Abstract
Cyclooxygenase-2 activity is required for the development of lithium-induced polyuria. However, the involvement of a specific, terminal prostaglandin (PG) isomerase has not been evaluated. The present study was undertaken to assess lithium-induced polyuria in mice deficient in microsomal prostaglandin E synthase-1 (mPGES-1). A 2-wk administration of LiCl (4 mmol·kg−1·day−1 ip) in mPGES-1 +/+ mice led to a marked polyuria with hyposmotic urine. This was associated with elevated renal mPGES-1 protein expression and increased urine PGE2 excretion. In contrast, mPGES-1 −/− mice were largely resistant to lithium-induced polyuria and a urine concentrating defect, accompanied by nearly complete blockade of high urine PGE2 and cAMP output. Immunoblotting, immunohistochemistry, and quantitative (q) RT-PCR consistently detected a significant decrease in aquaporin-2 (AQP2) protein expression in both the renal cortex and medulla of lithium-treated +/+ mice. This decrease was significantly attenuated in the −/− mice. qRT-PCR detected similar patterns of changes in AQP2 mRNA in the medulla but not in the cortex. Similarly, the total protein abundance of the Na-K-2Cl cotransporter (NKCC2) in the medulla but not in the cortex of the +/+ mice was significantly reduced by lithium treatment. In contrast, the dowregulation of renal medullary NKCC2 expression was significantly attenuated in the −/− mice. We conclude that mPGES-1-derived PGE2 mediates lithium-induced polyuria likely via inhibition of AQP2 and NKCC2 expression.
Keywords: PGE2, acquired nephrogenic diabetes insipidus, AQP2, collecting duct
first introduced to clinical practice 50 years ago, lithium remains the cornerstone treatment of manic-depressive and other psychiatric illnesses (23). It is estimated that ∼1 of every 1,000 individuals in the population is on lithium treatment (58). Despite the proven efficacy, lithium can cause a number of deleterious side effects, with the most prominent one being acquired nephrogenic diabetes insipidus (NDI) (40, 58). Acquired NDI is caused by a urine concentrating defect that leads to polyuria and polydipsia, accompanied by reduced urine osmolality. The occurrence of acquired NDI is estimated to be in as many as 70% of patients on lithium therapy (2, 56). Although diverse mechanisms might be involved, the downregulation of renal aquaporin-2 (AQP2) is considered to play a major role in the pathogenesis of this disorder (28, 32).
PGE2 is a major prostanoid produced in the kidney with the highest amount detected in the inner medulla where it modulates collecting duct (CD) water permeability (5). When added to the vasopressin (AVP)-prestimulated CD, PGE2 potently inhibits water absorption and cAMP accumulation (12, 13, 15), consistent with their in vivo diuretic effects (22). Additionally, PGE2 may indirectly regulate water excretion by a reduction of the corticomedullary osmotic gradient via inhibition of solute transport in the thick ascending limbs (TAL) (53) or by increase in renal medullary blood flow. Urine PGE2 excretion is elevated following lithium treatment (24, 37, 47). In clinical practice, inhibition of PG synthesis with nonsteroidal anti-inflammatory drugs (NSAIDs) remains an effective therapy for lithium-induced polyuria (3, 29, 33).
Prostaglandin E2 synthase (PGES) terminally converts cyclooxygenase (COX)-derived PGH2 into PGE2. Three PGES have been cloned and are referred to as microsomal PGES (mPGES-1), mPGES-2, and cytosolic PGES (cPGES) (34). Among these isoforms, mPGES-1 has emerged as a novel PGES critically involved in pain and the inflammatory response as well as in physiological processes, including regulation of renal salt and water excretion (20, 21, 51, 52, 60). In addition, the PGE2 synthase activity of mPGES-2 and cPGES cannot be confirmed by gene knockout (KO) studies (17, 31, 36). The goal of the present study was to investigate the role of mPGES-1 in lithium-induced NDI.
METHODS
Animals.
mPGES-1 null mice were originally generated by Trebino et al. (57). This mouse colony was propagated at the University of Utah and maintained on a mixed DBA/1lacJ*C57BL/6/129/SV background. All protocols employing mice were conducted in accordance with the principles and guidance of the University of Utah Institutional Animal Care and Use Committee.
Lithium treatment.
Lithium treatment was performed as previously described (47). Briefly, male 3- to 4-mo-old mPGES-1 +/+ and −/− mice were administered vehicle (equivalent amount of saline) or LiCl (4 mmol·kg−1·day−1 ip) daily for 14 days. On days 0, 7, and 14, mice were placed in metabolic cages (Hatteras Instruments) for determination of 24-h urine output and water intake. All animals were fed a normal-sodium diet with free access to tap water. At the end of the experiment, the kidneys were harvested and blood samples were collected. One kidney was dissected into the cortex and the medulla (containing inner medulla and inner stripe of outer medulla), and half of the tissues were snap-frozen in liquid nitrogen and the other half stored in RNAlater; another kidney was fixed in 4% paraformaldehyde and embedded in paraffin. Urine and plasma osmolality was measured by using an osmometer (Osmett II, Precision Systems, Natick, MA).
