 10.5) | |||
| Object decision | 20 | 17.1 (6.1) | 16.5 (3.1) | 17.3 (2.5) | 0.8 (−1.4 to 3.4) | −0.3 (−1.2 to 0.7) | −1.1 (−3.7 to 1.0) |
| NVDF (sum of scaled scores) | – | 6.4 (1.8) | 8.6 (2.5) | 12.4 (2.4) | −2.2 (−4.2 to −0.2) | −5.9 (−7.2 to −4.7) | −3.7 (−5.4 to −2.0) |
| Recognition memory (pictorial) | 30 | 27.4 (3.3) | 24.8 (7.4) | 29.6 (0.7) | 3.1 (−1.1 to 9.4) | −2.0 (−4.2 to −0.7) | −5.1 (−11.6 to −1.1) |
| Synonyms—concrete (2nd error) | 25 | 10.8 (6.1) | 3.9 (3.6) | 21.5 (5.3) | 8.7 (4.1 to 13.0) | −10.1 (−13.5 to −5.6) | −18.8 (−21.2 to −15.2) |
| Synonyms—abstract (2nd error) | 25 | 9.3 (6.3) | 4.4 (7.4) | 22.1 (4.8) | 4.9 (−3.0 to 10.1) | −13.1 (−16.1 to −9.2) | −18.0 (−22.2 to −11.0) |
Significant differences between groups are in bold. Controls comprised a previous age- and gender-matched sample.
BPVS = British Picture Vocabulary Scale; NVDF = Non-Verbal Design Fluency; TROG = Test of Reception of Grammar. CI = confidence interval.
Experimental assessment of auditory cognition
General testing procedure
All experimental neuropsychological tests were run under Matlab 7.3® (www.mathworks.com) on a notebook computer. Subject responses were entered directly by the experimenter and saved for offline analysis. Sounds were delivered using a high-fidelity external soundcard (Edirol® UA-4FX) and linear headphones (Sennheiser® HD265) at comfortable listening level (peak absolute sound pressure levels between 70 and 100 dB). Images were presented on a 17 in. high-resolution monitor. For all tests, performance on each test item was probed using a simple question with two alternative responses. Answers could be given verbally, or in the case of speech output difficulty, by pointing to a prompt sheet displaying the two responses. Each test was prefaced with a brief example phase to ensure that the subject understood the test.
Early perceptual level
This test was designed to assess early perceptual processing of auditory stimuli beyond the level of elementary sensory encoding in the auditory periphery, based on the discrimination of complex sound properties. Most natural sounds contain energy distributed across multiple frequencies with variable energy (intensity). This patterning of frequency and intensity is the ‘spectral shape’ of the sound (Warren et al., 2005a) and is presented schematically in Fig. 1A. Spectral shape is one important determinant of timbre, a key factor in the perception of sound identity. Since spectral shape perception necessitates the integration of intensity information across multiple frequency bands, it is operationally analogous to shape perception in vision, which requires the integration of information across two (spatial) dimensions. Here, we designed tests to manipulate shape information in auditory and visual objects, respectively.
Figure 1.
Schematic of experimental stimuli and presentation sequences (A and B). Schematics of auditory and visual early perceptual stimuli, and the presentation sequence used. (C) Schematic of spectral inversion of a complex sound, as used in the auditory apperceptive test. (D and E) Examples of auditory and visual semantic stimulus pairs, and a schematic of the presentation sequence used. t = time (s).
Stimuli
Sounds were digitally generated using a Matlab-based signal-synthesis algorithm (Warren et al., 2005a) enabling generation of harmonic series with specified spectral shape. Different ‘trapezoidal’ spectral shapes were created in the frequency domain by varying the gradient of the ‘ascending’ slope of the frequency trapezoid (see Fig. 1A and example sound 1, available as supplementary material online). Frequency bandwidth, sound duration and temporal envelope were held constant. Fundamental frequency and average intensity (root mean square level) varied across the stimulus set, to reduce any tendency for subjects to use the absolute intensity level in a particular frequency band to perform the test. Thirty-two sound pairs were created: 16 ‘same’ pairs comprising identical sounds, and 16 ‘different’ pairs comprising sounds that differed only in spectral shape. Sounds in each pair were presented sequentially (inter-stimulus interval 1 s).
As visual analogues of the spectral shape stimuli, rectangles of varying dimensions were generated by holding total flux (area) constant, while varying the height/length ratio. Rectangles had constant hue and were presented on a uniform black background (Fig. 1B). Thirty-two rectangle pairs were created (16 same, 16 different). To minimize differences in working memory load between stimulus modalities, rectangles within each pair were presented sequentially with the same inter-stimulus interval as the sound pairs.
Task
Stimulus pairs were presented in a fixed balanced order: experimental conditions were evenly distributed in a non-predictable fashion throughout the test sequences. For each test, after presentation of each pair, the subject was asked ‘Are they the same or different?’
Apperceptive level
This test was designed to assess the status of ‘apperceptive’ processing for complex sounds. The existence of an apperceptive level of object processing is well-established in vision, and corresponds to a post-sensory stage of perceptual categorization that generates (or accesses) structural representations: sets of distinctive geometric and volumetric features that enable object identity to be abstracted despite changing contexts and viewpoints. Deficits at this level produce ‘apperceptive agnosia’, in which patients characteristically have difficulty in identifying the objects presented from unusual (non-canonical) viewpoints or under degraded viewing conditions. While limited evidence suggests that apperceptive deficits also exist in the auditory modality (e.g. in music: Peretz et al., 1994), their generality remains uncertain. In order to assess the integrity of putative pre-semantic object representations for complex sounds, we devised a test requiring differentiation of real (possible) and novel (impossible) sounds that might be considered an auditory ‘object decision’ test, analogous to the object decision test in vision (Warrington and James, 1991). The key experimental manipulation here was spectral inversion (Blesser, 1972). The spectral inversion procedure flips the energetic frequencies present in a broadband sound (i.e. exchanges the energy present between higher and lower frequencies) about a user-specified frequency value (Fig. 1C) to create a frequency structure that is ‘impossible’ in a natural sound. Example stimuli are available online: sound 2a is a natural animal call and sound 2b is the same call after spectral inversion. This procedure retains the spectrotemporal complexity of a natural sound but produces a percept of an artificial or ‘alien’ sound in normal listeners (Scott et al., 2000). While spectral inversion animal calls (for example) sound highly artificial, the procedure preserves many acoustic features of the original sound, such that spectral inversion and natural sounds are not differentiated by spectral content or temporal envelope alone. Rather, spectral inversion alters more complex acoustic features, including spectral and joint spectrotemporal modulations that are likely to be critical for disambiguating natural from synthetic sounds (e.g. Chi et al., 2005). We also wished to investigate whether this process of auditory object representation might be modulated by the relative ease or difficulty with which individual stimuli are identified (the procedure used to quantify sound identifiability is described in Appendix B, available as supplementary material online).
