Abstract
Smooth muscle contraction is regulated by phosphorylation of the myosin light chain (MLC) catalyzed by MLC kinase and dephosphorylation catalyzed by MLC phosphatase. Agonist stimulation of smooth muscle results in the inhibition of MLC phosphatase activity and a net increase in MLC phosphorylation and therefore force. The two pathways believed to be primarily important for inhibition of MLC phosphatase activity are protein kinase C (PKC)-catalyzed CPI-17 phosphorylation and Rho kinase (ROCK)-catalyzed myosin phosphatase-targeting subunit (MYPT1) phosphorylation. The goal of this study was to determine the roles of PKC and ROCK and their downstream effectors in regulating MLC phosphorylation levels and force during the phasic and sustained phases of carbachol-stimulated contraction in intact bladder smooth muscle. These studies were performed in the presence and absence of the PKC inhibitor bisindolylmaleimide-1 (Bis) or the ROCK inhibitor H-1152. Phosphorylation levels of Thr38-CPI-17 and Thr696/Thr850-MYPT1 were measured at different times during carbachol stimulation using site-specific antibodies. Thr38-CPI-17 phosphorylation increased concurrently with carbachol-stimulated force generation. This increase was reduced by inhibition of PKC during the entire contraction but was only reduced by ROCK inhibition during the sustained phase of contraction. MYPT1 showed high basal phosphorylation levels at both sites; however, only Thr850 phosphorylation increased with carbachol stimulation; the increase was abolished by the inhibition of either ROCK or PKC. Our results suggest that during agonist stimulation, PKC regulates MLC phosphatase activity through phosphorylation of CPI-17. In contrast, ROCK phosphorylates both Thr850-MYPT1 and CPI-17, possibly through cross talk with a PKC pathway, but is only significant during the sustained phase of contraction. Last, our results demonstrate that there is a constitutively activate pool of ROCK that phosphorylates MYPT1 in the basal state, which may account for the high resting levels of MLC phosphorylation measured in rabbit bladder smooth muscle.
Keywords: MLC phosphatase, myosin phosphatase-targeting subunit, CPI-17, bisindolylmaleimide-1, H-1152, MLC phosphorylation
it is well accepted that phosphorylation of the 20-kDa regulatory myosin light chain (MLC) catalyzed by the Ca2+/calmodulin-dependent MLC kinase and dephosphorylation catalyzed by MLC phosphatase play a primary role in the regulation of smooth muscle contraction and relaxation (12, 37). Historically, most studies have been aimed at the regulation of the MLC kinase as it was assumed that the MLC phosphatase was simply a constitutively active enzyme. However, it is now known that the MLC phosphatase is a regulated enzyme and plays an important role in the control of smooth muscle contraction (10). The smooth muscle MLC phosphatase is composed of three subunits: a 38-kDa catalytic subunit of type 1 phosphatase (δ isoform; PP1cδ), a 110-kDa noncatalytic myosin phosphatase-targeting subunit (MYPT1), and a 20-kDa noncatalytic subunit (M20).
Agonist stimulation of smooth muscle has been shown to initiate signaling pathways that result in inhibition of MLC phosphatase and a net increase in MLC phosphorylation levels. The net increase in MLC phosphorylation levels in turn increases force development at any given level of Ca2+. Therefore, agonist stimulation produces a state of enhanced myofilament Ca2+ sensitivity (8, 38).
Two mechanisms have been proposed to account for the agonist-dependent inhibition of MLC phosphatase activity in smooth muscle. The first is inhibition of the catalytic subunit PP1cδ by a phosphorylatable inhibitory protein, CPI-17, while the second is Rho kinase (ROCK)-catalyzed phosphorylation of the regulatory subunit MYPT1 (8, 10, 38). CPI-17 is the first identified PP1cδ-inhibitory protein in smooth muscle (4, 5). Phosphorylation of CPI-17 at Thr38 greatly increases its inhibitory potency toward the MLC phosphatase (4). Upon agonist stimulation, an increase in Thr38-CPI-17 phosphorylation was found to correlate with increases in force and MLC phosphorylation in several smooth muscles, suggesting an important role for CPI-17 in Ca2+ sensitization (15, 16, 27). It has also been reported that the role of phosphorylated CPI-17 in the regulation of MLC phosphatase activity is smooth muscle tissue specific (49). The primary kinase believed to phosphorylate CPI-17 is protein kinase C (PKC), although other kinases, such as ROCK and p21-activated protein kinase (PAK), have been suggested to phosphorylate CPI-17, depending on the types of smooth muscle tissue studied (16, 17, 27, 44).
Phosphorylation of MYPT1 at two specific residues has been shown to inhibit MLC phosphatase activity. These residues are Thr696 and Thr850, corresponding to the human and chicken sequences, respectively (38). Several kinases have been shown to phosphorylate MYPT1 in vitro; however, the primary physiologically relevant kinase is believed to be ROCK (6, 13, 14, 46). Phosphorylation of MYPT1 at Thr696 and Thr850 inhibits MLC phosphatase activity by decreasing the function of PP1cδ and interrupting substrate binding to the phosphatase. Whether Thr696- and Thr850-MYPT1 are the endogenous targets regulating MLC phosphatase activity during agonist stimulation still remains controversial and depends on the smooth muscle tissue and the agonist used in the stimulation.
The pathways involving PKC-catalyzed CPI-17 phosphorylation and ROCK-catalyzed MYPT1 phosphorylation in the inhibition of MLC phosphatase activity have been well described in vascular and some nonvascular smooth muscles (8, 38). The pathways important for MLC phosphatase inhibition in bladder smooth muscle, however, are not as well understood. It has been shown that both PKC and ROCK are involved in muscarinic receptor-dependent contractions of bladder smooth muscle (11, 33). In addition to receptor-mediated kinases, constitutively active PKC (26, 33) and ROCK (9, 31) have been shown to be present in vascular and bladder smooth muscles. Therefore, the goal of this study was to test the hypothesis that both CPI-17 phosphorylation and MYPT1 phosphorylation are important in a carbachol-induced contraction of rabbit bladder smooth muscle. Specifically, we were interested in testing the hypothesis that the CPI-17 pathway predominates in the early phasic component of a contraction while the MYPT1 pathway plays a greater role in the later tonic-like phase of a bladder smooth muscle contraction.
MATERIALS AND METHODS
Intact bladder smooth muscle preparation.
