Abstract
We investigated the role of dopamine in working memory by examining effects of withdrawing dopaminergic medication in patients with Parkinson’s disease. Resistance to distraction during a delayed response task was abnormally enhanced in Parkinson’s disease patients OFF medication relative to controls. Conversely, performance on a backward digit span test was impaired in these same Parkinson’s disease patients OFF medication. Dopaminergic medication reinstated susceptibility to distraction and backward digit span performance, so that performance of Parkinson’s disease patients ON medication did not differ from that of controls. We hypothesize that the enhanced distractor resistance and impaired backward digit span in Parkinson’s disease reflects low dopamine levels in the striatum, and perhaps upregulated frontal dopamine levels. Dopaminergic medication may reinstate distractibility by normalizing the balance between striatal and prefrontal dopamine transmission.
Keywords: working memory, cognitive deficits, dopamine, Parkinson’s disease, basal ganglia
Introduction
Brain dopamine has been implicated in cognitive processes such as working memory and cognitive flexibility. The effects of dopamine on these cognitive processes have been most commonly associated with the prefrontal cortex. Thus, lesions in the prefrontal cortex of monkeys impair performance on the classic delayed response test of working memory, almost to the same extent as do ablations of the prefrontal cortex. Furthermore, iontophoretic application of dopamine receptor agents onto prefrontal cortex neurons modulates delay-period activity in awake behaving monkeys (Sawaguchi and Goldman-Rakic, 1991). Such empirical data are captured by biology-based theoretical models of prefrontal cortex dopamine function, which suggest that dopamine in the prefrontal cortex enhances the stability of task-relevant representations by promoting distractor resistance (Durstewitz et al., 2000; Durstewitz and Seamans, 2008).
Although the role of the striatum in the dopaminergic modulation of working memory and cognitive flexibility has not generally been emphasized, much evidence and theoretical work indicates its key contribution to the cognitive effects of dopamine (Frank, 2005; Gruber et al., 2006; Cools, 2008). For example, working memory deficits and cognitive inflexibility have been frequently observed in patients with Parkinson’s disease (Bowen et al., 1975; Lees and Smith, 1983; Cools et al., 1984, 2006; Owen et al., 1992; Lewis et al., 2005; Moustafa et al., 2008). Such deficits are found even in the earliest stages of the disease, when its characteristic dopamine depletion is still relatively restricted to the striatum and does not yet extend to the prefrontal cortex (Agid et al., 1993; Rakshi et al., 1999; Kaasinen et al., 2001; Sawamoto et al., 2008). This is perhaps not surprising given the strong anatomical connections between the prefrontal cortex and the striatum. Indeed, the pattern of cognitive deficits in mild Parkinson’s disease patients has been argued to resemble that seen in patients with frontal lobe damage (Owen et al., 1992, 1995), perhaps reflecting disruption of striatal output to the prefrontal cortex (Owen et al., 1998; Hazy et al., 2006; Moustafa et al., 2008).
However, recent evidence indicates that the behavioural consequences of the dopaminergic modulation of prefrontal cortex output differ from those of the modulation of striatal output. Such target region specificity is supported by work with marmosets, which has revealed contrasting effects of 6-ODHA lesions in the prefrontal cortex and in the striatum (Crofts et al., 2001). Specifically, in keeping with the classic work on the role of prefrontal cortex dopamine in working memory, 6-hydroxydopamine lesions of the prefrontal cortex impaired performance on a delayed response task with high demands for the maintenance of information (Collins et al., 1998). These lesions also impaired set maintenance in a visual discrimination task, presumably by reducing distractor resistance (Crofts et al., 2001). In contrast, 6-hydroxydopamine lesions in the striatum actually improved set maintenance by enhancing resistance to distraction beyond that in control marmosets (Crofts et al., 2001), thus inducing a form of behavioural rigidity that can also be expressed as impaired set shifting (Collins et al., 2000). This opposition between the behavioural effects of 6-hydroxydopamine lesions in the prefrontal cortex and in the striatum maps well onto the supposed neurochemical reciprocity between dopamine in the prefrontal cortex and dopamine in the striatum. Increases and decreases in prefrontal cortex dopamine lead to decreases and increases, respectively, in striatal dopamine (Pycock et al., 1980; Roberts et al., 1994; Akil et al., 2003; Meyer-Lindenberg et al., 2005). Furthermore, there are also some reports of the reverse effect: dopamine levels in the prefrontal cortex of Parkinson’s disease patients have been found to be upregulated, possibly reflecting compensation of the severe dopamine depletion in the striatum (Rakshi et al., 1999; Kaasinen et al., 2001).
An intriguing implication of these observations is that mild Parkinson’s disease, which is characterized primarily by striatal dopamine depletion with relatively intact or even upregulated dopamine levels in the prefrontal cortex, might be accompanied by cognitive benefits as well as cognitive impairment. Specifically, here we hypothesize that mild Parkinson’s disease patients show abnormal increases in distractor resistance during working memory performance, a process that has been associated primarily with dopamine in the prefrontal cortex.
To test this counterintuitive hypothesis, we assessed performance in a group of mild Parkinson’s disease patients and age- and education-matched controls on a delayed response task, which we had used previously in a pharmacological functional MRI study (Cools et al., 2007). During the delay of this task, subjects are presented with distractors, which they are instructed to ignore. The data from our previous functional MRI study revealed that administration of the mixed D1/D2 dopamine receptor agonist bromocriptine modulated distractor-related neural activity selectively in the prefrontal cortex but not in the striatum. This finding concurred with the above-described literature on prefrontal cortex dopamine and working memory, and suggests that the effects of dopaminergic drugs on distractor resistance, also in this specific task, reflect modulation of the prefrontal cortex rather than of the striatum. To investigate whether the predicted cognitive benefits were dopamine dependent, we tested the Parkinson’s disease patients on two occasions, once ON and once OFF their dopaminergic medication.