Stimuli
Twenty animal and human vocalizations were selected from online sound databases (e.g. www.sonomic.com; www.soundrangers.co.uk). Individual items were chosen to vary in the ease with which they are identified by normal subjects: this effect was quantified in a second group of healthy age-matched controls who did not participate in the experiment proper [n = 18, 17 females; age: mean = 68.7 years [standard deviation (SD) = 6.7]; National Adult Reading Test IQ: mean = 122.6 (SD = 4.5)]. For each item, subjects were asked (i) ‘What is it?’ and (ii) ‘How difficult was that to recognize?’ (subjects answered using a 6-point Likert scale: 0 = did not recognize; 1 = very difficult; 2 = difficult; 3 = moderate; 4 = easy; 5 = very easy). Across the set of sounds, subject responses to (i) provided an index of frequency of correct identification while (ii) provided a rating of difficulty of identification for each sound. Further details about this procedure, the complete stimulus list and their corresponding ratings are presented in Appendix B, available as supplementary material online. For the experimental test, each natural sound was modified using a method of spectral inversion to create an additional set of 20 novel sounds.
As a visual analogue of this novel auditory apperceptive test, patients completed an established and normed test of visual apperception (Object Decision, Warrington and James, 1991) based on the discrimination of real from novel 2D silhouettes. The test comprises 20 arrays of four silhouettes.
Task
For the auditory apperceptive test, the 40 sounds (20 non-spectral inversion, 20 spectral inversion) were presented individually in a fixed balanced order: conditions were randomly distributed throughout the test sequence. For each sound, the subject was asked: ‘Is it a real thing or not a real thing?’ The visual apperceptive test was administered in standard fashion (Warrington and James, 1991): on each trial, the subject was shown the four silhouettes in an array, and asked to point to the real object.
Semantic level
This test was designed to assess the association of conceptual meaning with complex sounds (semantic level processing). The status of ‘associative agnosia’ is less well-established in the auditory than the visual modality (e.g. De Renzi et al., 1969; Taylor and Warrington, 1971; Anaki et al., 2007), particularly in the context of degenerative disease (e.g. Bozeat et al., 2000; Garrard and Carroll, 2006). Here, we used a within-modality test to assess semantic processing of sounds and their visual analogues.
Stimuli
Environmental sounds were obtained from online sound databases (e.g. www.sonomic.com; www.soundrangers.co.uk). Thirty-two individual sounds representing a range of human and animal sounds and environmental noises were chosen and arranged to constitute 32 pairs of sequentially presented sounds (see Table B2, Appendix B, available as supplementary material online). Picture analogues of the sound pairs were obtained using online image search engines and image databases (e.g. http://images.google.co.uk, www.flckr.co.uk). Pictures were 32 visual object parts, chosen such that each object part was easily recognizable as a distinct entity in isolation from the rest of the larger object to which it belongs. The identifiability of the sounds and pictures was assessed using the same procedure as for the stimuli used in the apperceptive test (Appendix B, available as supplementary material online) in the same group of untrained healthy age-matched controls. Both auditory and visual semantic stimuli were highly recognizable: identifiability ratings showed that although pictures were overall easier to identify than sounds, sounds were nonetheless frequently identified successfully, and moreover, stimulus identification difficulty ratings were similar between the two modalities.
In the experimental test, sounds were paired such that the individual sounds in a pair had dissimilar acoustic characteristics, to reduce the availability of perceptual matching cues. In 16 ‘same’ pairs, sounds were produced by the same source (e.g. horse neighing, horse galloping; example sound 3, available as supplementary material online). In 16 ‘different’ pairs, sounds were produced by different sources (e.g. horse neighing, human coughing). The test design is presented schematically in Fig. 1D. All 32 sounds appeared once in the ‘same’ and once in the ‘different’ condition, to control for item-specific effects. From the set of 32 pictures, 16 ‘same’ and 16 ‘different’ pairs were created such that pictures within a pair had dissimilar visual perceptual characteristics (Fig. 1E). All 32 pictures appeared once in the ‘same’ and once in the ‘different’ condition. To minimize differences in working memory load between stimulus modalities, pictures within each pair were presented sequentially with the same inter-stimulus interval as the sound pairs. All sound and picture pairs, together with their normative data, are listed in Table B2 in Appendix B (available as supplementary material online).
Task
Stimulus pairs were presented in a fixed balanced order: conditions were randomly presented throughout the test sequence. To reduce any effects from semantic priming between modalities, subjects completed the semantic picture test first, followed by at least one other unrelated test and then the semantic sound test. On each sound trial, the subject was asked: ‘Are the sounds made by the same thing or different things?’ On each picture trial the subject was asked: ‘Are the pictures part of the same thing or different things?’
Analysis of behavioural data
Group data
Linear regression models were used to relate scores for each test (general neuropsychological and experimental) to group membership (PNFA, semantic dementia or healthy control). Each model included age and gender as covariates, with the exception of the models for non-verbal design fluency and trail making since these are internally corrected for age and gender. Since normality assumptions were not satisfied, bootstrap confidence intervals (95% CIs, bias-corrected, accelerated with 2000 replications) are reported and used to infer statistical significance. The subset of ‘real’ (non-spectral inversion) sounds in the auditory apperceptive test was submitted to a further analysis: a mixed effects logistic regression model was used to relate, for each sound, the probability of a correct response to its corresponding difficulty rating (quantified using the procedure described in Appendix B). The model included fixed effects (sound difficulty rating, group membership and their interaction) and crossed random effects (individual subjects, individual sounds). The model was fitted using a Laplacian approximation. All analyses were carried out using Stata 10™.