Male New Zealand White rabbits, weighing 2–2.5 kg, were used in this study. All animal studies and procedures were approved by the Drexel University College of Medicine's Institutional Animal Care and Use Committee. Rabbits were euthanized by a pentobarbital overdose administered via the ear vein. The bladders were then quickly removed and placed in ice-cold physiological salt solution (PSS). PSS contained (in mM) 140 NaCl, 4.7 KCl, 1.2 MgSO4, 1.6 CaCl2, 1.2 Na2HPO4, 2 MOPS (pH 7.4), 5 d-glucose, and 0.02 EDTA. The bladder neck, trigone, and base region were removed, leaving only the middle detrusor body for experimentation. The detrusor body was dissected in cold PSS under a dissecting microscope. The majority of the mucosal and serosal layers were carefully removed, and muscle strips (∼1.5 × 6 mm) were cut along the central axis of the bladder in the longitudinal orientation as previously described (43) and stored in PSS at 4°C until used. Storage was never longer than 48 h.
Isometric force recording.
The bladder strips were mounted between a Grass FT.03 force transducer and a stationary clip in water-jacketed muscle organ baths containing PSS at 37°C and aerated with 100% O2. The strips were stretched to a force of ∼5–6 g and allowed to stress-relax until a basal force of ∼1 g was achieved. Typically, the tissues achieve a stable basal force of ∼1 g within 1 h; additional time does not alter the level of basal force. We have performed length/tension experiments to demonstrate that this level of basal force approximates the optimal length for maximal active stress development (Lo). These studies were performed by stretching the tissues, allowing them to stress-relax to a stable force, performing a semiquick release to a lower force, and then subjecting them to a 110 mM KCl-PSS (equal-molar substitution of KCl for NaCl) depolarizing solution. Once a stable contractile force was attained, the tissues were relaxed and allowed to return to baseline. The tissues were then stretched to a greater force, and the procedure was repeated. These stretch-release, contraction cycles were repeated until active force began to decrease. From these studies, it was determined that a basal force of ∼1 g provided a preload that resulted in maximal active force being attained. After the strips reached a stable force of 1 g, they were then allowed to equilibrate for at least 40 min.
After the equilibration period, the strips were stimulated with 110 mM KCl-PSS, then relaxed with PSS; this contraction-relaxation cycle was repeated four times. The peak force in each equilibrated muscle strip was used to normalize the force generated with carbachol stimulation. Our bladder preparation devoid of mucosal and serosal layers does not exhibit spontaneous activity. Therefore, peak force is calculated by simply subtracting the force before a stimulus from the total force in response to the stimulus. Data therefore are presented as a percentage of the maximal response to 110 mM KCl. Bisindolylmaleimide-1 (Bis; 3 μM) and H-1152 (1 μM) were used to inhibit PKC and ROCK activity, respectively. We have previously used 10 μM Bis to inhibit PKC activity (39). In preliminary experiments we found that 3 μM Bis inhibited a phorbol 12,13-dibutyrate contraction of bladder smooth muscle. To minimize any nonspecific effects of the putative PKC inhibitor, we chose to use the lower concentration of Bis in this study. The H-1152 concentration was chosen from a concentration-inhibition curve generated using phospho-MYPT1 as the end point (data not shown). The inhibitors were added to the muscle bath 20 min before the addition of the stimulus. Tissues were allowed to contract for 5 min and then relaxed by rinsing with PSS.
Measurement of MLC phosphorylation.
MLC phosphorylation levels were measured by standard techniques previously published by our laboratory (22, 23, 42). Briefly, strips were rapidly frozen in a dry ice/acetone slurry containing 6% trichloroacetic acid and 10 mM DTT at various time points (0, 40 s, 3 and 5 min) of carbachol stimulation. The strips were then slowly thawed to room temperature, rinsed in acetone, air dried, and then subjected to homogenization in a 1% SDS, 10% glycerol, and 1 mM DTT solution using glass/glass homogenizers. Samples were centrifuged at 12,000 rpm for 6 min and subjected to two-dimensional gel electrophoresis and transferred to nitrocellulose membranes for quantification of MLC phosphorylation as previously described (23). Transferred proteins were visualized with Colloidal Gold Stain (Amersham Biosciences, Piscataway, NJ) and digitized by densitometric analysis using a Bio-Rad GS-800 quantitative densitometer (Bio-Rad, Hercules, CA). MLC phosphorylation levels were calculated by dividing the densitometric analysis of the spot corresponding to the phosphorylated MLC by the sum of the densitometric analyses of the spots corresponding to the phosphorylated and unphosphorylated MLC. Values were presented as moles Pi/mole MLC.
Quantification of CPI-17 phosphorylation.
Tissue strips, resting or stimulated, were rapidly frozen and then homogenized in a 1% SDS, 10% glycerol, and 1 mM DTT containing homogenization buffer with glass/glass homogenizers. The samples were then subjected to SDS-PAGE gel electrophoresis (4% SDS stacking gel, 14% separating gels) and transferred to a nitrocellulose membrane at 0.8 A for 2.5 h. The membranes were blocked with 5% BSA (Sigma-Aldrich, St. Louis, MO) in a PBS solution for 1 h, and then incubated with an antibody against Thr38-phospho-CPI-17 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. Membranes were briefly rinsed with distilled water and incubated with a polyclonal goat anti-rabbit antibody conjugated to horseradish peroxidase (1:5,000; Upstate Cell Signaling, Billerica, MA). Immunoreactive bands were detected using enhanced chemiluminescence (ECL, Amersham Biosciences, Piscataway, NJ). The blots were then stripped and reprobed using a primary antibody against CPI-17 (1:400; Santa Cruz Biotechnology) followed by washes in a PBS-0.05% Tween 20 solution. Control experiments measuring phospho-CPI-17 levels a second time after stripping were performed to ensure that no protein was lost during the stripping protocol. The washed membranes were incubated with a monoclonal sheep anti-mouse antibody conjugated to horseradish peroxidase (1:10,000; Amersham Biosciences). Densitometry was performed to analyze protein band intensity. The ratio of the density of the band corresponding to the Thr38-phospho-CPI-17 to that of the band corresponding to total CPI-17 was used to determine CPI-17 phosphorylation levels. In most cases protein loadings were similar, but in all cases a direct comparison of phosphorylated protein levels to total protein levels was obtained in the same blot by stripping and reprobing. We have successfully used this technique to quantify ERK kinase phosphorylation levels and demonstrated that phosphorylation levels measured are linearly related to actual ERK kinase phosphotransfer activity (7).
Quantification of MYPT1 phosphorylation.