In addition, to assess whether any changes reflect non-specific effects, such as motivation, arousal or attention, we also assessed performance on task manipulations that were predicted to induce deficits rather than benefits. These included standard neuropsychological tests, such as the backward digit span test, as well as a task-switching manipulation built into the task of interest itself (Cools et al., 2001a, b, 2003).
Methods
Participants
Twenty-nine adults (15 Parkinson’s disease patients, 14 healthy control subjects) consented and participated in the study. Patient participants were recruited from the Movement Disorders Clinic at the University of California San Francisco, as well as through community support groups. All subjects were given a written informed consent form and were paid for participation. Subjects were interviewed for psychiatric and neurological history as well as current and past medication. The study procedures were approved by the University of California Berkeley Committee for the Protection of Human Subjects.
Patients with Parkinson’s disease
Fifteen early- to moderate-stage Parkinson’s disease patients (seven males) aged between 54 and 77 years [mean (SD) 64.5 (8.5) years] participated in and completed the study. All subjects were right-handed, and were natives or fluent English speakers (two non-natives). They had on average 16.3 (2.0) years of education. Duration of Parkinson’s disease varied from 10 months to 22 years from the time of initial diagnosis [mean (SD) 8.1 (6.1) years]. Patients were on various regimens of anti-Parkinsonian medications; 13 subjects were taking levodopa/carbidopa; 2 were receiving dopamine receptor agonists only. Total daily dose of levodopa/carbidopa varied from 50/200 mg to 225/500 mg [mean (SD) 121/423 (58/165) mg]. Medications are listed in Table 1.
Table 1.
Medication chart
| Parkinson’s disease (n = 15) | Control (n = 14) | |
|---|---|---|
| Sinemet | 13 | 0 |
| Pramipexole (D3 receptor agonist) | 9 | 0 |
| Comtan (COMT inhibitor) | 3 | 0 |
| Selegiline | 2 | 0 |
| Amantadine | 2 | 0 |
| Ropinirole | 2 | 0 |
| Methylphenidate | 1 | 0 |
| Trihexyphenidyl | 1 | 0 |
| Thyroid replacement (levothyroxine) | 4 | 2 |
| Statin | 6 | 3 |
| Anti-hypertensive | 6 | 1 |
| Anti-depressant (SSRIs) | 4 | 0 |
COMT = Catechol-O-methyltransferase; SSRI = selective serotonin reuptake inhibitor.
Control group
Fourteen control subjects (five males) aged between 59 and 80 years [mean (SD) 66.5 (6.2) years] with no current major health problems or history of neurological/psychiatric illness participated in and completed the study. All subjects were right-handed, and were natives or fluent English speakers (two non-natives). They had on average (SD) 18 (2.7) years of education. Current medications included thyroid replacement (two subjects), anti-hypertensive drugs (one subject) and statin (three subjects) (Table 1).
General procedure
Both groups completed a computerized delayed response task, which is discussed in detail below.
The study session consisted of a battery of neuropsychological tests, which included (in the order given) (i) an attentional shifting task (Cools et al., 2004, 2006) (data not reported); (ii) a paper and pen version of the Stroop task (Stroop, 1935); (iii) the experimental task of interest, i.e. an adapted delayed response task (Cools et al., 2007); (iv) a letter fluency task (Benton, 1968); (v) the forward and backward digit span; (vi) the Beck Depression Inventory (Beck et al., 1961); (vii) the North American Adult Reading Test (Nelson, 1982); (viii) the Mini Mental State Examination to assess cognitive impairment (Folstein et al., 1975); and (ix) the Barratt Impulsiveness Scale (Patton et al., 1995). In addition, the severity of clinical symptoms was assessed in the Parkinson’s disease group according to the Hoehn and Yahr (1967) five-point rating scale, and using the Unified Parkinson’s Disease (44-point) Rating Scale (UPDRS) (Fahn et al., 1987).
Parkinson’s disease subjects completed two test sessions—once after they had taken their regular dopaminergic medication, and once after a minimum of 18 h withdrawal from all dopaminergic medication. The session order was approximately counterbalanced across patients (eight patients were tested first in their ON state) and the two sessions were separated by at least 48 h. Control subjects completed one session.
UPDRS scores of the Parkinson’s disease patients are presented in Table 2 and ranged between 16 and 72. The average Hoehn and Yahr rating was 1.25 (SD = 1.0). Clinical symptoms were significantly worsened after withdrawal of medication as measured with the UPDRS at the time of testing (total UPDRS: T14 = 5.9; P < 0.001).
Table 2.
UPDRS scores for the Parkinson’s disease group
| ON | OFF | |
|---|---|---|
| Mentation, behaviour and mood | 1.5 (1.5) | 1.9 (1.6)* |
| Activities of daily living | 6.7 (4.2) | 9.5 (4.2)* |
| Motor examination | 13.2 (9.8) | 20.1 (11.7)* |
| UPDRS total | 21.3 (13.7) | 31.8 (15.9)* |
Values represent means (SDs).
*Significantly different from the ON state at P < 0.01.