In order to assess factors influencing performance on particular components of the experimental auditory battery, patient performance on individual auditory tests was assessed in relation to other tests in the battery, general neuropsychological functions and general measures of disease severity (clinical disease duration, MMSE) using a correlation analysis (Spearman's ρ). This analysis was carried out separately in the PNFA and semantic dementia groups, to take into account the different auditory profiles of each PPA subgroup.
Individual data: Auditory and visual cost analyses
Individual subject performance profiles were examined for modality specific effects. For both the perceptual and semantic levels of assessment, individual subjects were categorized according to whether their performance showed an ‘auditory cost’ (performance worse on the auditory than the analogous visual test) or no auditory cost (performance equivalent between modalities or worse in the visual modality). Subjects were also categorized according to whether thei performance showed a ‘visual cost’ at each test level using analogous criteria. Proportions of subjects showing costs were compared between groups using exact logistic regression adjusting for age and gender.
Results
Brain imaging findings
Individual brain magnetic resonance findings for patients in the PNFA and semantic dementia groups are presented in Fig. 2. Inspection of sections aligned to show key auditory cortical areas in and surrounding the superior temporal plane gives an impression of the range of variation in the distribution and severity of structural damage involving these areas in PNFA and semantic dementia. In PNFA, atrophy showed wide variation both in the degree of leftward cerebral asymmetry and, within each hemisphere, the relative involvement of anterior and posterior areas. In contrast, the semantic dementia group showed a more uniform atrophy pattern with involvement chiefly of the anterior temporal lobes, initially with predominant involvement of the left temporal lobe and increasingly bitemporal involvement with increasing disease duration.
Figure 2.
MRI brain sections showing auditory cortices in PNFA and semantic dementia (SD) patients. Sections of each patient's volumetric T1-weighted magnetic resonance brain volume are shown. Sections have been tilted to run along the superior temporal plane (STP) to show key auditory cortical areas: the site of primary auditory cortex in Heschl's gyrus (HG), and surrounding non-primary areas in anterior temporal lobe (aTL), posterior superior temporal gyrus and planum temporale (posterior temporal lobe: pTL), insula (ins) and inferior parietal lobe (iPL). For all brain images, the left hemisphere is shown on the left. For reference normal auditory cortical anatomy is shown on the inset sections (lower right) from the brain of a healthy younger individual. Brain images from the PNFA group are shown above and the semantic dementia group below. Above each image is shown the patient's age (left) and clinical disease duration (right) in years at the time of the scan. Within each group brain images have been arranged loosely in order of disease duration; the PNFA group had an older age range and a wider variation in age, and to reflect this, images have been further clustered to show younger patients above and older patients below.
Subcortical auditory function
Abnormal PTA profiles were documented in 2/12 patients in the PNFA group (both 3FA; bilateral), 2/8 patients in the semantic dementia group (one 3FA, one HFA; bilateral), and one healthy control subject (HFA; bilateral). Otoacoustic emissions were consistent with PTA thresholds for all individuals. Abnormal auditory brain-stem responses were recorded in 4/6 patients (two bilateral) in the PNFA group and 2/4 patients (none bilateral) in the semantic dementia group. PTA and auditory brain-stem response data are summarized in Table A1 (Appendix A, online supplementary material).
General neuropsychological assessment
On baseline assessment of general neuropsychological functions, the PNFA and semantic dementia groups had profiles consistent with their clinical diagnoses (Table 2): the PNFA group showed impairments chiefly affecting naming, single word repetition, reading, executive function and attention, while the semantic dementia group showed more severe impairment of naming with additional deficits of single word comprehension and face recognition but normal single word repetition and executive functions. On contemporaneous general neuropsychological assessment, both groups showed normal performance in the visual object decision task but impaired performance on other measures relative to healthy controls (Table 2). The PNFA group performed significantly less well than the semantic dementia group on non-verbal design fluency, while the semantic dementia group performed significantly less well than the PNFA group on the concrete words component of the synonyms test.
Experimental assessment of auditory cognition
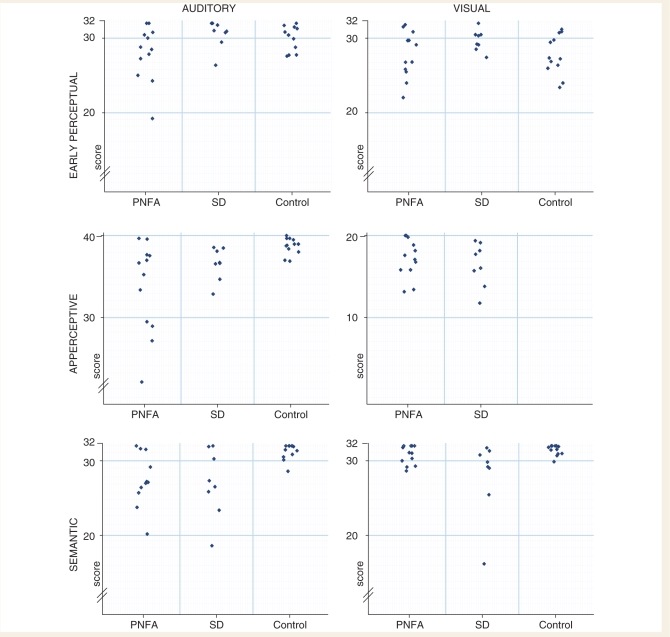
Raw behavioural data are shown in Fig. 3. Bootstrap analyses as described in the Methods section were used to determine the significance of group differences and are presented in Table 3. The overall patterns of disease group performance across the set of experimental tests are summarized in Table 4.
Figure 3.
Performance on experimental subtests: raw data. SD = semantic dementia
Table 3.