Tissue strips, resting or stimulated, were rapidly frozen and then homogenized in a homogenization buffer, as described above, including a Protease Inhibitor Cocktail solution (1:100; Sigma-Aldrich). The samples were then subjected to SDS-PAGE (4% SDS stacking gel, 7.5% separating gel), and transferred to a nitrocellulose membrane at 0.8 A for 2.5 h. The membranes were blocked with 3% nonfat milk in a PBS solution for 40 min and then incubated with a primary antibody against either Thr696 or Thr850 phospho-MYPT1 (1:2,000 Upstate Cell Signaling) overnight at 4°C. Membranes were briefly washed with deionized water and then incubated with a polyclonal goat anti-rabbit antibody conjugated to horseradish peroxidase (1:5,000; Upstate Cell Signaling). Immunoreactive bands were detected using ECL (Amersham Biosciences). The blots were stripped and then reprobed using a primary MYPT1 antibody (1:10,000; Covance, Princeton, NJ) followed by washes in a PBS-0.05% Tween 20 solution and incubated with a polyclonal goat anti-rabbit antibody conjugated to horseradish peroxidase (1:5,000; Upstate Cell Signaling). Densitometry was performed to analyze protein band intensity. The ratio of the density of the bands corresponding to either the phospho-Thr696 or phospho-Thr850 to the bands corresponding to total MYPT1 was used to determine MYPT1 phosphorylation levels. In most cases protein loadings were similar, but in all cases a direct comparison of phosphorylated protein levels to total protein levels was obtained in the same blot by stripping and reprobing.
Materials and statistics.
All reagents for solutions described above, unless otherwise specified below, were purchased from Fisher Scientific (Pittsburgh, PA) and were of analytic grade or better. All electrophoretic and blotting reagents were obtained from Bio-Rad Laboratories. Bis and H-1152 were purchased from Calbiochem (San Diego, CA). Carbachol was purchased from Sigma-Aldrich. Membrane antibody stripping solutions were purchased from Pierce Biotechnology (Rockford, IL).
Statistical significance between means was determined using Student's t-test. A P value <0.05 was taken as significant. All “n” values refer to the number of bladder muscle strips; each strip was taken from a different animal.
RESULTS
Isometric force in response to carbachol stimulation.
The addition of carbachol results in a dose-dependent contraction in urinary bladder smooth muscle. We used 30 μM carbachol in the present study to induce a maximal contraction. We chose this concentration based on dose-response curves in rabbit bladder smooth muscle strips previously published by our laboratory in which we demonstrated that both 30 and 100 μM carbachol produced maximal forces (43). Carbachol was chosen as the agonist so we could compare our findings with previous studies from our laboratory. Isometric force was measured at 0 s, 40 s, 3 min, and 5 min of stimulation and expressed as a percentage of the strips' maximal response to 110 mM KCl. The results are shown in Fig. 1. Smooth muscle strips developed a quick, phasic-like contraction in response to carbachol. Force rapidly decreased to a maintained value of 40–50% of the maximal force at 3–5 min. Preincubation of the bladder strips with either the PKC inhibitor Bis or the ROCK inhibitor H-1152 decreased both the peak and maintained phases of carbachol-stimulated contraction. It is well accepted that carbachol stimulation activates both PKC and Rho/ROCK signaling through G protein-coupled pathways in bladder smooth muscle (30). Our results suggest that both PKC and ROCK pathways are involved in the regulation of carbachol-stimulated transient and maintained phases of contraction.
Fig. 1.
Carbachol-stimulated bladder smooth contraction in the presence and absence of the PKC inhibitor Bis or the Rho kinase (ROCK) inhibitor H-1152. Intact bladder smooth muscle strips were contracted for 5 min with carbachol (30 μM) in the presence and absence of Bisindolylmaleimide-1 (Bis; 3 μM) or H-1152 (1 μM). Isometric force was measured at 0 s, 40 s, 3 min, and 5 min of carbachol stimulation, and the results are expressed as a percentage of the maximal response to 110 mM KCl-physiological salt solution (PSS). Strips contracted in response to carbachol alone (●) generated high levels of force at the peak (40 s) and then maintained force during the sustained phase of contraction (3 min and 5 min). Inhibition with Bis (○) or H-1152 (▼) significantly decreased force at all time points. Values are means ± SE of 9–11 determinations. *P < 0.05 compared with values in the presence of carbachol alone.
Inhibition of PKC by Bis or ROCK by H-1152 also significantly decreased basal levels of force. As shown in Fig. 2, the addition of either inhibitor relaxed the tissues from basal force by ∼20–30%. These results suggest that in addition to stimulated levels of force, PKC and ROCK are also involved in the regulation of basal tone in bladder smooth muscle. It should be noted that our preparation of bladder smooth muscle devoid of mucosal and serosal layers rarely exhibits spontaneous activity. Therefore, basal levels of force are stable and the decreases in force in response to Bis or H-1152 can be easily measured.
Fig. 2.
Changes in bladder smooth muscle basal tone in response to the addition of the PKC inhibitor Bis or the ROCK inhibitor H-1152. Intact bladder smooth muscle strips were incubated with vehicle, Bis (3 μM), or H-1152 (1 μM) for 20 min. Basal forces were measured at 10 and 20 min of incubation with vehicle (black bars), Bis (open bars), or H-1152 (grey bars) and expressed as a percentage of basal force at 0 min. Inhibition with Bis and H-1152 for 10 and 20 min significantly decreased basal tone of the bladder smooth muscle strips. Values are means ± SE of 5 determinations. *P < 0.05 compared with values at 0 min.
MLC phosphorylation in response to carbachol stimulation.
MLC phosphorylation is one of the primary mechanisms in the initiation of smooth muscle contraction (12). MLC phosphorylation levels are determined by the balance between MLC kinase and MLC phosphatase activities (37). In our present study, MLC phosphorylation levels were measured to obtain a better understanding of the mechanism(s) responsible for carbachol-induced contractions as well as the mechanism(s) by which pharmacological inhibitors decrease stimulated force. As shown in Fig. 3, stimulation of bladder smooth muscle strips with carbachol increased both force and MLC phosphorylation levels during the early phase followed by a decrease to a quasi-steady-state plateau (Fig. 1). The addition of the pharmacological kinase inhibitor Bis or H-1152 significantly reduced carbachol-stimulated MLC phosphorylation levels and abolished any apparent correlation between force and MLC phosphorylation. In fact, in the presence of either Bis or H-1152, carbachol-stimulated MLC phosphorylation levels did not increase above resting values even though the two inhibitors did not completely abolish force generation. These results are consistent with the concept that carbachol-stimulated force generation results, in part, from elevated levels of MLC phosphorylation and the inhibitors of PKC and ROCK reduced force, in part, by decreasing MLC phosphorylation levels.