Demographics and performance on the background neuropsychological battery are presented in Table 3. Parkinson’s disease patients and controls were well matched in terms of age, education, pre-morbid IQ (as measured with the North American Adult Reading Test), clinical depression (on the Beck Depression Inventory) and dementia (on the Mini Mental State Examination) ratings. Barratt Impulsiveness Scale scores were significantly higher in the Parkinson’s disease group relative to the control group, as reported earlier (Lawrence et al., 2007). There were no statistically significant differences between the ON and OFF state in terms of background neuropsychological performance (Stroop, letter fluency, forward digit span and backward digit span). Similarly, in the ON medication state, Parkinson’s disease patients’ performance did not differ from that of controls on any of the background neuropsychological measures. However, in the OFF medication state, Parkinson’s disease patients performed more poorly on the backward digit span than did controls (T24 = 2.2; P = 0.04). This pattern of mild neuropsychological impairment in Parkinson’s disease patients OFF medication contrasted with, and thus cannot account for, their cognitive benefits on the delayed response task reported below.
Table 3.
Neuropsychological data sets
| Test | Parkinson’s disease ON/OFF (n = 15) | Control (n = 14) |
|---|---|---|
| Age, years | 64.5 (8.5) | 66.5 (6.2) |
| Education, years | 16.3 (2.0) | 18.0 (2.7) |
| Stroop incongruent | 38.3 (10.6)/36.8 (10.5) | 38.5 (6.5) |
| Letter fluencya | 86.1 (24.4)/83.8 (22.7) | 89.3 (22.5) |
| Forward digit spana,b | 10.1 (2.8)/9.2 (2.6) | 9.9 (2.0) |
| Backward digit spana | 7.4 (2.5)/7.0 (1.5)* | 8.4 (2.0) |
| BDI | 7.7 (3.5) | 4.5 (5.2) |
| NAART error | 5.3 (9.1) | 4.5 (5.8) |
| MMSE | 28.6 (1.8) | 29.4 (1.2) |
| BIS | 65.0 (9.4)* | 55.0 (8.6) |
Values represent mean (SD). BDI = Beck Depression Inventory; MMSE = Mini Mental State Examination; BIS = Barratt Impulsiveness Scale; NAART = North American Adult Reading Test.
a Twelve patients completed the letter fluency task on both sessions; missing data from one or both session(s) for three patients due to fatigue.
b n = 11 for Parkinson’s disease subjects completing the digit span task in both sessions; missing 12 data due to subject fatigue (6 ON, 6 OFF).
*Significantly different from controls at P < 0.05.
Delayed response task
For this patient study, we used the same paradigm that was employed in our previous functional MRI study (Cools et al., 2007), apart from three minor changes (see below).
Stimulus presentation and response recording were conducted using E-prime 1.1 (Psychology Software Tools, Inc., Pittsburgh, PA, USA). The computerized delayed response task was designed to measure distractor resistance, i.e. the ability to retain encoded information during a delay in the presence of a distractor, as well as attentional switching, i.e. the ability to flexibly switch attention between task relevant stimuli at encoding.
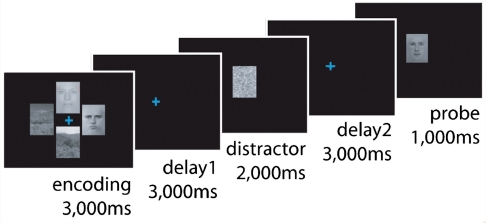
During the task, subjects had to encode, maintain and retrieve visual stimuli. Four such stimuli (two faces and two scenes, positioned around a coloured fixation cross in the centre of the screen; location randomized) were presented during the encoding period (3000 ms), which was followed by a delay period (3000 ms, only the fixation cross remained on the screen) when subjects had to maintain the relevant stimuli (either the faces or the scenes) in memory. Following this initial delay period, another stimulus was presented (for 2000 ms), which subjects were instructed to ignore. This distractor was either a scrambled image, the scrambled distractor (ScD), or a novel face or scene, the non-scrambled distractor (D). Non-scrambled distractors were always congruent with the trial-relevant stimulus category (i.e. if subjects memorized faces, then the distractor was a novel face), and was followed by a second delay (3000 ms), after which subjects were probed to respond with the right or left finger, depending on whether the probe stimulus (presented for 1000 ms) matched one of the two task-relevant encoding stimuli (Fig. 1). Critically, the colour of the fixation cue indicated to subjects whether to attend to the faces or the scenes. If the fixation cross was blue they had to memorize the faces; if it was green, then they had to memorize the scenes. The blue face trials and the green scene trials were randomized within blocks, enabling the measurement of the flexible switching of attention between faces and scenes.
Figure 1.
Schematic of the delayed response task. See text for details.
The details of the paradigm were identical to those used in our previous study (Cools et al., 2007), apart from three minor changes. First, we reduced the delay periods from 8000 ms to 3000 ms. Second, we lengthened the encoding period from 1000 ms to 3000 ms. Third, we used a different button box for collecting responses with larger buttons (horizontally arranged, 3 × 2 cm each; labelled with ‘match’ and ‘non-match’). These changes were made to ease the load on the patients and make sure that they were able to complete the task.
Subjects completed 128 trials of the task, divided in four blocks of 32 trials. Subjects were instructed to place their right and left index fingers on the buttons and encouraged to respond as quickly as possible; trials were self-paced. However, some subjects used a single hand due to unilateral motor symptoms associated with Parkinson’s disease (one subject used their non-dominant unaffected left hand, and two subjects used their dominant unaffected right hand; the same hand was used in the ON and OFF sessions).