Experimental data: differences in group means adjusted for age and gender
| Auditory |
Visual |
|||||
|---|---|---|---|---|---|---|
| 95% Confidence Interval |
95% Confidence Interval |
|||||
| Mean difference | Lower | Upper | Mean difference | Lower | Upper | |
| Auditory early perceptual | Visual early perceptual | |||||
| PNFA–Semantic dementia | −4.2 | −9.1 | −1.1 | −1.5 | −5.4 | 1.7 |
| PNFA–Control | −3.4 | −6.5 | −1.4 | −0.1 | −3.0 | 2.4 |
| Semantic dementia–Control | 0.8 | −1.5 | 3.3 | 1.4 | −1.0 | 3.9 |
| Auditory apperceptive | Visual apperceptivea | |||||
| PNFA–Semantic dementia | −1.5 | −5.2 | 1.8 | 0.9 | −1.8 | 4.5 |
| PNFA–Control | −5.9 | −9.7 | −3.4 | |||
| SD–Control | −4.4 | −7.2 | −2.0 | |||
| Auditory semantic | Visual semantic | |||||
| PNFA–Semantic dementia | 0.9 | −3.9 | 5.6 | 3.0 | 0.3 | 8.9 |
| PNFA–Control | −4.1 | −6.5 | −2.2 | −1.4 | −3.0 | −0.5 |
| Semantic dementia–Control | −5.0 | −9.6 | −1.2 | −4.4 | −11.1 | −1.7 |
Significant differences between groups are in bold.
a Although the visual apperceptive (Object Decision) test aimed to probe similar cognitive processes to the auditory apperceptive test, it is not precisely analogous: see text for details.
Table 4.
Summary of disease group performance patterns on experimental tests
| Disease group |
||||
|---|---|---|---|---|
| PNFA |
Semantic dementia |
|||
| Cognitive processing level | Auditory modality | Visual modality | Auditory modality | Visual modality |
| Early perceptual | ++ | − | − | − |
| Apperceptive | + | − | + | − |
| Semantic | + | + | + | ++ |
++ = significant deficit compared with alternate patient group and healthy controls;
+ = significant deficit compared with healthy controls; − = no significant deficit;
Early perceptual level
On the auditory early perceptual test, the PNFA group was significantly more impaired than both the healthy control group and the semantic dementia group. The performance of the semantic dementia group did not differ significantly from controls. Performance on the test did not differ materially for patients with and without peripheral hearing loss. On the analogous early visual perception test, performance was equivalent between disease groups and did not differ significantly from controls.
Apperceptive level
On the auditory apperceptive test, both the PNFA group and the semantic dementia group were impaired, relative to healthy controls. The performance of the PNFA group did not differ significantly overall from the semantic dementia group. However, inspection of individual data (Fig. 3) suggests that there may be a subgroup of patients with PNFA with more marked impairment on this test.
The performance patterns across the three groups were further assessed for any effect of recognition difficulty (identifiability) within the subset of ‘real’ (non-spectral inversion) sounds. Sound identifiability was significantly associated with performance in the healthy control group: a one unit reduction in the recognition difficulty of a sound (Appendix B, available as supplementary material online) was associated with a 110% increase in the odds of correctly stating that the sound was real (95% CI: 6–316%, P = 0.03). A similar magnitude of association was seen in the PNFA group [75% odds increase per unit difficulty reduction (95% CI: 8–183%, P = 0.02)], but not in the semantic dementia group [9% odds increase per unit difficulty reduction (95% CI: –52–144%, P = 0.8)]. Despite the variation in the significance of this association across the three groups (significant in the control and PNFA groups; non-significant in the semantic dementia group), a global test for a difference in the association among groups was not statistically significant, reflecting the wide CIs within each group.
On the standardized visual apperceptive (Object Decision) test, regression analysis did not show significant differences in mean performance between the disease groups. One of the 12 patients with PNFA and 1 of the 8 patients with semantic dementia scored below the 5th percentile of published age control norms (Warrington and James, 1991). Although this visual test and the experimental auditory apperceptive test were not directly comparable, it is noteworthy that on the corresponding auditory test 7/12 patients with PNFA and 5/8 patients with semantic dementia scored below the range of the healthy control sample. These findings would be in keeping with a more severe impairment of apperceptive processing within the auditory than the visual modality.
Semantic level
On the auditory semantic test, the PNFA and the semantic dementia groups were comparably impaired relative to healthy controls. The performance of the PNFA group did not differ significantly from the semantic dementia group. On the visual semantic test, both disease groups were impaired with respect to the control group; however, performance of the semantic dementia group was significantly worse than the PNFA group.
Correlation analyses
In the PNFA group, performance on both the auditory perceptual task and the auditory semantic task was positively associated (ρ 0.60; P < 0.05) with performance on the auditory apperceptive task. Performance on the auditory apperceptive task was also positively associated (ρ 0.70; P < 0.05) with performance on the visual object decision task. Experimental test performance was not significantly associated with other contemporaneous general neuropsychological or disease severity measures in the PNFA group. In the semantic dementia group (but not the PNFA group), performance on the auditory semantic task was strongly positively associated (ρ 0.97; P < 0.001) with performance on the visual semantic task, with some evidence of a positive association with performance on the Synonyms test (ρ 0.65; P = 0.08); performance on the auditory semantic task was also associated with general measures of disease severity (disease duration, ρ –0.97, P < 0.001; MMSE score, ρ 0.89, P < 0.001), but not with auditory apperceptive performance. In neither the PNFA nor the semantic dementia group was performance on any experimental auditory task significantly associated with a contemporaneous measure of executive function (non-verbal design fluency).
Individual data: auditory and visual cost
There was evidence (P < 0.05) that patients with PNFA were more likely than patients with semantic dementia to exhibit an auditory cost on the early perceptual test, but not on the semantic test (detailed results presented in Appendix C, Table C1, available as supplementary material online). Examining the individual data, on the early perceptual test, 7/12 patients with PNFA showed an auditory cost, compared with 1/8 patients with semantic dementia; and on the semantic test, 10/12 patients with PNFA showed an auditory cost, compared with 4/8 patients with semantic dementia. There was also borderline statistically significant evidence (0.05 < P < 0.1) that individuals with PNFA were less likely to exhibit a visual cost than each of the other groups.