Fig. 3.
Carbachol-stimulated myosin light chain (MLC) phosphorylation levels in the presence and absence of Bis and H-1152 in bladder smooth muscle strips. Intact bladder smooth muscle strips were contracted for 5 min with carbachol (30 μM) in the presence and absence of Bis (3 μM) or H-1152 (1 μM). Tissues were rapidly frozen at 0 s, 40 s, 3 min, and 5 min of contraction and then processed for quantification of MLC phosphorylation levels. Carbachol stimulation significantly increased MLC phosphorylation level at all time points (●). MLC phosphorylation values in strips incubated with either Bis (○) or H-1152 (▼) were significantly lower compared with carbachol stimulation alone. Values are means ± SE of at least 4 determinations. #P < 0.05 compared with values at rest in strips contracted with carbachol alone. *P < 0.05 compared with values at the same time points in strips contracted with carbachol alone.
CPI-17 phosphorylation in response to agonist stimulation.
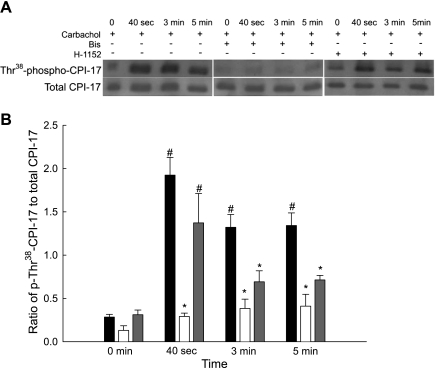
CPI-17 can be phosphorylated by either PKC or ROCK upon agonist stimulation of smooth muscle (15, 16, 27). Our current study addresses PKC- and ROCK-mediated CPI-17 phosphorylation in bladder smooth muscle by using a phosphorylation site-specific antibody (Thr38-phospho-CPI-17) to quantify levels of CPI-17 phosphorylation. Figure 4A shows representative Western blots of total and phospho-CPI-17 antibody binding, and Fig. 4B shows the averaged results of several such blots. In all experiments, a direct comparison of phosphorylated and total protein levels were made on the same blot. Levels of phospho-Thr38-CPI-17 were increased concurrently with carbachol stimulation at all time points. Carbachol-stimulated CPI-17 phosphorylation levels were significantly increased at 40 s of stimulation. Inhibition of PKC significantly decreased but did not completely abolish CPI-17 phosphorylation. Inhibition of ROCK, however, only significantly decreased CPI-17 phosphorylation at the sustained phase of contraction (3 and 5 min). The results suggest that PKC plays a major role in CPI-17 phosphorylation throughout carbachol stimulation, while ROCK is only involved in sustained contractile force, either by direct phosphorylation of CPI-17 or through interaction with the PKC signaling pathway.
Fig. 4.
Representative Western blots and quantification of phospho-Thr38-CPI-17 levels from bladder smooth strips contracted with carbachol in the presence and absence of Bis or H-1152. A: representative Western blots of strips stimulated with carbachol (30 μM) in the presence of vehicle (left), Bis (3 μM, middle), or H-1152 (1 μM, right). Strips were rapidly frozen at 0 s, 40 s, 3 min, and 5 min of carbachol contraction. B: results of several experiments of the type shown in A. Quantification of phosphorylated CPI-17 levels was taken as the ratio of the relative band intensities of phospho-Thr38-CPI-17 to total CPI-17. Carbachol stimulation significantly increased phospho-Thr38-CPI-17 levels at all time points in vehicle strips (black bars). Inhibition of PKC (open bars) significantly reduced CPI-17 phosphorylation levels at all time points. Inhibition of ROCK (grey bars) significantly reduced CPI-17 phosphorylation levels only at 3 and 5 min. Values are means ± SE of at least 4 determinations. #P < 0.05 compared with values at rest. *P < 0.05 compared with values in response to carbachol at the same time point.
MYPT1 phosphorylation in response to agonist stimulation.
Phosphorylation of MYPT1 has been shown to be one of the major mechanisms regulating MLC phosphatase activity and hence MLC phosphorylation levels and force generation in agonist-stimulated contraction of smooth muscle (8). Phosphorylation of Thr696-MYPT1 and Thr850-MYPT1 was investigated in this study during carbachol stimulation of bladder smooth muscle. Figures 5A and 6A show representative Western blots of carbachol-stimulated total and Thr696 and Thr850 phosphorylated MYPT1, respectively. Figures 5B and 6B show the averaged results of several such blots. In all experiments, a direct comparison of phosphorylated to total protein levels was made on the same blot.
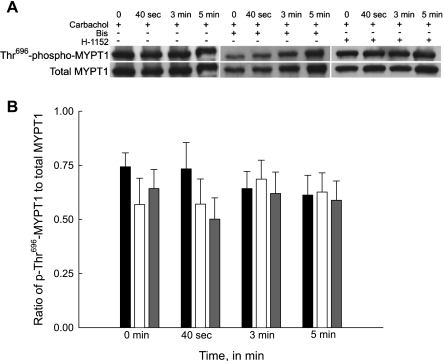
Fig. 5.
Representative Western blots and quantification of phospho-Thr696-MYPT1 levels from bladder smooth muscle strips contracted with carbachol in the presence and absence of Bis or H-1152. A: representative Western blots of strips stimulated with carbachol (30 μM) in the presence of either vehicle (left), Bis (3 μM, middle), or H-1152 (1 μM, right). Strips were rapidly frozen at 0 s, 40 s, 3 min, and 5 min of carbachol stimulation. B: results of several experiments of the type shown in A. Quantification of phospho-Thr696-myosin phosphatase-targeting subunit (MYPT1) was taken as the ratio of the relative band intensities of phospho-Thr696-MYPT1 to total MYPT1. Carbachol stimulation alone did not increase Thr696-MYPT1 phosphorylation levels (black bars). Inhibition of either PKC (open bars) or ROCK (grey bars) had no effect on Thr696-MYPT1 phosphorylation in all strips. Values are means ± SE of at least 4 determinations.
Fig. 6.