Data analysis
There were three trial-types: (i) non-switch with a non-scrambled distractor (D-NS: 36 trials); (ii) non-switch with a scrambled distractor (ScD-NS: 40 trials) and (iii) switch with a scrambled distractor (ScD-SW: 52 trials). Initially we focused our analyses on the effects of Parkinson’s disease and dopaminergic medication on two separate task measures: (i) distractor costs (performance after non-scrambled distractors minus performance after scrambled distractors), representing the degree of distractor resistance (cognitive stability) during the working memory delay, and (ii) switch costs, representing the degree of cognitive flexibility during working memory encoding. The switch cost was calculated by subtracting performance (measured at probe) on non-switch trials (ScD-NS) from that on switch trials (ScD-SW). For this calculation of switch costs we employed only trials with scrambled distractors.
In contrast to our prediction, our healthy controls did not respond significantly more slowly and did not make more errors on switch trials than on non-switch trials (Table 4; Fig. 2). A repeated measures ANOVA on reaction times and mean proportions of correct responses on trials with scrambled distractors only, with switch and stimulus category as within-subject factors, showed that there were no main effects of switch [reaction time: F(1,13) = 1.0, ns; accuracy: F(1,13) = 0.02, ns] or stimulus category [reaction time: F(1,13) = 0.001, ns; F(1,13) = 0.005, ns] or a switch × stimulus category interaction [reaction time: F(1,13) = 0.7, ns; accuracy: F(1,13) = 0.9, ns]. These data from healthy controls indicate that, unlike the version of the task employed in our previous study (Cools et al., 2007), the current version of the task was not sufficiently sensitive for detecting switch costs, perhaps as a result of the longer encoding period employed here (see ‘Discussion’). Accordingly, to test our primary hypothesis about distractor resistance, we collapsed data across non-switch trials with scrambled distractors (ScD-NS) and switch trials with non-scrambled distractors (ScD-SW) and focused our further analyses of patient data on our primary measure of interest, the distractor cost.
Table 4.
Raw data on the delayed response task
| Distractor accuracy |
Probe accuracy |
Probe reaction time |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| D-NS | ScD-NS | ScD-SW | D-NS | ScD-NS | ScD-SW | D-NS | ScD-NS | ScD-SW | |
| Parkinson’s disease OFF | |||||||||
| Face | 0.73 (0.1) | 0.99 (0.03) | 1.00 (0.01) | 0.76 (0.1) | 0.78 (0.1) | 0.74 (0.1) | 1875 (233) | 1848 (272) | 1866 (288) |
| Scene | 0.80 (0.1) | 0.99 (0.03) | 0.99 (0.02) | 0.84 (0.1) | 0.80 (0.1) | 0.81 (0.1) | 1655 (269) | 1828 (402) | 1840 (378) |
| Parkinson’s disease ON | |||||||||
| Face | 0.73 (0.1) | 1.00 (0.01) | 0.98 (0.03) | 0.73 (0.1) | 0.73 (0.1) | 0.76 (0.1) | 2218 (563) | 1968 (393) | 1986 (391) |
| Scene | 0.80 (0.1) | 0.98 (0.03) | 0.99 (0.02) | 0.81 (0.1) | 0.79 (0.1) | 0.79 (0.1) | 1920 (352) | 1956 (412) | 1920 (397) |
| CS | |||||||||
| Face | 0.74 (0.1) | 1.00 (0) | 1.00 (0) | 0.80 (0.1) | 0.82 (0.1) | 0.79 (0.1) | 1815 (271) | 1614 (263) | 1549 (171) |
| Scene | 0.76 (0.1) | 1.00 (0) | 1.00 (0) | 0.85 (0.1) | 0.79 (0.1) | 0.81 (0.1) | 1551 (249) | 1599 (303) | 1652 (266) |
Values represent mean reaction times and mean proportion of correct responses (standard errors of the mean).
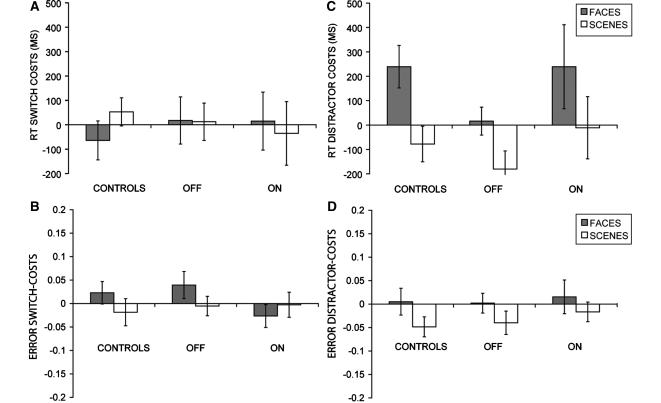
Figure 2.
Mean reaction time (RT) switch costs (A), error switch costs (B), reaction time distractor costs (C) and error distractor costs (D) at probe for faces (grey bars) and scenes (white bars) for controls and Parkinson’s disease patients (OFF and ON medication sessions plotted separately). Error bars represent (two times) the standard error of the difference.
Reaction time and mean proportions of correct responses at the probe and distractor periods were analysed with repeated measures ANOVAs (using Statistical Package for the Social Sciences 15.0 Inc., Chicago, IL, USA). Details of within- and between-subject factors are described in the ‘Results’ section.
For statistical purposes, a log10 transformation was applied to the mean reaction times, to maximize homogeneity of variances between groups. Levene’s test of homogeneity of variances revealed that this transformation was successful in equalizing variance between groups (all P’s > 0.1). The mean proportions of correct responses were not transformed, because Levene’s test of homogeneity of variances revealed no significant differences prior to transformation (all P’s > 0.2). One-tailed P-values are reported for simple main effects with clear directional hypotheses.
Results
In Table 4, we present the raw mean reaction times and accuracy rates at the distractor and probe period of all groups as a function of trial type (D-NS, ScD-NS, ScD-SW) and stimulus category (faces, scenes).