Discussion
Here, we have defined specific disorders of complex non-verbal sound processing in canonical subtypes of PPA; PNFA and semantic dementia. Both the PNFA and semantic dementia patient groups had deficits of non-verbal sound analysis compared with healthy age-matched individuals. There was evidence for relative specificity of deficits in PNFA and semantic dementia: deficits of early auditory perceptual analysis were more common in PNFA; deficits of semantic processing occurred in both syndromes but were relatively modality specific in PNFA and part of a more severe generic semantic deficit in semantic dementia; while deficits of apperceptive processing occurred in both PNFA and semantic dementia, albeit with evidence that the mechanism of the deficit differed between the two syndromes. Patients with PNFA were more likely to show more severe auditory than visual deficits as compared to patients with semantic dementia. The experimental design here ensured that our findings were not attributable to the effect of certain potentially confounding factors, such as cross-modal or verbal-response procedures. While it is likely that the experimental tests engaged other cognitive operations (for example, non-verbal working memory and executive processing) in addition to complex sound processing per se, we did not find evidence in a correlation analysis that group-specific effects were attributable to such generic cognitive deficits; nor did these differences simply reflect subcortical auditory dysfunction or disease duration.
The auditory profiles of the PNFA and semantic dementia groups suggest likely cognitive mechanisms in these two PPA syndromes. The more severe impairments at earlier stages of perceptual processing of complex sounds in PNFA versus semantic dementia are consistent with a core perceptual defect in the cortical processing of complex sound information in PNFA. The additional deficits of apperceptive and semantic levels of processing exhibited by patients with PNFA would follow as a consequence of the primary perceptual defect, if complex sound information is processed serially along a hierarchically organized cortical pathway (Griffiths and Warren, 2004). The observation of correlated performance on perceptual, apperceptive and semantic tests in the PNFA group here offers some support for such an interpretation. However, this evidence does not rule out the possibility of additional non-verbal semantic impairment in PNFA (we note, for example, that patients with PNFA did not perform normally on a visual semantic matching test, even though they performed significantly better than patients with semantic dementia). Cortical processing of complex sound information need not be exclusively serial: indeed, interactions between different processing stages are likely on both theoretical and empirical grounds (Griffiths and Warren, 2004; Rogers et al., 2004; Kveraga et al., 2007). In contrast to the situation in PNFA, auditory deficits exhibited by patients with semantic dementia were restricted to higher order processing stages and semantic deficits were more severe, with correlated involvement of the auditory and visual modalities: this is the pattern of deficits predicted to arise from a core defect of multimodal semantic knowledge, consistent with a growing body of neuropsychological work in semantic dementia (Bozeat et al., 2000; Lambon Ralph et al., 2001; Coccia et al., 2004; Hodges and Patterson, 2007; Rami et al., 2007).
The patterns of performance of the disease groups on the auditory apperceptive test may give further clues to the core cognitive deficits in each group: both groups were impaired; however the PNFA group, unlike the semantic dementia group, exhibited sensitivity to the identifiability of the stimuli, and auditory apperceptive performance was correlated with auditory semantic performance in the PNFA group but not the semantic dementia group. Further, in the PNFA group (but not the semantic dementia group), auditory apperceptive performance was correlated with visual apperceptive performance, raising the possibility that analogous cortical mechanisms might mediate object representation in each modality. Perceptual attributes of individual sounds are likely to have contributed substantially to the difficulty of identification factor that we have quantified here: cat calls, for example, have rather variable spectrotemporal characteristics despite belonging to a single, rather narrow, semantic field. We propose that loss of fidelity of perceptual representations affects categorization and ultimately recognition of complex sounds in PNFA, whereas sound recognition in semantic dementia is chiefly affected by a primary semantic level impairment. As the PNFA and semantic dementia groups were comparably impaired in their overall performance on the auditory apperceptive test, the processing of basic categorical information about the characteristics of natural sounds may depend both on perceptual and ‘top down’ semantic factors, as proposed in certain theoretical models of auditory object processing (Griffiths and Warren, 2004). Indeed, patients with semantic dementia have been shown to have deficits of visual object decision processes, and the relative dependence on semantic factors (e.g. processing of chimaeric versus nonsense objects) is likely to influence performance (Hovius et al., 2003). However, in line with previous experimental evidence from other modalities in semantic dementia, it may be that super-ordinate categorization of complex sounds can be achieved even where explicit identification is not possible (Hodges and Patterson, 2007; Crutch and Warrington, 2008). It is also possible that at least some patients with semantic dementia may develop a true apperceptive deficit for the representation of complex auditory objects, perhaps analogous to deficits of perceptual face analysis previously documented in some patients with progressive prosopagnosia and more posterior extension of the pathological process within the temporal lobe (Joubert et al., 2003). We do not argue for a simple dichotomy of perceptual and semantic auditory defects in PNFA versus semantic dementia: rather, it is likely that syndrome- and modality-specific profiles are relative rather than absolute, and phenomenologically similar deficits could have distinct cognitive mechanisms. This is an important issue for future study.
Visual inspection of the individual profiles of atrophy in PNFA and semantic dementia patients (Fig. 2) suggests possible anatomical bases for the group-level differences and within-group variation in auditory performance. The profiles observed—variable peri-Sylvian atrophy in PNFA and more focal and more uniform, leftward-asymmetric anterior temporal lobe atrophy in semantic dementia—are consistent with previous anatomical evidence in these PPA syndromes (Mesulam, 1982, 2003; Nestor et al., 2003; Gorno-Tempini et al., 2004; Hodges and Patterson, 2007; Rohrer et al., 2008a, b, 2009). The more marked involvement of posterior peri-Sylvian cortices in the PNFA group would predict deficits at earlier auditory cortical processing stages based on the evidence from normal subjects (Griffiths and Warren, 2002; Lewis et al., 2005; Warren et al., 2005b; Zaehle et al., 2008), while individual variation in the extent of posterior damage would allow for variation in the prominence of such deficits across the PNFA group (Fig. 3). It is also clear that patients with PNFA have involvement of higher order anterior peri-Sylvian and inferior parietal areas that might potentially contribute to conjoint deficits of semantic processing of complex sounds (Engelien et al., 1995, 2006; Lewis et al., 2004, 2005, 2009; Thierry and Price, 2006). In contrast, the more stereotypical involvement of the anterior left temporal lobe and anterior peri-Sylvian cortex in semantic dementia patients would provide a substrate for the more restricted, multimodal deficit of semantic processing exhibited by these patients (Bozeat et al., 2000; Lambon Ralph et al., 2001; Coccia et al., 2004; Hodges and Patterson, 2007; Rami et al., 2007). Quantitative cross-sectional and longitudinal analyses in larger PPA cohorts will be required to substantiate these functional anatomical relationships.