Enzyme immunoassay.
Urine samples were centrifuged for 5 min at 10,000 rpm and diluted 1:1 with enzyme immunoassay buffer. Concentrations of urinary PGE2 and cAMP were determined by enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's the instructions.
Immunoblotting.
The frozen kidney tissues were homogenized by a polytron in PBS containing 1% Triton X-100, 250 μM phenylmethanesulfonyl fluoride (PMSF), 2 mM EDTA, and 5 mM DTT (pH 7.5). Protein concentrations were determined by Coomassie reagent. Forty micrograms of protein were denatured in boiling water for 10 min, separated by SDS-PAGE gel, and transferred onto nitrocellulose membranes. The blots were blocked overnight with 5% nonfat dry milk in Tris-buffered saline (TBS), followed by incubation for 1 h with rabbit anti-mPGES-1 antibody (catalog no. 160140, Cayman Chemical) or anti-AQP2 antibody (42) (gift from Dr. Mark A. Knepper, National Heart, Lung, and Blood Institute). After washing with TBS, blots were incubated with a goat anti-horseradish peroxidase-conjugated secondary antibody and visualized using enhanced chemiluminescence methods. The immunoreactive bands were quantified using the Gel and Graph Digitizing System (Silk Scientific, Orem, UT).
Immunohistochemistry.
Kidney sections (4-μm thickness) were incubated in 3% H2O2 for 10 min at room temperature to block endogenous peroxidase activity. The slides were boiled in antigen retrieval solution (1 mmol/l Tris·HCl, 0.1 mmol/l EDTA, pH 8.0) for 15 min at high power in a microwave oven. The sections were incubated overnight at 4°C with an anti-AQP2 antibody at appropriate dilutions (41). After washing with PBS, the secondary antibody was applied and the signals were visualized using an ABC kit (Santa Cruz Biotechnology).
Quantitative RT-PCR.
The kidney tissues stored in RNAlater were subjected to total RNA isolation using TRIzol. One microgram of total RNA was denatured at 65°C for 5 min, and cDNA synthesis was then performed at 42°C for 1 h using Superscript reverse transcriptase (BRL, Gaithersburg, MD). Oligonucleotides were designed using Primer3 software (available at http://frodo.wi.mit.edu/primer3/). The sequences of the oligonucleotide primers in the public sequence are as follows: AQP2, 5′-GGACCTGGCTGTCAATGCT-3′ (sense) and 5′-ATCGGTGGAGGCAAAGATG-3′ (antisense) (GenBank accession no. NM_009699); and β-actin, 5′-GCTCTGGCTCCTAGCACCAT-3′ (sense) and 5′-GCCACCGATCCACACAGAGT-3′ (antisense; GenBank accession no.NM_007393). Quantitative (q) PCR amplification was performed using the SYBR Green Master Mix (Applied Biosystems) and the Prism 7500 Real-Time PCR Detection System (Applied Biosystems). Cycling conditions were 95°C for 10 min, followed by 40 repeats at 95°C for 15 s and 60°C for 1 min. Relative amounts of mRNA, normalized by β-actin, were calculated from threshold cycle numbers (CT; i.e., 2−δδCT) and expressed as a value relative to the vehicle group (44).
Statistical analysis.
All of the values are presented as means ± SE. Repeated-measures ANOVA was used to analyze data from the time course studies with an unpaired Student's t-test to identify differences at a single time point. For the end point studies, statistical analysis was performed by using one-way ANOVA followed by a Bonferroni posttest. Differences were considered to be significant when the P value was <0.05.
RESULTS
Effect of mPGES-1 deletion on the lithium-induced urine concentrating defect.