Representative Western blots and quantification of phospho-Thr850-MYPT1 levels from bladder smooth muscle strips contracted with carbachol in the presence and absence of Bis or H-1152. A: representative Western blots of strips stimulated with carbachol (30 μM) in the presence of either vehicle (left), Bis (3 μM, middle), or H-1152 (1 μM, right). Strips were rapidly frozen at 0 s, 40 s, 3 min, and 5 min of carbachol stimulation. B: results of several experiments of the type shown in A. Quantification of phospho-Thr850-MYPT1 levels was taken as the ratio of the relative band intensities of phospho-Thr850-MYPT1 to total MYPT1. Carbachol stimulation significantly increased Thr850-MYPT1 phosphorylation levels in the sustained phase (5 min) of contraction (black bars). Inhibition of PKC (open bars) did not affect Thr850-MYPT1 phosphorylation at any time point. Inhibition of ROCK (grey bars) significantly decreased Thr850-MYPT1 phosphorylation at all time points measured. Values are means ± SE of at least 4 determinations. #P < 0.05 compared with values at rest. *P < 0.05 compared with values in strips contracted with carbachol at the same time points.
A high basal level of Thr696-MYPT1 phosphorylation was observed in all bladder smooth muscle strips and was not increased with carbachol stimulation (Fig. 5B). Inhibition with either PKC or ROCK inhibitors had no significant effect on phospho-Thr696-MYPT in the presence or absence of carbachol. The second phosphorylation site, Thr850-MYPT1, also exhibited high basal phosphorylation levels (Fig. 6B). Figure 6B shows that Thr850-MYPT1 phosphorylation increased slowly following carbachol stimulation, only achieving significance compared with basal at 5 min of stimulation. Inhibition of PKC did not significantly decrease the carbachol-stimulated increase in Thr850-MYPT1 phosphorylation levels. Conversely, inhibition of ROCK not only abolished the carbachol-dependent increase in Thr850-MYPT1 phosphorylation, it also significantly decreased basal phosphorylation levels. Our results suggest that only Thr850-MYPT1 phosphorylation is involved in the regulation of carbachol-stimulated bladder smooth muscle contraction.
Bladder smooth muscle basal tone and protein phosphorylation levels.
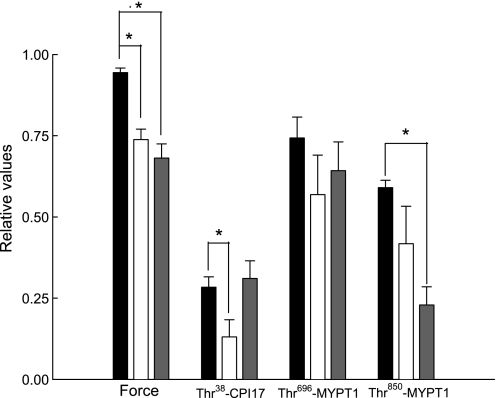
Incubation of bladder smooth muscle strips with either a PKC or ROCK inhibitor significantly decreased bladder smooth muscle basal tone (Fig. 7). Inhibition of PKC also decreased basal CPI-17 phosphorylation levels whereas inhibition of ROCK had no effect. Inhibition of ROCK significantly decreased basal Thr850-MYPT1 phosphorylation levels but had no effect on basal levels of Thr696-MYPT1 phosphorylation. Our results suggest that ROCK is important in the regulation of bladder smooth muscle basal tone mediated by phosphorylation of Thr850-MYPT1 while PKC may be involved through the CPI-17 phosphorylation pathway.
Fig. 7.
Effect of Bis and H-1152 on force, CPI-17 phosphorylation, and Thr696- and Thr850-MYPT1 phosphorylation levels in resting bladder smooth muscle. Equilibrated intact bladder smooth muscle strips were incubated with vehicle (black bars), Bis (3 μM, open bars), or H-1152 (1 μM, grey bars) for 20 min in PSS at 37°C. Either vehicle, Bis, or H-1152 was added, and basal force was recorded 20 min later. The strips were then rapidly frozen for quantitation of protein phosphorylation levels. All strips exposed to vehicle or the PKC inhibitor showed high levels of basal Thr696 and Thr850-MYPT1 phosphorylation. Incubation with the PKC inhibitor significantly decreased basal force and Thr38-CPI-17 phosphorylation levels. Incubation with the ROCK inhibitor significantly decreased basal force and Thr850-MYPT but not Thr696-MYPT1 phosphorylation levels. Values are means ± SE of at least 4 determinations. *P < 0.05 compared with values in strips treated with vehicle.
DISCUSSION
Carbachol stimulation activates the Gq/11-phospholipase Cβ-PKC and the G12/13-RhoGEF-ROCK pathways through binding to muscarinic receptors in bladder smooth muscle (30). It is currently believed that the end point for both pathways is inhibition of MLC phosphatase activity which results in an increase in myofilament Ca2+ sensitivity, MLC phosphorylation, and force (38). Two downstream effectors of PKC and ROCK, CPI-17 and MYPT1, respectively, were proposed to play important roles in the regulation of MLC phosphatase activity in several smooth muscle types (4, 16, 48). However, it is unclear how these two pathways work to regulate the MLC phosphatase in agonist-stimulated bladder smooth muscle contraction. Recently, our laboratory reported an abolishment of receptor-G protein-dependent Ca2+ sensitization, alterations in the MLC phosphorylation-force relationship, and loss of phorbol ester-induced contractions in a pathophysiological state of rabbit bladder smooth muscle (39, 40, 41). Unfortunately, the precise mechanisms underlying these important aspects of bladder smooth muscle contraction in a normal physiological state are not well understood. Therefore, the goal of this study was to test the hypothesis that these two signaling pathways involving CPI-17 and MYPT1 phosphorylation are important during agonist-stimulated contractions of normal intact bladder smooth muscle strips. Our major findings in this report are 1) there are changes in CPI-17 phosphorylation that occur in response to carbachol and there are decreases in basal levels of CPI-17 phosphorylation in the presence of an inhibitor of PKC; 2) there is a dissociation of MLC phosphorylation levels and force by either inhibition of PKC or ROCK; 3) Thr696-MYPT1 is not involved in carbachol-induced contractions of bladder smooth muscle; and 4) inhibition of ROCK decreases CPI-17 phosphorylation levels during the tonic phase of a contraction. Our studies also confirm previous reports that inhibition of PKC and ROCK decreases basal and stimulated force production in bladder smooth muscle and that a constitutively active ROCK is present in bladder smooth muscles (9, 11, 31, 32, 33).