This study has addressed deficits of auditory processing identified in a consecutive series of patients with PPA: i.e. we have used a ‘lesion-led’ approach. However, an uncertain proportion of patients with PPA syndromes present with prominent symptoms of central auditory dysfunction: a number of cases have been described with progressive word deafness or agnosia for non-verbal sounds as leading features, many in the Japanese literature (Confavreux et al., 1992; Otsuki et al., 1998; Kuramoto et al., 2002; Kaga et al., 2004; Yamamoto et al., 2004; Uttner et al., 2006; Iizuka et al., 2007; Jörgens et al., 2008). The auditory deficits in these cases have not been systematically characterized; however, the available evidence suggests that most have a clinical syndrome aligned with PNFA, comprising speech production failure with variably salient accompanying features, including dysprosody, dysarthria, working memory impairment, parietal signs and behavioural disturbance. Anatomically, such cases have bilateral, often asymmetric peri-Sylvian atrophy or hypometabolism. The defect of early perceptual analysis of non-verbal sounds identified in the PNFA group here suggests a possible basis for clinical syndromes of word deafness and auditory agnosia that develop in some patients. In this regard, we note the wide variation in performance of our PNFA patients on the early perceptual and apperceptive auditory tests (Fig. 3), raising the possibility of discrete subgroups with more severe auditory impairment within the PNFA spectrum. This would be consistent with the considerable anatomical and pathological heterogeneity of PNFA, which is in contrast to the relatively uniform profile of semantic dementia (Rohrer et al., 2008a).
The relationship between auditory dysfunction and impaired speech output is of considerable interest in those patients with clinically evident auditory agnosias and in the PNFA group more broadly. There are a number of potential mechanisms by which deficits of complex sound analysis could impair speech production. Anatomically, analysis of incoming auditory signals, speech output and monitoring of own voice are linked via the dorsal auditory cortical pathway(s) between frontal, parietal and posterior superior temporal cortices (Warren et al., 2005b). Functionally, sensori–motor interactions mediated by this dorsal pathway have been shown to modulate spoken output in healthy individuals (Wilson et al., 2006) and in patients with focal brain damage (Racette et al., 2006), perhaps by transforming, or failing to transform faithfully, stored templates for auditory objects (in particular, phonemes) into motor programmes. By a mechanism of this kind, degraded processing of complex sounds from cortical degeneration in the region of the posterior temporal lobe/temporo-parietal junction might, via linked cortical processing stages, affect mechanisms of speech output mediated by more anterior cortical regions. This possibility does not of course exclude concurrent primary involvement of the speech output mechanisms proper (indeed, that would be anticipated with a neurodegenerative process).
Taken together, the present findings argue for the existence of core disorders of complex non-verbal sound perception and recognition in PPA and for specific disorders at perceptual and semantic levels of analysis in PNFA and semantic dementia, respectively. Our findings have clear clinical and pathophysiological implications. Clinically, the findings define the PPA syndromes more fully and provide a framework for understanding the symptoms of altered auditory function reported by a proportion of patients with PPA (Confavreux et al., 1992; Bozeat et al., 2000; Uttner et al., 2006; Griffiths et al., in press) Disorders of non-verbal sound processing in the PPA spectrum may be more widespread and significant than previously recognized: auditory complaints in these ‘language-based dementias’ should not be uncritically ascribed to peripheral hearing loss. Pathophysiologically, the existence of non-verbal auditory agnosias in these PPA subtypes argues for the existence of fundamental disorders of cortical information processing, affecting other kinds of complex auditory information besides speech. In the case of semantic dementia, this interpretation is constant with a multimodal deficit of knowledge stores anticipated by substantial neuropsychological evidence; in the case of PNFA, it raises the possibility that a generic derangement of complex sound analysis might underpin at least a proportion of cases of progressive disintegration of speech processing. To establish a precise brain basis for the auditory signatures identified here is likely to be challenging, particularly for PNFA: previous evidence from the study of focal lesions in aphasic stroke suggests a close correlation between verbal and non-verbal dysfunction but only a loose correlation between particular non-verbal deficits and anatomical substrates (Adriani et al., 2003; Saygin et al., 2003), and this issue is likely to be amplified in degenerative pathologies. We propose non-verbal analogues of cortical language network dysfunction in PPA syndromes (Sonty et al., 2007): verbal and non-verbal dysfunction might jointly result from the degraded exchange of information between distributed cortical areas in the temporal and frontal lobes. Clear directions for future work include more detailed analysis of component processes that underpin complex sound defects in different PPA syndromes; the application of anatomical, functional and connectivity based brain imaging modalities that can delineate areas of pathophysiological as well as structural damage; systematic clinico-pathological correlation across the PPA spectrum; and tracking of the evolution of non-verbal deficits in relation to the language deficits that characterize the PPA syndromes.
Funding
Medical Research Council UK; Medical Research Council Capacity Building PhD Studentship (to J.C.G.); Alzheimer's Research Trust Fellowship (to S.J.C.); Wellcome Trust Research Training Fellowship (to J.D.R.); Wellcome Trust Intermediate Clinical Fellowship (to J.D.W.).
Supplementary material
supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
We are grateful to the subjects for their participation. We thank Prof. Elizabeth Warrington, Dr Josephine Barnes, and Dr Gerard Ridgway for helpful discussion. This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. The Dementia Research Centre is an Alzheimer's Research Trust Co-ordinating Centre.