Healthy control subjects
Distractor resistance during the distractor period
Healthy control subjects were significantly less successful in withholding responding during the non-scrambled distractor than during the scrambled distractor [main effect of distractor: F(1,13) = 5.7, Pone-tailed = 0.02]. Three subjects failed to inhibit a (mostly ‘non-match) response for the majority (>94%) of non-scrambled distractors, suggesting that they had not processed the instruction of not to respond during distraction. The mean percentage of responses to non-scrambled distractors in the remaining 11 subjects was <6%. The main effect of distractor type on distractor resistance (i.e. response withholding) during the distractor period remained significant after the exclusion of these three subjects [main effect of distractor: F(1,10) = 15.7; Pone-tailed = 0.002].
Distractor resistance at probe
Subjects responded more slowly at probe after a non-scrambled distractor than after a scrambled distractor, but only for faces [F(1,13) = 8.5; Pone-tailed = 0.01], and not for scenes [F(1,13) = 1.5, ns]. A repeated measures ANOVA with distractor type (non-scrambled versus scrambled) and stimulus category (face versus scene) as within-subject factors confirmed a significant distractor type × stimulus category interaction [F(1,13) = 34.0; Ptwo-tailed < 0.0001]. Therefore, we focused further analyses on patient data from the face trials only.
We considered the possibility that this distractor-related slowing was driven by trials on which subjects failed to withhold responding during the distractor period. Thus, failure to withhold responding might reflect confusion about the order of (distractor and probe) events, inducing slowing owing to surprise when seeing the actual probe rather than owing to distraction. To assess this, we also calculated the distractor cost from only those trials on which subjects successfully withheld responding and, in addition, included accuracy during the distractor period as a covariate. Data from subjects who failed to follow instructions and consistently responded ‘non-match’ to almost all distractors were included in this analysis, based on the assumption that they were not surprised at probe but rather treated the task as having two probes per trial. The effect of distractor type on subsequent probe reaction time for faces was highly significant [F(1,12) = 32.6; P < 0.001].
For accuracy data at probe, distractor costs were numerically greater for face trials than for scene trials, suggesting that the reaction time effect does not reflect a bias in the speed–accuracy trade-off. However, these accuracy distractor costs were not significant [main effect of distractor type: F(1,13) = 2.0, ns; distractor type × stimulus category interaction: F(1,13) = 1.9, ns; effect of distractor type for faces only: F(1,13) = 0.2, ns], and thus we focus our further analyses on the reaction time costs. Note that, for completeness, we have presented all accuracy and reaction time data in Table 4.
Effect of Parkinson’s disease
To investigate the effects of Parkinson’s disease rather than of dopaminergic medication, we focused this set of analyses on the data from the OFF medication session and compared them with the data from controls (see below for analyses of the ON session).
At probe, Parkinson’s disease patients responded more slowly than did controls, with an overall mean reaction time of 1824 ms for patients and 1626 ms for controls, although the difference did not reach statistical significance [F(1,27) = 1.1, ns]. In addition, overall accuracy was numerically but not significantly lower in patients (79%) than in controls (81%) [F(1,27) = 0.3, ns].
Distractor resistance during the distractor period
The tendency to withhold responding did not differ significantly between the Parkinson’s disease group (OFF medication) and the control group. Two patients failed to follow instructions and responded to >92% of non-scrambled distractors. The remaining 13 patients responded to <12% of non-scrambled distractors. Like control subjects, Parkinson’s disease patients failed to withhold responding during the non-scrambled distractor to a significantly greater extent than during the scrambled distractor [simple main effect of distractor type: F(1,14) = 6.4; Pone-tailed = 0.01], and this effect of distractor type did not differ between groups [group × distractor-type interaction: F(1,27) = 0.5, ns].
Distractor resistance at probe
Unlike control subjects, Parkinson’s disease patients (OFF medication) did not respond more slowly after a non-scrambled distractor than after a scrambled distractor (Fig. 2). Thus, they did not exhibit a reaction time distractor cost and were more resistant to distraction than controls [group × distractor-type interaction: F(1,27) = 5.9; Ptwo-tailed = 0.02]. Although there was no difference between patients and controls in terms of accuracy during the distractor period (see above), we also performed a supplementary analysis, as in controls, in which this distractor accuracy measure was included as a covariate, and in which we included only those trials on which subjects successfully withheld responding. The group × distractor-type interaction remained significant [F(1,26) = 5.2; Ptwo-tailed = 0.03]. There were no effects in terms of accuracy.
Effect of dopaminergic medication in Parkinson’s disease
To assess whether the enhanced distractor resistance in the OFF medication state was affected by dopaminergic medication, we compared data from the ON medication session with those from healthy age- and education-matched controls. In addition, we also compared data from the ON and OFF sessions directly.
At probe, Parkinson’s disease patients responded more slowly when they were in the ON medication state (overall mean reaction time: 1989 ms) than when they were in the OFF medication state (overall mean reaction time: 1824 ms), although the difference did not reach statistical significance (T14 = −1.7; Ptwo-tailed = 0.11). In addition, overall accuracy was numerically but not significantly higher in the OFF medication state (79%) relative to the ON medication state (77%) (T14 = 1.6; Ptwo-tailed = 0.12).
Distractor resistance during the distractor period
Two patients ON medication failed to follow instructions and responded to >97% of non-scrambled distractors. The remaining 13 responded to <12% of non-scrambled distractors. Like control subjects, Parkinson’s disease patients ON medication failed to withhold responding during the non-scrambled distractor to a greater extent than during the scrambled distractor [simple main effect of distractor type: F(1,14) = 6.9; Pone-tailed = 0.01]. The effect of distractor type during the distractor period did not differ from that of controls [F(1,27) = 0.04, ns], or from that from the OFF medication state [F(1,28) < 0.001, ns].