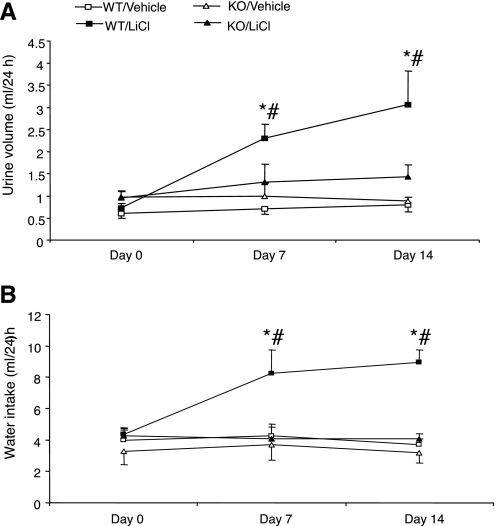
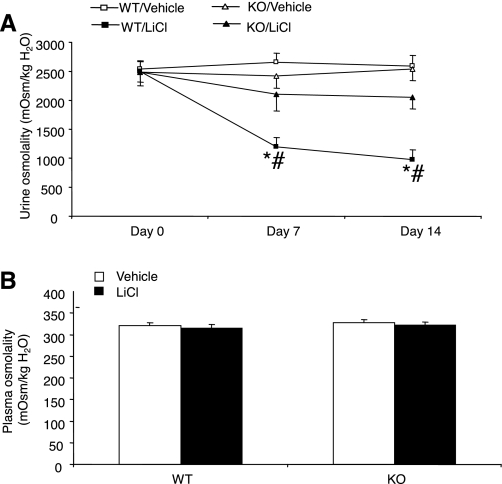
As shown in Fig. 1A, daily intraperitoneal injection of LiCl over 14 days induced time-dependent increases in urine volume in mPGES-1 +/+ mice (day 0: 0.73 ± 0.1; day 7: 2.3 ± 0.33, P < 0.01; day 14: 3.07 ± 0.77 ml/24 h, P < 0.01). In contrast, this treatment did not significantly affect urine output in mPGES-1 −/− mice (day 0: 0.95 ± 0.16; day 7: 1.3 ± 0.4, P > 0.05; day 14: 1.44 ± 0.26 ml/24 h, P > 0.05). In parallel with increased urine output, water intake markedly increased in lithium-treated +/+ mice (day 0: 4.35 ± 0.4; day 7: 8.3 ± 1.52, P < 0.01; day 14: 8.96 ± 0.8 ml/24 h, P < 0.01), but not in lithium-treated mPGES-1 −/− mice (day 0: 4.25 ± 0.6; day 7: 4.1 ± 0.93; day 14: 4.09 ± 0.33 ml/24 h) (Fig. 1B). Figure 2 summarizes changes in urinary vs. plasma osmolality in response to lithium treatment. Urine osmolality significantly decreased in lithium-treated +/+ mice (day 0: 2,498 ± 179; day 7: 1,200 ± 157, P < 0.01; day 14: 972 ± 177 mosmol/kgH2O, P < 0.01) (Fig. 2A), while, lithium-treated mPGES-1 −/− mice only exhibited a trend of reduction of urine osmolality at day 14 (day 0: 2,492 ± 23; day 7: 2,285 ± 195; day 14: 2,049 ± 196 mosmol/kgH2O, P = 0.077). Plasma osmolality was determined on day 14 and was unaltered irrespective of lithium treatment or the genotype (Fig. 2B). We also examined the AVP-induced urine concentrating ability between the genotypes. In response to chronic infusion of 1-desamino-8-d-arginine vasopressin (dDAVP; a synthetic analog of vasopressin) at 0.25 ng·kg−1·day−1 via osmotic minipumps, urine osmolality significantly increased in +/+ mice on day 1 and remained slightly elevated on day 3 (day 0: 1,936 ± 65; day 1: 2,311 ± 156, P < 0.05; day 3: 2,180 ± 64 mosmol/kgH2O, n = 6–8). A similar pattern of changes in urine osmolality was observed in dDAVP-treated mPGES-1 −/− mice (day 0: 2,040 ± 158; day 1: 2,319 ± 122, P < 0.05; day 3: 2,066 ± 62 mosmol/kgH2O, n = 6–8). In response to dDAVP infusion, a small decrease in urine volume was noticed in both genotypes on day 1, but this decrease did not reach statistical significance irrespective of genotype (data not shown).
Fig. 1.
Water intake and urine volume in microsomal prostaglandin E synthase-1 (mPGES-1) wild-type (WT) and knockout (KO) mice following a 14-day treatment with vehicle (equivalent amount of saline, ip once a day) or LiCl (4 mmol·kg−1·day−1 ip once a day). A: urine volume. B: water intake. Values are means ± SE; n = 5–7/group. *P < 0.01 vs. WT/vehicle. #P < 0.01 vs. KO/LiCl.
Fig. 2.
Urine and plasma osmolality in mPGES-1 WT and KO mice following treatment with vehicle or LiCl. A: urine osmolality. B: plasma osmolality. Values are means ± SE; n = 5–7/group. *P < 0.01 vs. WT/vehicle. #P < 0.01 vs. KO/LiCl.
Effect of mPGES-1 deletion on lithium-induced downregulation of renal transporters.