Glossary
Abbreviations
- CI
confidence interval
- FA
frequency average
- HFA
high frequency average
- MMSE
Mini-Mental State Examination
- PNFA
progressive non-fluent aphasia
- PPA
primary progressive aphasias
- PTA
pure tone audiometry
References
- Adriani M, Maeder P, Meuli R, Thiran AB, Frischknecht R, Villemure JG, et al. Sound recognition and localization in man: specialized cortical networks and effects of acute circumscribed lesions. Exp Brain Res. 2003;153:591–604. doi: 10.1007/s00221-003-1616-0. [DOI] [PubMed] [Google Scholar]
- Anaki D, Kaufman Y, Freedman M, Moscovitch M. Associative (prosop) agnosia without (apparent) perceptual deficits: a case-study. Neuropsychologia. 2007;45:1658–71. doi: 10.1016/j.neuropsychologia.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Test for reception of grammar. Cambridge: MRC Applied Psychology Unit; 1989. [Google Scholar]
- Blesser B. Speech perception under conditions of spectral transformation. I. Phonetic characteristics. J Speech Hear Res. 1972;15:5–41. doi: 10.1044/jshr.1501.05. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–15. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Chi T, Ru P, Shamma SA. Multiresolution spectrotemporal analysis of complex sounds. J Acoust Soc Am. 2005;118:887–906. doi: 10.1121/1.1945807. [DOI] [PubMed] [Google Scholar]
- Clarke S, Bellmann A, De Ribaupierre F, Assal G. Non-verbal auditory recognition in normal subjects and brain-damaged patients: evidence for parallel processing. Neuropsychologia. 1996;34:587–603. doi: 10.1016/0028-3932(95)00142-5. [DOI] [PubMed] [Google Scholar]
- Coccia M, Bartolini M, Luzzi S, Provinciali L, Lambon Ralph MA. Semantic memory is an amodal, dynamic system: evidence from the interaction of naming and object use in semantic dementia. Cognitive Neuropsychology. 2004;21:513–27. doi: 10.1080/02643290342000113. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Croisile B, Garassus P, Aimard G, Trillet M. Progressive amusia and aprosody. Arch Neurol. 1992;49:971–6. doi: 10.1001/archneur.1992.00530330095023. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Warrington EK. The Influence of refractoriness upon comprehension of non-verbal auditory stimuli. Neurocase. 2008;14:494–507. doi: 10.1080/13554790802498955. [DOI] [PubMed] [Google Scholar]
- Davis A. Hearing in adults: the prevalence and distribution of hearing impairment and reported hearing disability in the MRC Institute of Hearing Research's National Study of Hearing. London: Whurr Publishers; 1995. [Google Scholar]
- De Renzi E, Scotti G, Spinnler H. Perceptual and associative disorders of visual recognition. Relationship to the side of the cerebral lesion. Neurology. 1969;19:634–42. doi: 10.1212/wnl.19.7.634. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system. San Antonio: The Psychological Corporation; 2001. D-KEFS). S.l. [Google Scholar]
- Dunn LM, Dunn LM, Whetton C, Pintilie D. British Picture Vocabulary Scale. Windsor: NFER-Nelson; 1982. [Google Scholar]
- Engelien A, Silbersweig D, Stern E, Huber W, Doring W, Frith C, et al. The functional anatomy of recovery from auditory agnosia. A PET study of sound categorization in a neurological patient and normal controls. Brain. 1995;118(Pt 6):1395–409. doi: 10.1093/brain/118.6.1395. [DOI] [PubMed] [Google Scholar]
- Engelien A, Tuscher O, Hermans W, Isenberg N, Eidelberg D, Frith C, et al. Functional neuroanatomy of non-verbal semantic sound processing in humans. J Neural Transm. 2006;113:599–608. doi: 10.1007/s00702-005-0342-0. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Barbier A, Marra C. Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain. 2003;126:792–803. doi: 10.1093/brain/awg092. [DOI] [PubMed] [Google Scholar]
- Garrard P, Carroll E. Lost in semantic space: a multi-modal, non-verbal assessment of feature knowledge in semantic dementia. Brain. 2006;129:1152–63. doi: 10.1093/brain/awl069. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths TD, Bamiou D-E, Warren JD. Disorders of the auditory brain. In: Palmer AR, Rees A, editors. Oxford Handbook of Auditory Science: The Auditory Brain. Oxford: Oxford University Press ; (in press) [Google Scholar]
- Griffiths TD, Rees A, Green G. Disorders of human complex sound processing. Neurocase. 1999;5:365–78. [Google Scholar]
- Griffiths TD, Warren JD. The planum temporale as a computational hub. Trends Neurosci. 2002;25:348–53. doi: 10.1016/s0166-2236(02)02191-4. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD. What is an auditory object? Nat Rev Neurosci. 2004;5:887–92. doi: 10.1038/nrn1538. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6:1004–14. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- Hovius M, Kellenbach ML, Graham KS, Hodges JR, Patterson K. What does the object decision task measure? Reflections on the basis of evidence from semantic dementia. Neuropsychology. 2003;17:100–7. [PubMed] [Google Scholar]
- Iizuka O, Suzuki K, Endo K, Fujii T, Mori E. Pure word deafness and pure anarthria in a patient with frontotemporal dementia. Eur J Neurol. 2007;14:473–5. doi: 10.1111/j.1468-1331.2007.01671.x. [DOI] [PubMed] [Google Scholar]
- Jörgens S, Biermann-Ruben K, Kurz MW, Flügel C, Daehli Kurz K, Antke C, et al. Word deafness as a cortical auditory processing deficit: a case report with MEG. Neurocase. 2008;14:307–16. doi: 10.1080/13554790802363738. [DOI] [PubMed] [Google Scholar]
- Joubert S, Felician O, Barbeau E, Sontheimer A, Barton JJ, Ceccaldi M, et al. Impaired configurational processing in a case of progressive prosopagnosia associated with predominant right temporal lobe atrophy. Brain. 2003;126:2537–50. doi: 10.1093/brain/awg259. [DOI] [PubMed] [Google Scholar]
- Kaga K, Nakamura M, Takayama Y, Momose H. A case of cortical deafness and anarthria. Acta Otolaryngol. 2004;124:202–5. doi: 10.1080/00016480310015975. [DOI] [PubMed] [Google Scholar]
- Kuramoto S, Hirano T, Uyama E, Tokisato K, Miura M, Watanabe S, et al. A case of slowly progressive aphasia accompanied with auditory agnosia. Rinsho Shinkeigaku. 2002;42:299–303. [PubMed] [Google Scholar]