Distractor resistance at probe
Unlike the OFF state, Parkinson’s disease patients in the ON state were equally vulnerable to distraction as controls (Fig. 2). There was no group difference in reaction time costs after the non-scrambled distractor relative to the scrambled distractor [group × distractor type: F(1,27) = 0.15, ns]. However, their distractor cost was not completely normalized and remained insignificant (simple main effect of distractor type: T14 = 1.7, Pone-tailed = 0.1). A direct comparison between data from the ON and OFF states revealed that the medication × distractor-type interaction was not significant for reaction time [F(1,14) = 1.6, ns] or accuracy [F(1,14) = 0.1, ns].
Summary
Healthy elderly control subjects exhibited a significant distractor cost in terms of probe reaction time when both task relevant encoding stimuli and distractors were faces. As found previously, there was no distractor cost for scenes (Yoon et al., 2006). This distractor cost was abolished in Parkinson’s disease patients who were OFF their medication: their response speed was unaffected by distraction. However, when these same patients were tested ON medication, their distractor cost was reinstated and differed no longer from that of controls. In contrast to their cognitive benefit on the delayed response task, patients in the OFF state actually exhibited impaired performance on the backward digit span test.
Discussion
The present data reveal significant vulnerability of working memory performance to intervening distraction in healthy volunteers. Specifically, response was slowed during the probe period of a delayed response task, when a face distractor was presented during the delay relative to when a scrambled distractor was presented. This distractor-related slowing was absent in Parkinson’s disease patients OFF medication, leading to relatively faster responding after distraction than in controls. Thus, Parkinson’s disease patients exhibited enhanced distractor resistance when they were OFF their medication. Slowing was reinstated by dopaminergic medication, as evidenced by the finding that responding of the same patients in the ON medication state did not differ from that of controls, although the direct comparison between the ON and OFF medication states did not reach significance. The improved performance on the delayed response task of patients OFF medication relative to controls contrasts with their impairment on the backward digit span test. The finding suggests that Parkinson’s disease can enhance or impair working memory performance depending on task demands. Specifically, in Parkinson’s disease patients, working memory representations were more resistant, not only to distraction, leading to improvement on the delayed response task, but also to backward reordering, leading to impairment on the backward digit span test.
The finding that Parkinson’s disease patients exhibited enhanced distractor resistance significantly refines our understanding of the mechanisms of cognitive deficits in Parkinson’s disease. These cognitive deficits have often been argued to resemble the consequences of frontal lesions (Lees and Smith, 1983; Brown and Marsden, 1988; Owen et al., 1992, 1993a, 1995; Dubois and Pillon, 1997: but see Owen et al., 1993b), possibly reflecting disruption of striatal output (Owen et al., 1998). However, studies with lateral frontal lesion patients have revealed disruption rather than facilitation of distractor resistance during working memory (Malmo, 1942; Chao and Knight, 1995). Thus, in contrast, the current data suggest that some types of frontal function might be enhanced even beyond normal function rather than reduced in mild Parkinson’s disease.
The enhancement might reflect deficient dopamine levels in the striatum, and/or upregulated dopamine levels in the prefrontal cortex (Rakshi et al., 1999). A functional division has been proposed to exist between dopamine-dependent striatal updating processes and dopamine-dependent prefrontal maintenance processes (Bilder et al., 2004). Poor striatum-dependent updating might confer benefits in terms of distractor resistance, due to reduced responsiveness to new input, and a striatal locus of modulation is suggested by recent imaging data showing transient under-activation of the striatum during set shifting and updating in working memory (an n-back task) (Monchi et al., 2007; Marklund et al., 2009). It might be noted that our data are less consistent with other recent theorizing, positing an important role for the striatum in dopamine-induced increases in filtering and distractor resistance in working memory (Gruber et al., 2006; McNab and Klingberg, 2008).
Alternatively, the current finding might reflect indirect modulation of prefrontal cortex output, rather than striatal output, given recent models of dopamine function in the prefrontal cortex (Seamans and Yang, 2004; Durstewitz and Seamans, 2008) and known neurochemical reciprocity between dopamine in the prefrontal cortex and dopamine in the striatum. According to these models, dopamine facilitates the stabilization of currently relevant representation in the face of intervening distractors by directly acting at the level of the prefrontal cortex. The hypothesis that the enhanced distractor resistance reflects modulation of the prefrontal cortex is further strengthened by evidence from our recent pharmacological functional MRI study, in which the same task was employed. In this study distractor-related neural activity in healthy young volunteers was modulated by dopaminergic drugs only in the prefrontal cortex, and not in the striatum (Cools et al., 2007).
Our finding that Parkinson’s disease patients exhibit normal delayed response performance in the absence of significant distraction is consistent with previous reports, which have also shown intact performance on delayed response tasks that do not require complex processing (Fournet et al., 2000; Ketcham et al., 2003; Lewis et al., 2003; Fern-Pollak et al., 2004; for review see Cools, 2006). On the other hand, the present finding differs from those described in some other recent studies of working memory deficits in Parkinson’s disease. For example, Moustafa et al. (2008) recently reported that Parkinson’s disease patients were impaired when ignoring distractors during the delay of an adapted version of the AX-CPT (continuous performance task). However, this deficit was found only in patients ON medication and not in patients OFF medication. An important difference between the present study and that previous study is that the previous effect was obtained in a shifting phase of the task, when subjects had to shift attention (and responding) away from previously relevant stimuli (i.e. the current distractors) towards newly relevant stimuli. The present data suggest that the deficit in Parkinson’s disease patients ON medication, but not OFF medication, observed in that study might reflect a combination of both a Parkinson’s disease-related shifting impairment as well as a selective enhancement of distractor resistance in patients OFF medication.