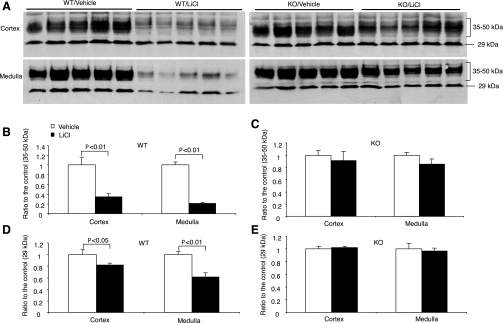
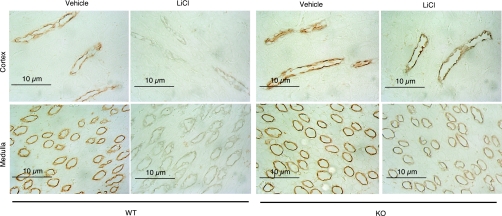
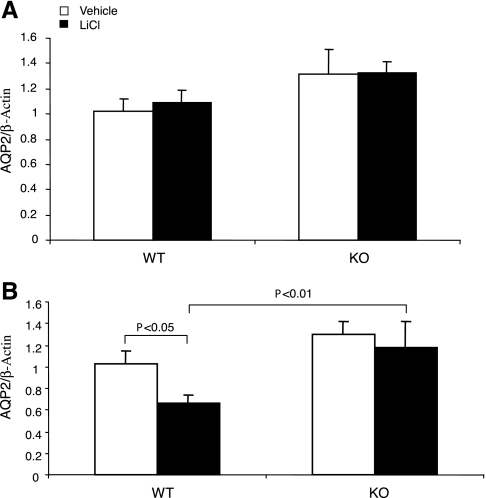
The downregulation of renal AQP2 expression plays a pivotal role in mediating lithium-induced polyuria. Moreover, the suppressed AQP2 expression may be attributable to elevated renal PG synthesis. Therefore, we performed a comprehensive analysis of renal AQP2 expression by immunoblotting, immunohistochemistry, and qRT-PCR. By immunoblotting, as expected, AQP2 protein was detected as multiple bands, consisting of the major band of 29 kDa and the glycosylated bands of 35–50 kDa. The abundance of the 35- to 50-kDa bands in the cortex was reduced by 67% and the abundance of the 29-kDa bands by 18% in lithium-treated +/+ mice (Fig. 3). In sharp contrast, none of these bands was significantly affected in lithium-treated mPGES-1 −/− mice (Fig. 3). Similar results were also seen in the medulla. Consistent with the immunoblotting results, immunohistochemistry detected drastically reduced labeling of AQP2 in the CD of lithium-treated +/+ mice. This reduction was also seen in mPGES-1 −/− mice but appeared to be less prominent compared with their +/+ controls (Fig. 4). Renal cortical AQP2 mRNA showed no changes in response to lithium treatment, irrespective of the genotype (Fig. 5A). In contrast, renal medullary AQP2 expression exhibited a 40% reduction in lithium-treated +/+ mice, but this reduction was less significant in lithium-treated mPGES-1 −/− mice (Fig. 5B). Compared with lithium-treated +/+ controls, renal medullary AQP2 mRNA was not significantly reduced in lithium-treated mPGES-1 −/− mice (Fig. 5B).
Fig. 3.
Renal aquaporin-2 (AQP2) protein abundance in mPGES-1 WT and KO mice following treatment with vehicle or LiCl. A: representative immunoblots of AQP2 in the cortex (top) and the medulla (bottom). B–E: densitometric analysis: WT/35–50 kDa (B), KO/35–50 kDa (C), WT/29 kDa (D), and KO/29 kDa (E). Values are means ± SE; n = 5.
Fig. 4.
AQP2 immunostaining in the cortex and medulla of mPGES-1 WT and KO mice following treatment with vehicle or LiCl. Shown are representatives from at least 3 animals in each group.
Fig. 5.
Quantitative (q) RT-PCR analysis of AQP2 expression in the cortex (A) and medulla (B) of mPGES-1 WT and KO mice following treatment with vehicle or LiCl. Values are means ± SE; n = 5–7/group.
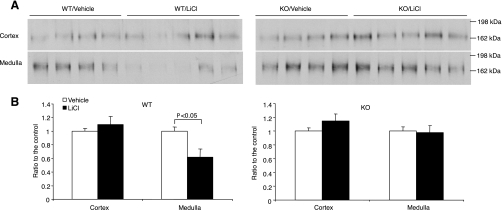
Besides AQP2, NKCC2 has also been implicated to play a role in lithium-induced NDI (24). Further evidence indicates that during lithium-induced NDI, NKCC2 may be subjected to downregulation by COX-2-derived products in a similar way to AQP2. Therefore, we performed immunoblot analysis of NKCC2 expression in the kidneys of lithium-treated mPGES-1 +/+ and −/− mice. Lithium treatment of mPGES-1 +/+ mice led to a 35% reduction of NKCC2 selectively in the medulla but not in the cortex (Fig. 6). In contrast, mPGES-1 −/− mice were resistant to lithium-induced reduction of renal medullary NKCC2 expression (Fig. 6).
Fig. 6.