- Kveraga K, Ghuman AS, Bar M. Top-down predictions in the cognitive brain. Brain Cogn. 2007;65:145–68. doi: 10.1016/j.bandc.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. J Cogn Neurosci. 2001;13:341–56. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Brefczynski JA, Phinney RE, Janik JJ, DeYoe EA. Distinct cortical pathways for processing tool versus animal sounds. J Neurosci. 2005;25:5148–58. doi: 10.1523/JNEUROSCI.0419-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Talkington WJ, Walker NA, Spirou GA, Jajosky A, Frum C, et al. Human cortical organization for processing vocalizations indicates representation of harmonic structure as a signal attribute. J Neurosci. 2009;29:2283–96. doi: 10.1523/JNEUROSCI.4145-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Wightman FL, Brefczynski JA, Phinney RE, Binder JR, DeYoe EA. Human brain regions involved in recognizing environmental sounds. Cereb Cortex. 2004;14:1008–21. doi: 10.1093/cercor/bhh061. [DOI] [PubMed] [Google Scholar]
- McCarthy R, Warrington EK. A two-route model of speech production. Evidence from aphasia. Brain. 1984;107(Pt 2):463–85. doi: 10.1093/brain/107.2.463. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–8. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia—a language-based dementia. N Engl J Med. 2003;349:1535–42. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S13–21. [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–18. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Otsuki M, Soma Y, Sato M, Homma A, Tsuji S. Slowly progressive pure word deafness. Eur Neurol. 1998;39:135–40. doi: 10.1159/000007923. [DOI] [PubMed] [Google Scholar]
- Peretz I, Kolinsky R, Tramo M, Labrecque R, Hublet C, Demeurisse G, et al. Functional dissociations following bilateral lesions of auditory cortex. Brain. 1994;117:1283–301. doi: 10.1093/brain/117.6.1283. [DOI] [PubMed] [Google Scholar]
- Racette A, Bard C, Peretz I. Making non-fluent aphasics speak: sing along! Brain. 2006;129:2571–84. doi: 10.1093/brain/awl250. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven's Progressive Matrices and Vocabulary Scales. San Antonio, TX: General Overview.Harcourt Assessment; 2003. Section 1. [Google Scholar]
- Rami L, Loy CT, Hailstone J, Warren JD. Odour identification in frontotemporal lobar degeneration. J Neurol. 2007;254:431–5. doi: 10.1007/s00415-006-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. A manual for the administrating and scoring of the Trail Making Test. Indianapolis, IN, USA: Indiana University Press; 1959. [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, et al. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–35. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Knight WD, Warren JE, Fox NC, Rossor MN, Warren JD. Word-finding difficulty: a clinical analysis of the progressive aphasias. Brain. 2008a;131:8–38. doi: 10.1093/brain/awm251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, McNaught E, Foster J, Clegg SL, Barnes J, Omar R, et al. Tracking progression in frontotemporal lobar degeneration: serial MRI in semantic dementia. Neurology. 2008b;71:1445–51. doi: 10.1212/01.wnl.0000327889.13734.cd. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, et al. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology. 2009;72:1562–9. doi: 10.1212/WNL.0b013e3181a4124e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin AP, Dick F, Wilson SM, Dronkers NF, Bates E. Neural resources for processing language and environmental sounds: evidence from aphasia. Brain. 2003;126:928–45. doi: 10.1093/brain/awg082. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123(Pt 12):2400–6. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Weintraub S, Johnson NA, Parrish TB, Gitelman DR. Altered effective connectivity within the language network in primary progressive aphasia. J Neurosci. 2007;27:1334–45. doi: 10.1523/JNEUROSCI.4127-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Warrington EK. Visual agnosia: a single case report. Cortex. 1971;7:152–61. doi: 10.1016/s0010-9452(71)80011-4. [DOI] [PubMed] [Google Scholar]
- Thierry G, Price CJ. Dissociating verbal and nonverbal conceptual processing in the human brain. J Cogn Neurosci. 2006;18:1018–28. doi: 10.1162/jocn.2006.18.6.1018. [DOI] [PubMed] [Google Scholar]
- Uttner I, Mottaghy FM, Schreiber H, Riecker A, Ludolph AC, Kassubek J. Primary progressive aphasia accompanied by environmental sound agnosia: a neuropsychological, MRI and PET study. Psychiatry Res. 2006;146:191–7. doi: 10.1016/j.pscychresns.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Warren JD, Jennings AR, Griffiths TD. Analysis of the spectral envelope of sounds by the human brain. Neuroimage. 2005a;24:1052–7. doi: 10.1016/j.neuroimage.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Warren JE, Wise RJ, Warren JD. Sounds do-able: auditory-motor transformations and the posterior temporal plane. Trends Neurosci. 2005b;28:636–43. doi: 10.1016/j.tins.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol. 1975;27:635–57. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The Camden memory tests. East Sussex, UK: manual. Psychology Press; 1996. [Google Scholar]
- Warrington EK, James M. An experimental investigation of facial recognition in patients with unilateral cerebral lesions. Cortex. 1967;3:317–26. [Google Scholar]
- Warrington EK, James M. The visual object and space perception battery. Bury St. Edmunds: Thames Valley Test Company; 1991. [Google Scholar]
- Warrington EK, McKenna P, Orpwood L. Single Word Comprehension: A Concrete and Abstract Word Synonym Test. Neuropsychol Rehabil. 1998;8:143–54. [Google Scholar]
- Wilson SM, Iacoboni M. Neural responses to non-native phonemes varying in producibility: evidence for the sensorimotor nature of speech perception. Neuroimage. 2006;33:316–25. doi: 10.1016/j.neuroimage.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kikuchi T, Nagae J, Ogata K, Ogawa M, Kawai M. Dysprosody associated with environmental auditory sound agnosia in right temporal lobe hypoperfusion—a case report. Rinsho Shinkeigaku. 2004;44:28–33. [PubMed] [Google Scholar]
- Zaehle T, Geiser E, Alter K, Jancke L, Meyer M. Segmental processing in the human auditory dorsal stream. Brain Res. 2008;1220:179–90. doi: 10.1016/j.brainres.2007.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.