Significant distractor costs in healthy controls were observed only for face trials, and not for scene trials. This observation replicates previous findings (Yoon et al., 2006) and might reflect disproportionate distractibility by biologically salient stimuli. Although we refrain from emphasizing effects of disease or medication on the scene distractor costs, which are difficult to interpret, we report for completeness that such effects were not significant (both P’s > 0.2).
The observation that distractor resistance was affected only in terms of reaction times, and not in terms of accuracy, probably reflects a lack of sensitivity of the current task to detecting error distractor costs. Indeed the distractor cost surfaced only in terms of reaction times and not in terms of errors even in healthy controls. The finding that Parkinson’s disease patients OFF medication nevertheless exhibited enhanced distractor resistance, if only in terms of reaction times, suggests that the disease potentiated the robustness or strength of current task-relevant representations. Future studies should employ tasks that are sensitive to error distractor costs to investigate whether Parkinson’s disease also prevents the disruption of these representations qualitatively, which should lead to higher relative accuracy as well as higher relative reaction times after distraction. Such a more sensitive paradigm might also be more adequate for definitively testing the hypothesis that distractor vulnerability in Parkinson’s disease is sensitive to restoration by dopaminergic medication.
The current study was designed to assess not only distractor costs but also switch costs. Previous results indicate that healthy subjects make more errors at probe when they had to switch attention between faces and scenes than when they attended to the same stimulus category on two consecutive (non-switch) trials (Cools et al., 2007). In the current study no such switch costs were obtained. On hindsight, this is perhaps not surprising, because we increased the duration of the encoding period from 1000 ms to 3000 ms, in order to prevent presumed limits on general cognitive speed in the patients. We argue that the short duration of 1000 ms was in fact essential for these error switch costs to surface at probe, presumably because the error costs reflected incomplete reconfiguration of attentional set. The duration of 3000 ms in the current study must simply have been long enough for all subjects to complete this reconfiguration process.
In conclusion, the present data demonstrate enhanced resistance to distraction in Parkinson’s disease patients, but only when they are OFF their medication. Thus, mild Parkinson’s disease is accompanied not only by cognitive inflexibility, as evidenced by impaired backward digit span as well as deficient set shifting and task switching observed in many previous studies (Cools et al., 2001a, b, 2003), but also by aberrant cognitive stability in the face of distraction. We hypothesize that this pattern reflects deficient dopamine levels in the striatum, and/or upregulated dopamine levels in the prefrontal cortex. Preliminary support was obtained for this enhanced distractor resistance to be sensitive to restoration by dopaminergic medication, perhaps reflecting a restoration of the balance between dopamine in the striatum and dopamine in the prefrontal cortex.
Funding
This work was supported by the National Institutes of Health (DA02060 to M.D. and R.C.)
Acknowledgements
The authors report no biomedical financial interests or potential conflicts of interest. The work was conducted within the Helen Wills Neuroscience Institute at UC Berkeley, and we thank Dr C. Christine and Dr M. Aminoff from the movement disorder clinic at UC San Francisco for help with patient referral and Prof. Richard Ivry for helpful comments on a previous draft.
Glossary
Abbreviations
- D
non-scrambled distractor
- NS
non-switch trial
- ScD
scrambled distractor
- SW
switch trial
- UPDRS
Unified Parkinson’s Disease (44-point) Rating Scale
References
- Agid Y, Ruberg M, Javoy-Agid F, Hirsch E, Raisman-Vozari R, Vyas S, et al. Are dopaminergic neurons selectively vulnerable to Parkinson’s disease? Adv Neurol. 1993;60:148–64. [PubMed] [Google Scholar]
- Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–13. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatr. 1961;11:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–61. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Bowen FP, Kamienny RS, Burns MM, Yahr MD. Parkinsonism: effects of levodopa treatment on concept formation. Neurology. 1975;25:701–4. doi: 10.1212/wnl.25.8.701. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Brown R, Marsden C. ‘Subcortical dementia’: the neuropsychological evidence. Neurosci. 1988;25:363–87. doi: 10.1016/0306-4522(88)90246-1. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown R, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in the prefrontal cortex of rhesus monkeys. Science. 1979;205:929–31. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Chao L, Knight R. Human prefrontal lesions increase distractibility to irrelevant sensory inputs. NeuroReport. 1995;6:1605–10. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. Perseveration and strategy in a novel spatial self-ordered sequencing task for nonhuman primates: effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. J Cogn Neurosci. 1998;10:332–54. doi: 10.1162/089892998562771. [DOI] [PubMed] [Google Scholar]
- Collins P, Wilkinson LS, Everitt BJ, Robbins TW, Roberts AC. The effect of dopamine depletion from the caudate nucleus of the common marmoset (Callithrix jacchus) on tests of prefrontal cognitive function. Behav Neurosci. 2000;114:3–17. doi: 10.1037//0735-7044.114.1.3. [DOI] [PubMed] [Google Scholar]