ROCK is the major kinase that was previously reported to be responsible for phosphorylation of the myosin binding subunit of MLC phosphatase (13–15, 34). CPI-17, another regulatory factor of MLC phosphatase in smooth muscle, is primarily activated by PKC-catalyzed phosphorylation (5, 16). However, PKC can also be involved through cross talk with ROCK (27). Because the basal CPI-17 phosphorylation levels were low, it is reasonable to assume that CPI-17 phosphorylation may not be physiologically important for the basal force regulation in smooth muscle. Where PKC and CPI-17 phosphorylation in the basal state may be important is in a pathological state such as benign prostatic hyperplasia. Work from Chacko's laboratory (2) recently demonstrated that resting CPI-17 phosphorylation levels are reduced in decompensated bladders from a model of benign prostatic hyperplasia.
First, we demonstrated that both PKC and ROCK pathways are involved in the regulation of carbachol-stimulated contraction. This was shown by the fact that both the PKC inhibitor Bis and the ROCK inhibitor H-1152 decreased force at the transient and prolonged phases of contraction. These findings are consistent with previous reports from Ratz's laboratory (11, 33) as well as Suzuki's laboratory (9) showing that inhibition of either PKC or ROCK depressed contractions of bladder smooth muscle. A concomitant decrease in MLC phosphorylation levels in our study indicated that the two pathways regulated force through affecting MLC phosphorylation levels, which is determined by the balance between MLC kinase and MLC phosphatase activities. MLC kinase activity, however, is not believed to be affected by inhibition of either pathway, according to a recent report by Mizuno and colleagues (21). Therefore, based on the observations of Mizuno et al. (21), the decrease in MLC phosphorylation with PKC and ROCK inhibition is not likely due to MLC kinase inhibition. Since all of the evidence linked the two pathways to the regulation of the MLC phosphatase, we further investigated the two major regulators of MLC phosphatase activity, CPI-17 and MYPT1, in bladder smooth muscle.
Involvement of PKC and CPI-17 in a carbachol-mediated contraction.
CPI-17 was the first identified PP1cδ-inhibitory protein in smooth muscle (4, 5, 17). Phosphorylation of CPI-17 at Thr38 greatly increases its inhibitory potency toward the MLC phosphatase (15). PKC is the major kinase responsible for CPI-17 phosphorylation; however, the ROCK pathway has also been suggested to catalyze CPI-17 phosphorylation (4, 17, 27). We addressed this potential pathway in carbachol stimulated bladder smooth muscle contraction using a phosphorylation-site-specific antibody. Our results showed a significant increase in Thr38-CPI-17 phosphorylation with carbachol stimulation during both the transient, phasic-like contraction and the latter sustained phase of contraction. PKC, as expected, played a major role in the phosphorylation of CPI-17. ROCK appeared to only be involved during the sustained phase of contraction. However, because inhibition of PKC almost completely abolished CPI-17 phosphorylation, it is unlikely that ROCK alone directly phosphorylates CPI-17 in bladder smooth muscle. Thus it is reasonable to believe that there is cross talk between ROCK and PKC in the regulation of CPI-17 phosphorylation. A synergistic action between PKC and ROCK has been suggested in the regulation of resting tone and in the maintenance of stimulated tonic tone in bladder smooth muscle (33). Although CPI-17 phosphorylation levels were not measured in this study (33), it is likely that CPI-17 was phosphorylated, at least in the stimulated state. Previous protein-protein interaction studies have shown a specific high binding affinity between Rho and PKC-α and that Rho can regulate activation of PKC-α (36). Using cells expressing dominant negative RhoA and PKC-α, Patil et al. (29) suggested cross talk between the two pathways in the regulation of CPI-17 phosphorylation in rabbit colon smooth muscle cells. It was also reported by Mehta et al. (20) that PKC can phosphorylate the guanine nucleotide dissociation inhibitor to increase activity of the Rho/ROCK pathway. Thus there are two possible explanations for the phosphorylation of CPI-17 in the sustained phase of contraction. First, in addition to the initial phosphorylation by PKC, CPI-17 may also be phosphorylated by a second isoform(s) of PKC, which is activated by ROCK in the prolonged phase of contraction. Second, the ability of ROCK to phosphorylate CPI-17 in the prolonged phase of contraction may be caused by activation via PKC. Further studies are required to clarify the precise details of the cross talk between these two pathways.
ROCK and MYPT1 phosphorylation during a carbachol-mediated contraction.
MYPT1 is a myosin binding subunit that maintains the physical and functional integrity of the MLC phosphatase. There are two major phosphorylation sites of MYPT1 which have been suggested to regulate the activity of MLC phosphatase upon agonist stimulation: Thr696 and Thr850 (38). Carbachol stimulation activates ROCK, which is thought to be the primary kinase to catalyze phosphorylation of MYPT1 at both Thr696 and Thr850. However, it has been controversial as to whether both Thr696- and Thr850-MYPT1 are endogenous targets of ROCK in agonist-stimulated contraction of bladder smooth muscle. Given our finding that rabbit smooth muscle expresses ROCK (data not shown), the present study was designed to address whether carbachol-induced, ROCK-mediated phosphorylation of Thr696 and Thr850 in bladder smooth muscle occurs by using phosphorylation site-specific antibodies for MYPT1.
Thr696-MYPT1 phosphorylation.
Our results clearly show that, although high basal Thr696-MYPT1 phosphorylation levels were observed, carbachol stimulation did not increase the levels of phosphorylation in rabbit bladder smooth muscle. Previous reports have provided different results in terms of agonist-stimulated Thr696-MYPT1 phosphorylation. For example, it has been reported that an agonist stimulates an increase in Thr696-MYPT1 phosphorylation in rat ileal smooth muscle tissues and cultured vascular aorta smooth muscle cells (28, 34). Other reports are consistent with our findings that there is no change with agonist stimulation in several smooth muscle tissues from several different species such as rabbit vas deferens smooth muscles and rat femoral and caudal arterial smooth muscle (16, 27, 47). Even in studies using the same smooth muscle preparation, the results can vary depending on the agonist employed or the laboratory reporting the findings (16, 35). In terms of bladder smooth muscle, Mizuno et al. (21) reported that carbachol stimulation increased Thr696-MYPT1 phosphorylation in mice. Except for the difference in animal species, the reason for this apparent discrepancy with our current results is not known.