Renal Na-K-2Cl cotransporter (NKCC2) protein abundance in mPGES-1 WT and KO mice following treatment with vehicle or LiCl. A: representative immunoblots of NKCC2 in the cortex (top) and medulla (bottom). B: densitometric analysis. Values are means ± SE; n = 4–5/group.
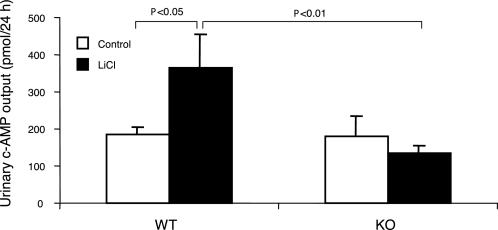
Elevated urinary cAMP excretion has been reported in lithium-induced NDI in rats despite unclear functional implications (55). We examined urinary cAMP excretion in lithium-treated mPGES-1 +/+ and −/− mice. The +/+ mice exhibited increased urinary cAMP excretion in response to lithium treatment that was not seen in the −/− mice (Fig. 7).
Fig. 7.
Urinary cAMP excretion in mPGES-1 WT and KO mice following treatment with vehicle or LiCl. The cAMP measurements were performed using ELISA on day 14. Values are means ± SE; n = 5–7/group.
Effect of lithium treatment on urinary PGE2 excretion and renal mPGES-1 expression.
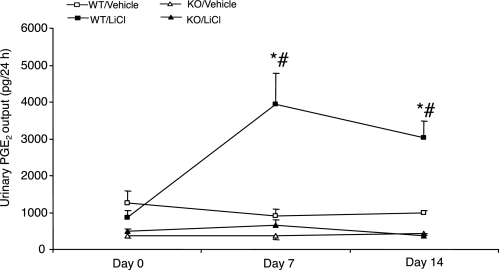
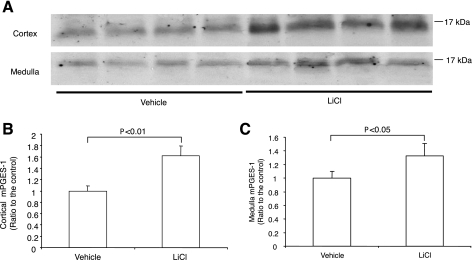
Lithium treatment in +/+ mice significantly elevated urinary PGE2 excretion (day 0: 876 ± 182; day 7: 3,947 ± 832, P < 0.01; day 14: 3,035 ± 448 pg/24 h, P < 0.01). mPGES-1 −/− mice had reduced baseline PGE2 excretion that was totally unresponsive to lithium treatment (day 0: 500 ± 41; day 7: 660 ± 150, P > 0.05; day 14: 364 ± 59 pg/24 h, P > 0.05) (Fig. 8). On day 14, the animals were killed and the kidneys from +/+ mice were subjected to immunoblotting for mPGES-1 expression. mPGES-1 protein abundance exhibited a 62% increase in the renal cortex and a 33% increase in the medulla in response to lithium treatment (Fig. 9).
Fig. 8.
Urinary PGE2 excretion in mPGES-1 WT and KO mice following treatment with vehicle or LiCl. Urinary PGE2 concentration was determined by enzyme immmunoassay. Values are means ± SE; n = 5–7/group. *P < 0.01 vs. WT/vehicle. #P < 0.01 vs. KO/LiCl for the corresponding period.
Fig. 9.
Immunoblotting analysis of mPGES-1 expression in the cortex and medulla of WT mice following a 14-day treatment with vehicle or LiCl. A: representative blots. B: densitometric analysis. The expression was normalized to the vehicle group. Values are means ± SE; n = 4/group.
DISCUSSION
mPGES-1 is a recently characterized PGE synthase critically involved in pain and the inflammatory response and is therefore considered as a viable target for development of the next generation of analgesic drugs. However, the potential role of this enzyme in other physiological or pathological processes is poorly characterized. mPGES-1 is abundantly expressed in the kidney, with a substantially higher level in the renal medulla than in the renal cortex (49), a pattern similar to COX-1 and COX-2 (19). Within the kidney, mPGES-1 expression is found in macula densa, distal convoluted tubule, collecting duct, and renal medullary interstitial cells, corresponding to the nephron distribution of PGE2 synthesis. Our previous studies employing mPGES-1 KO have identified a physiological role of this enzyme in mediating the natriuretic and diuretic responses during salt and water loading (21) (51). Lithium-induced NDI is a well-described disorder characterized by polyuria, polydipsia, and a urine concentrating defect, accompanied by increased PGE2 synthesis. The present study demonstrated that overactivation of renal mPGES-1 is the underlying pathogenesis of lithium-induced NDI. Clear-cut data showed that mPGES-1 KO mice were resistant to lithium-induced polyuria, accompanied by almost completely blockade of increased urinary PGE2 excretion. In parallel with the improved urine concentrating ability, renal AQP2 expression was preserved in the lithium-treated mPGES-1 KO mice. Moreover, lithium treatment significantly elevated mPGES-1 protein expression in the kidney.