- Cools AR, Van Den Bercken JHL, Horstink MWI, Van Spaendonck KPM, Berger HJC. Cognitive and motor shifting aptitude disorder in Parkinson’s disease. J Neurol Neurosurg Psychiatr. 1984;47:443–53. doi: 10.1136/jnnp.47.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R. Role of dopamine in the motivational and cognitive control of behaviour. The Neuroscientist. 2008;14:381–95. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001a;11:1136–43. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain. 2001b;124:2503–12. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41:1431–41. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–35. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Ivry R, D’Esposito M. The human striatum is necessary for responding to changes in stimulus relevance. J Cogn Neurosci. 2006;18:1959–72. doi: 10.1162/jocn.2006.18.12.1973. [DOI] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D’Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–14. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Van Denderen JCM, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cerebral Cortex. 2001;11:1015–26. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Cooney JW, Gazzaley A, Gibbs SE, Postle BR. Is the prefrontal cortex necessary for delay task performance? Evidence from lesion and FMRI data. J Int Neuropsychol Soc. 2006;12:248–60. doi: 10.1017/S1355617706060322. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Knight RT. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. J Neurosci. 2005;25:8333–7. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans J, Sejnowski T. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83:1733–50. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64:739–49. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Committee MotUD. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Caine D, Goldstein M, editors. Recent developments in Parkinson’s disease. Florham Park, NJ: McMillan Health Care Information; 1987. [Google Scholar]
- Fern-Pollak L, Whone AL, Brooks DJ, Mehta MA. Cognitive and motor effects of dopaminergic medication withdrawal in Parkinson’s disease. Neuropsychologia. 2004;42:1917–26. doi: 10.1016/j.neuropsychologia.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fournet N, Moreaud O, Roulin JL, Naegele B, Pellat J. Working memory functioning in medicated Parkinson’s disease patients and the effect of withdrawal of dopaminergic medication. Neuropsychology. 2000;14:247–53. doi: 10.1037//0894-4105.14.2.247. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Dayan P, Gutkin BS, Solla SA. Dopamine modulation in the basal ganglia locks the gate to working memory. J Comput Neurosci. 2006;20:153–66. doi: 10.1007/s10827-005-5705-x. [DOI] [PubMed] [Google Scholar]
- Hazy T, Frank M, O’Reilly R. Banishing the homunculus: making working memory work. Neuroscience. 2006;139:105–18. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nurmi E, Bruck A, Eskola O, Bergman J, Solin O, et al. Increased frontal [(18)F]fluorodopa uptake in early Parkinson's; disease: sex differences in the prefrontal cortex. Brain. 2001;124:1125–30. doi: 10.1093/brain/124.6.1125. [DOI] [PubMed] [Google Scholar]
- Ketcham CJ, Hodgson TL, Kennard C, Stelmach GE. Memory-motor transformations are impaired in Parkinson’s disease. Exp Brain Res. 2003;164:30–9. doi: 10.1007/s00221-002-1332-1. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Blackwell AD, Barker RA, Spagnolo F, Clark L, Aitken MR, et al. Predictors of punding in Parkinson’s disease: results from a questionnaire survey. Mov Disord. 2007;22:2339–45. doi: 10.1002/mds.21702. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Smith E. Cognitive deficits in the early stages of Parkinson’s disease. Brain. 1983;106:257–70. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Cools R, Robbins TW, Dove A, Barker RA, Owen AM. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. 2003;41:645–54. doi: 10.1016/s0028-3932(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Lewis S, Slabosz A, Robbins T, Barker R, Owen A. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia. 2005;43:823–32. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Malmo R. Interference factors in delayed response in monkeys after removal of frontal lobes. J Neurophysiol. 1942;5:295–308. [Google Scholar]
- Marklund P, Larsson A, Elgh E, Linder J, Åhlström Riklund K, Forsgren L, et al. Temporal dynamics of basal ganglia under-recruitment in Parkinson’s disease: transient caudate abnormalities during updating of working memory. Brain. 2009;132:336–46. doi: 10.1093/brain/awn309. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–7. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–6. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson’s disease during–executive processing depends on striatal involvement. Brain. 2007;130:233–44. doi: 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa AA, Sherman SJ, Frank MJ. A dopaminergic basis for working memory, learning and attentional shifting in Parkinsonism. Neuropsychologia. 2008;46:3144–56. doi: 10.1016/j.neuropsychologia.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Nelson HE. Test Manual. Windsor (UK): NFER-Nelson; 1982. National Adult Reading Test (NART) [Google Scholar]
- Owen AM, Beksinska M, James M, Leigh PN, Summers BA, Marsden CD, et al. Visuo-spatial memory deficits at different stages of Parkinson’s disease. Neuropsychologia. 1993a;31:627–44. doi: 10.1016/0028-3932(93)90135-m. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson’s disease identified with PET. Implications for higher cortical functions. Brain. 1998;121:949–65. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh JM, Summers BA, Marsden CD, Quinn NP, et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115:1727–51. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson’s disease. Brain. 1993b;116:1159–79. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Hodges JR, Summers BA, Polkey CE, Robbins TW. Dopamine-dependent frontostriatal planning deficits in early Parkinson’s disease. Neuropsychology. 1995;9:126–140. [Google Scholar]
- Patton J, Stanford M, Barratt E. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74–7. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- Rakshi J, Uema T, Ito K, Bailey D, Morrish P, Ashburner J, et al. Frontal, midbrain and striatal dopamergic function in early and advanced Parkinson’s disease. A 3D [(18)F]dopa-PET study. Brain. 1999;122 doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, et al. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort test: possible interactions with subcortical dopamine. J Neurosci. 1994;14:2531–44. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–50. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain. 2008;131:1294–302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–62. [Google Scholar]
- Yoon JH, Curtis CE, D’Esposito M. Differential effects of distraction during working memory on delay-period activity in the prefrontal cortex and the visual association cortex. Neuroimage. 2006;29:1117–26. doi: 10.1016/j.neuroimage.2005.08.024. [DOI] [PubMed] [Google Scholar]