Inhibition of ROCK or PKC had no effect on either agonist-induced or basal levels of Thr696-MYPT1 phosphorylation. This suggests that Thr696-MYPT1 is not an endogenous target of either kinase. Previous reports have shown that MYPT1 phosphorylation, especially at Thr696, is highly resistant to dephosphorylation by protein phosphatases (45). This may imply that Thr696-MYPT1 phosphorylation-based regulation of MLC phosphatase activity may be more suitable for the long-term regulation of basal tone rather than the acute regulation such as during a single smooth muscle contraction-relaxation cycle. However, it should be noted that several other kinases have been suggested to phosphorylate Thr696-MYPT1. For example, ZIP-like kinase and integrin-linked kinase were reported to be potential kinases that phosphorylate MLC phosphatase in vivo (18, 24). Myotonic dystrophy protein kinase, p21-activated protein kinase, and Raf-1 have also been suggested to catalyze Thr696-MYPT1 phosphorylation and inhibit MLC phosphatase activity in vitro (1, 25, 44). Whether any of these kinases regulate the basal phosphorylation level of Thr696-MYPT1 in bladder smooth muscle requires further investigation.
Thr850-MYPT1 phosphorylation.
Our results demonstrate that levels of phosphorylation at the other inhibitory phosphorylation site, Thr850-MYPT1, were increased in the sustained phase of carbachol stimulation. These results indicate that ROCK expressed in bladder smooth muscle is active. Thr850-MYPT1 phosphorylation, instead of directly inhibiting MLC phosphatase activity, disrupts the binding of the phosphatase to myosin, thus decreasing its activity (46). Several groups have also reported an increase in Thr850-MYPT1 phosphorylation with agonist stimulation (16, 27, 47, 49). However, instead of preceding or even concomitant with the increase in agonist-induced force, Thr850-MYPT1 phosphorylation is only significantly increased during the later, tonic phase of contraction. In contrast, during KCl-stimulated contractions of bladder smooth muscle, Thr853-MYPT1 (equivalent to Thr850-MYPT1 in our present study) phosphorylation levels were demonstrated as early as 30 sec following stimulation (33).
Constitutively active ROCK.
Inhibition of ROCK not only abolished the carbachol-stimulated phosphorylation of Thr850-MYPT1, but it also decreased its basal phosphorylation level. Inhibition of PKC, however, abolished the carbachol-stimulated Thr850-MYPT1 phosphorylation but did not affect the basal levels of phosphorylation. Previous studies have suggested that ROCK is the major kinase that phosphorylates Thr850-MYPT1 and that PKC does not directly phosphorylate Thr850-MYPT1 (38, 49). ROCK also showed a significant contribution to the basal phosphorylation level of Thr850-MYPT1. This would suggest that a constitutively active ROCK is present in bladder smooth muscle. Our results are consistent with previous reports clearly demonstrating the presence of a constitutively active form of ROCK in bladder smooth muscle (9, 31, 33). Interestingly a constitutively active PKC has also been shown to be present in bladder smooth muscle (33) and vascular smooth muscle (26). The relatively high basal phosphorylation level of MYPT1 at both sites, with the presumed concurrent inhibition of MLC phosphatase activity, may explain the unusually high basal MLC phosphorylation levels we have observed in bladder smooth muscle. This would produce an increase in basal tone, which may in turn be important in regulating the volume-pressure relationship of the bladder as it fills.
Potential problems.
Although we believe our interpretations are consistent with the results of our studies, there are potential pitfalls with the tools used. It is always possible that techniques to measure protein phosphorylation levels are not sufficiently sensitive to measure stimulation-induced increases. Therefore, although we did not measure any carbachol-induced increases in Thr696-MYPT1 phosphorylation, it is possible that any increase was below our ability to detect. We do not believe this to be the case, as similar techniques were used to measure increases in Thr850-MYPT1 phosphorylation levels. A second potentially more serious pitfall is the fact that most if not all kinase inhibitors have nonspecific effects. We chose Bis and H-1152 because they are suggested to have the best specificity against PKC and ROCK, respectively. It is possible that Bis and H-1152 exhibited nonspecific effects, as the concentrations we used were higher than those shown to be effective on purified kinases or cells (3, 19). However, higher inhibitor concentrations are typically needed when tissues are used. We did not use alternative kinase inhibitors due to their known lack of specificity. Nonspecific effects of other kinase inhibitors, we believe, would have seriously diminished the ability to properly interpret the results.
Summary.
It is believed that the fast transient contraction of smooth muscle following agonist stimulation is due to Ca2+ mobilization, calmodulin binding, and the resultant activation of the MLC kinase. Inhibition of MLC phosphatase is more important in maintaining MLC phosphorylation during the sustained phase of contraction when Ca2+ concentrations have decreased toward basal values. Our results suggest that inhibition of MLC phosphatase activity is involved in both the early phasic and the later tonic phase of bladder smooth muscle contraction.
In summary, our results demonstrate that during the early phase of a carbachol-induced bladder smooth muscle contraction, the PKC-mediated CPI-17 phosphorylation pathway is the primary regulator of MLC phosphatase activity. In the later phase of contraction, PKC and ROCK act in tandem to regulate contraction through both CPI-17 and Thr850-MYPT1 phosphorylation. During the resting state, high levels of Thr850-MYPT1 are presumably due to the presence of a constitutively active ROCK. Thr696-MYPT1 phosphorylation appears to have little to no role in the agonist-induced contraction of at least rabbit bladder smooth muscle. We propose that the high levels of resting MYPT1 phosphorylation can account for the high levels of basal MLC phosphorylation seen in most bladder smooth muscles. This may be important in setting the volume-pressure curve during bladder filling.