A rich literature has documented PGE2 as an important regulator of both Na+ and water transport along the nephron (1, 5). In vitro microperfusion studies demonstrate that PGE2 directly inhibits Na+ and Cl− transport in the TAL (53, 54) and Na+ transport and antagonizes the hydrosmotic action of AVP in the collecting duct (11–14, 54). PGE2 may also indirectly regulate urinary Na+ and water excretion by regulating renal medullary blood flow (45). Evidence exists to link increased PGE2 synthesis and lithium-induced polyuria. A 7-day lithium treatment in rats induced a marked polyuria with significant excretion of urinary PGE2, which typically reflects renal synthesis (55). Administration of indomethacin to the lithium-treated rats diminished urine volume by 80% and urinary PGE2 by 85%, suggesting a requirement of COX activity in producing the renal effect of lithium. In vitro and in vivo studies showed that lithium stimulated renal COX-2 expression via suppressing glycogen synthase kinase-3 activity but that COX-1 expression was unaffected (46, 47). The finding seems to favor a preferential involvement of COX-2, while administration of the COX-2 inhibitor SC58236 in lithium-treated wild-type mice only produced partial relief of polyuria and high urine PGE2 output. Interestingly, administration of SC58236 in the background of COX-1 deficiency resulted in a nearly complete relief in these parameters, suggesting participation of both COX isoforms (47).
COX activity generates an unstable endoperoxide intermediate PGH2 that is finally converted to bioactive prostanoids, including PGE2, prostacyclin (PGI2), PGF2α, PGD2, and thromboxane A2, by distinct terminal synthases (16, 50). To date, no studies are available to test the involvement of terminal synthases in the renal effect of lithium. Among terminal PG synthases, PGES specifically catalyzes the conversion of PGH2 to PGE2. The present study is the first to demonstrate a role of mPGES-1 in lithium-induced NDI. As reported by the study of Rao et al. (47), daily intraperitoneal injection of LiCl for 2 wk induced a marked increase in urine PGE2 excretion and renal mPGES-1 expression that corresponds to increased urine volume in mice. Strikingly, mPGES-1 deletion nearly completely abolished the increases in urine PGE2 and urine volume. These findings demonstrated that mPGES-1 nonredundantly contributed to lithium-induced NDI. Indeed, with the recent gene KO studies failing to prove PGE2 synthase activities of mPGES-2 (18) and cPGES (31, 36), mPGES-1 appears to represent the only enzymatic pathway capable of synthesizing PGE2 in vivo. The magnitude of inhibition of lithium-induced polyuria and PGE2 excretion in mPGES-1 KO mice was similar to that achieved by concomitant inhibition of COX-1 and COX-2 (47). Therefore, it seems reasonable to speculate that mPGES-1 may couple to both COX isoforms to mediate lithium-induced PGE2 synthesis in the kidney. It is somewhat puzzling, however, that lithium treatment in rats induced a region-dependent reduction of COX-1, COX-2, and mPGES-1 despite increased urine PGE2 excretion (27). The difference in species is unlikely accountable since a recent study demonstrated a pathogenic role of COX-2 in lithium-induced NDI (24) in rats, as reported earlier in mice (47). It seems possible that the discrepancy may be related to differences in experimental protocols, such as the dose or route of lithium administration.
The primary target of PGE2 in lithium-induced NDI appears to be AQP2. In this regard, the downregulation of renal AQP2 expression in lithium-treated +/+ mice was confirmed at the protein and mRNA levels, and this downregulation was significantly attenuated in mPGES-1 KO mice. This finding is in agreement with the observation that a COX-2 inhibitor improved polyuria and restored renal AQP2 expression in lithium-treated rats (24). In another NDI model induced by ureteral obstruction, COX-2 inhibitors exhibited similar effects (6, 43). Moreover, evidence from a human study suggests an inverse relationship between urinary AQP2 excretion and local PGE2 production in postobstructed kidneys (35). In our NDI model, mPGES-1 deletion preserved the total abundance of renal AQP2 protein and mRNA. Thus PGE2 regulation of AQP2 may occur primarily at the gene expression level although other studies suggested an influence of PGE2 on AQP2 trafficking in several cell culture systems (38, 61).
In response to lithium treatment, the changes in AQP2 protein appear to be more prominent than that in AQP2 mRNA. The dissociation between AQP2 protein and mRNA is particularly obvious in the cortex, where AQP2 protein was markedly suppressed by lithium, to a similar extent as in the inner medulla, contrasting to unchanged levels of renal cortical AQP2 mRNA. A similar phenomenon was also seen in the mouse model of acute water loading (51). Together, these findings emphasize the importance of posttranscriptional regulation of AQP2.
Although the changes in AQP2 are considered to play a major role in the development of lithium-induced polyuria and the urine concentrating defect, other mechanisms may also be responsible. These mechanisms may involve the reduction of the medullary osmotic gradient as a result of the downregulation of Na+ and urea transporters (24, 26). Indeed, renal expression of NKCC2, a key player responsible for generation of the medullary osmotic gradient, exhibited a similar pattern of changes as AQP2. The complexity is revealed by microarray and proteomic screening showing that lithium treatment altered renal medullary expression of a large number of transcripts and proteins, particularly those involved in cellular proliferation and cytoskeleton organization (7, 8, 25, 39, 48). It remains elusive whether mPGES-1-derived PGE2 influences cellular proliferation and cytoskeleton organization during lithium-induced NDI and whether this effect is linked to the alteration in renal transporters.
Increased urinary cAMP excretion has been reported in lithium-induced NDI in rats with unclear functional implications (55). The phenomenon cannot be explained with AVP; AVP-dependent renal generation of cAMP is impaired (4, 9, 10) or unaffected (30) in lithium-induced NDI. Other explanations include the increased production or action of parathyroid hormone, another important contributor of urinary cAMP excretion (55). In the present study, the increased urinary cAMP excretion in lithium-treated +/+ mice confirms the original observation in rats (55). Interestingly, the increase was completely abolished in mPGES-1 −/− mice. Whatever the underlying mechanism, this finding substantiates the difference between the genotypes in the response to lithium treatment.
Based on the in vitro perfusion studies, PGE2 is believed to play an important role in the regulation of CD function by antagonizing AVP-dependent water permeability and cAMP generation (11–14, 54). The availability of mPGES-1 KO mice provides a novel tool for assessing the role of endogenous PGE2 in modulating the urine concentrating action of AVP. To our surprise, mPGES-1 deletion had no effect on the urine osmolality response to dDAVP infusion, arguing against the well-accepted concept that inhibition of the hydrosmotic action of AVP underlies the diuretic effect of PGE2. It should be pointed out, however, that the expected reduction of urine volume after dDAVP infusion was not observed in either genotype possibly due to the technical difficulty in accurately collecting the small amount of urine under basal or antidiuresis condition. Another possibility is that after 24 h of dDAVP infusion, the animals may be already in a steady state.
Perhaps the most exciting aspect of the present study may lie in the therapeutic implication concerning the development of mPGES-1 inhibition as a novel intervention for the NDI caused by lithium or other etiologies. Due to the cardiovascular consequences of COX-2 inhibitors, mPGES-1 is considered as a promising target for the development of the next generation of analgesic drugs. A selective mPGES-1 inhibitor, MF63, has recently been developed by Merck and is reported to block pain and inflammatory responses in guinea pig and knock-in mice expressing human mPGES-1 (59). Our results will prompt future evaluation of mPGES-1 inhibitors the in treatment of the NDI state.
GRANTS
This work was supported by a Veterans Affairs Merit Review and National Institutes of Health Grants HL079453 and DK079162 (to T. Yang). T. Yang is an Established Investigator of the American Heart Association.
Acknowledgments:
The authors thank John McNeish (Pfizer, Groton, CT) for providing breeder pairs of mPGES-1 KO mice, Mark A. Knepper (National Institutes of Health/National Heart, Lung, and Blood Institute) for providing the anti-AQP2 antibody, and Shannon J. Odelberg and Ivor J. Benjamin (Cardiology, University of Utah) for technical assistance.