GRANTS
This study was supported, in part, by National Institutes of Health Grants HL 37956, DK 57252, and DK 69898. D. M. Kendig was funded by a Drexel University College of Medicine Aging Initiative Graduate Fellowship.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Broustas CG, Grammatikakis N, Eto M, Dent P, Brautigan DL, Kasid U. Phosphorylation of the myosin-binding subunit of myosin phosphatase by Raf-1 and inhibition of phosphatase activity. J Biol Chem 277: 3053–3059, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Chang S, Hypolite JA, Mohanan S, Zderic SA, Wein AJ, Chacko S. Alteration of the PKC-mediated signaling pathway for smooth muscle contraction in obstruction-induced hypertrophy of the urinary bladder. Lab Invest 89: 823–832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C isolation from porcine aorta media and characterization. J Biochem 118: 1104–1107, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Eto M, Senba S, Morita F, Yazawa M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI-17) in smooth muscle: it's specific localization in smooth muscle. FEBS Lett 410: 356–360, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem 274: 37385–37290, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Gorenne I, Su X, Moreland RS. Inhibition of p42 and p44 MAP kinase activity does not alter smooth muscle contraction in swine carotid artery. Am J Physiol Heart Circ Physiol 275: H131–H138, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Hartshorne DJ. Myosin phosphatase: subunits and interactions. Acta Physiol Scand 164: 483–493, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Hashitani H, Brading AF, Suzuki H. Correlations between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem 259: 197–209, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Jezior JR, Brady JD, Rosenstein DI, McCammon KA, Minor AS, Ratz PH. Dependency of detrusor contractions on calcium sensitization and calcium entry through LOE-98-sensitive channels. Br J Pharmacol 134: 78–87, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol 25: 593–620, 1985 [DOI] [PubMed] [Google Scholar]
- 13.Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol 147: 1023–38, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem 275: 9897–9900, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol 546: 879–889, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama M, Ito M, Feng J, Seko T, Shiraki K, Takase K, Hartshorne DJ, Nakano T. Phosphorylation of CPI-17, an inhibitory phosphoprotein of smooth muscle myosin phosphatase, by Rho-kinase. FEBS Lett 475: 197–200, 2000 [DOI] [PubMed] [Google Scholar]
- 18.MacDonald JA, Borman MA, Murányi A, Somlyo AV, Hartshorne DJ, Haystead TA. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc Natl Acad Sci USA 98: 2419–2424, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 268: 9194–9197, 1993 [PubMed] [Google Scholar]
- 20.Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem 276: 22614–22620, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Mizuno Y, Isotani E, Huang J, Ding H, Stull JT, Kamm KE. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am J Physiol Cell Physiol 295: C358–C364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreland S, Moreland RS. Effects of dihydropyridines on stress, myosin phosphorylation, and Vo in smooth muscle. Am J Physiol Heart Circ Physiol 252: H1049–H1058, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Moreland S, Nishimura J, van Breemen C, Ahn HY, Moreland RS. Transient myosin phosphorylation at constant Ca2+ during agonist activation of permeabilized arteries. Am J Physiol Cell Physiol 263: C540–C544, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Murányi A, MacDonald JA, Deng JT, Wilson DP, Haystead TA, Walsh MP, Erdodi F, Kiss E, Wu Y, Hartshorne DJ. Phosphorylation of the myosin phosphatase target subunit by integrin-linked kinase. Biochem J 366: 211–216, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murányi A, Zhang R, Liu F, Hirano K, Ito M, Epstein HF, Hartshorne DJ. Myotonic dystrophy protein kinase phosphorylates the myosin phosphatase targeting subunit and inhibits myosin phosphatase activity. FEBS Lett 493: 80–84, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Navedo MF, Amberg GC, Votaw VS, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci USA 102: 1112–1117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niiro N, Koga Y, Ikebe M. Agonist-induced changes in the phosphorylation of the myosin- binding subunit of myosin light chain phosphatase and CPI17, two regulatory factors of myosin light chain phosphatase, in smooth muscle. Biochem J 369: 117–128, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem 278: 48794–48804, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Patil SB, Bitar KN. RhoA- and PKC-α-mediated phosphorylation of MYPT and its association with HSP27 in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 290: G83–G95, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Peters SL, Schmidt M, Michel MC. Rho kinase: a target for treating urinary bladder dysfunction? Trends Pharmacol Sci 27: 492–497, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Poley RN, Dosier CR, Speich JE, Minor AS, Ratz PH. Stimulated calcium entry and constitutive RhoA kinase activity cause stretch-induced detrusor contraction. Eur J Pharmacol 599: 137–145, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratz PH, Minor AS. Length-dependent regulation of basal mysoin phosphorylation and force in detrusor smooth muscle. Am J Physiol Regul Integr Comp Physiol 284: R1063–R1070, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Ratz PH, Minor AS. Role of protein kinase Cζ and calcium entry in KCl-induced vascular smooth muscle calcium sensitization and feedback control of cellular calcium levels. J Pharmacol Exp Ther 328: 399–408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, Nakano T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res 92: 411–418, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Shin HM, Je HD, Gallant C, Tao TC, Hartshorne DJ, Ito M, Morgan KG. Differential association and localization of myosin phosphatase subunits during agonist-induced signal transduction in smooth muscle. Circ Res 90: 546–553, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Slater SJ, Seiz JL, Stagliano BA, Stubbs CD. Interaction of protein kinase C isozymes with Rho GTPases. Biochemistry 40: 4437–4445, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature 372: 231–236, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Stanton MC, Austin JC, Delaney DP, Gosfield A, Marx JO, Zderic SA, Chacko S, Moreland RS. Partial bladder outlet obstruction selectively abolishes protein kinase C induced contraction of rabbit detrusor smooth muscle. J Urol 176: 2716–2721, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Stanton MC, Clement M, Macarak EJ, Zderic SA, Moreland RS. Partial bladder outlet obstruction alters Ca2+ sensitivity of force, but not of MLC phosphorylation, in bladder smooth muscle. Am J Physiol Renal Physiol 285: F703–F710, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Stanton MC, Delaney D, Zderic SA, Moreland RS. Partial bladder outlet obstruction abolishes the receptor- and G protein-dependent increase in calcium sensitivity in rabbit bladder smooth muscle. Am J Physiol Renal Physiol 287: F682–F689, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Su X, Smolock EM, Marcel KN, Moreland RS. Phosphatidylinositol 3-kinase modulates vascular smooth muscle contraction by calcium and myosin light chain phosphorylation-independent and -dependent pathways. Am J Physiol Heart Circ Physiol 286: H657–H666, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Su X, Stein R, Stanton MC, Zderic S, Moreland RS. Effect of partial outlet obstruction on rabbit urinary bladder smooth muscle function. Am J Physiol Renal Physiol 284: F644–F652, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Takizawa N, Koga Y, Ikebe M. Phosphorylation of CPI17 and myosin binding subunit of type 1 protein phosphatase by p21-activated kinase. Biochem Biophys Res Commun 297: 773–778, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Takizawa N, Niiro N, Ikebe M. Dephosphorylation of the two regulatory components of myosin phosphatase, MBS and CPI17. FEBS Lett 515: 127–132, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1 M at Thr850 induces its dissociation from myosin. FEBS Lett 527: 101–104, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J 389: 763–774, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca2+ sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol 535: 553–564, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao D, Longo LD, Zhang L. α1-Adrenoceptor-mediated phosphorylation of MYPT-1 and CPI-17 in the uterine artery: role of ERK/PKC. Am J Physiol Heart Circ Physiol 288: H2828–H2835, 2005 [DOI] [PubMed] [Google Scholar]