REFERENCES
- 1..Anderson RJ, Berl T, McDonald KM, Schrier RW. Prostaglandins: effects on blood pressure, renal blood flow, sodium and water excretion. Kidney Int 10: 205–215, 1976 [DOI] [PubMed] [Google Scholar]
- 2.Bedford JJ, Leader JP, Walker RJ. Aquaporin expression in normal human kidney and in renal disease. J Am Soc Nephrol 14: 2581–2587, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bendz H, Aurell M. Drug-induced diabetes insipidus: incidence, prevention and management. Drug Saf 21: 449–456, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis 10: 329–345, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol 279: F12–F23, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Cheng X, Zhang H, Lee HL, Park JM. Cyclooxygenase-2 inhibitor preserves medullary aquaporin-2 expression and prevents polyuria after ureteral obstruction. J Urol 172: 2387–2390, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Christensen BM, Kim YH, Kwon TH, Nielsen S. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Christensen BM, Marples D, Kim YH, Wang W, Frokiaer J, Nielsen S. Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am J Physiol Cell Physiol 286: C952–C964, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Christensen S, Kusano E, Yusufi AN, Murayama N, Dousa TP. Pathogenesis of nephrogenic diabetes insipidus due to chronic administration of lithium in rats. J Clin Invest 75: 1869–1879, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cogan E, Abramow M. Inhibition by lithium of the hydroosmotic action of vasopressin in the isolated perfused cortical collecting tubule of the rabbit. J Clin Invest 77: 1507–1514, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culpepper RM, Andreoli TE. PGE2, forskolin, and cholera toxin interactions in modulating NaCl transport in mouse mTALH. Am J Physiol Renal Fluid Electrolyte Physiol 247: F784–F792, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Grantham JJ, Orloff J. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3′,5′-monophosphate, and theophylline. J Clin Invest 47: 1154–1161, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert RL, Jacobson HR, Breyer MD. PGE2 inhibits AVP-induced water flow in cortical collecting ducts by protein kinase C activation. Am J Physiol Renal Fluid Electrolyte Physiol 259: F318–F325, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Hebert RL, Jacobson HR, Breyer MD. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin Invest 87: 1992–1998, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebert RL, Jacobson HR, Fredin D, Breyer MD. Evidence that separate PGE2 receptors modulate water and sodium transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F643–F650, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta 1299: 125–140, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Jania LA, Chandrasekharan S, Backlund MG, Foley NA, Snouwaert J, Wang IM, Clark P, Audoly LP, Koller BH. Microsomal prostaglandin E synthase-2 is not essential for in vivo prostaglandin E2 biosynthesis. Prostaglandins Other Lipid Mediat 88: 73–81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jania LA, Chandrasekharan S, Backlund MG, Foley NA, Snouwaert J, Wang IM, Clark P, Audoly LP, Koller BH. Microsomal prostaglandin E synthase-2 is not essential for in vivo prostaglandin E(2) biosynthesis. Prostaglandins Other Lipid Mediat 88: 73–81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen BL, Kurtz A. Differential regulation of renal cyclooxygenase mRNA by dietary salt intake. Kidney Int 52: 1242–1249, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Jia Z, Guo X, Zhang H, Wang MH, Dong Z, Yang T. Microsomal prostaglandin synthase-1-derived prostaglandin E2 protects against angiotensin II-induced hypertension via inhibition of oxidative stress. Hypertension 52: 952–959, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res 99: 1243–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Johnston HH, Herzog JP, Lauler DP. Effect of prostaglandin E1 on renal hemodynamics, sodium and water excretion. Am J Physiol 213: 939–946, 1967 [DOI] [PubMed] [Google Scholar]
- 23.Khanna A. Acquired nephrogenic diabetes insipidus. Semin Nephrol 26: 244–248, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Kim GH, Choi NW, Jung JY, Song JH, Lee CH, Kang CM, Knepper MA. Treating lithium-induced nephrogenic diabetes insipidus with a COX-2 inhibitor improves polyuria via upregulation of AQP2 and NKCC2. Am J Physiol Renal Physiol 294: F702–F709, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Kim YH, Kwon TH, Christensen BM, Nielsen J, Wall SM, Madsen KM, Frøkiær J, Nielsen S. Altered expression of renal acid-base transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol 285: F1244–F1257, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Klein JD, Gunn RB, Roberts BR, Sands JM. Down-regulation of urea transporters in the renal inner medulla of lithium-fed rats. Kidney Int 61: 995–1002, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Kotnik P, Nielsen J, Kwon TH, Krzisnik C, Frøkiær J, Nielsen S. Altered expression of COX-1, COX-2, and mPGES in rats with nephrogenic and central diabetes insipidus. Am J Physiol Renal Physiol 288: F1053–F1068, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Kwon TH, Laursen UH, Marples D, Maunsbach AB, Knepper MA, Frøkiær J, Nielsen S. Altered expression of renal AQPs and Na+ transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol 279: F552–F564, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Lam SS, Kjellstrand C. Emergency treatment of lithium-induced diabetes insipidus with nonsteroidal anti-inflammatory drugs. Ren Fail 19: 183–188, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Shaw S, Kamsteeg EJ, Vandewalle A, Deen PM. Development of lithium-induced nephrogenic diabetes insipidus is dissociated from adenylyl cyclase activity. J Am Soc Nephrol 17: 1063–1072, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Lovgren AK, Kovarova M, Koller BH. cPGES/p23 is required for glucocorticoid receptor function and embryonic growth but not prostaglandin E2 synthesis. Mol Cell Biol 27: 4416–4430, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marples D, Christensen S, Christensen EI, Ottosen PD, Nielsen S. Lithium-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla. J Clin Invest 95: 1838–1845, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez EJ, Sinnott JTt Rodriguez-Paz G, Oehler RL. Lithium-induced nephrogenic diabetes insipidus treated with indomethacin. South Med J 86: 971–973, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Murakami M, Kudo I. Prostaglandin E synthase: a novel drug target for inflammation and cancer. Curr Pharm Des 12: 943–954, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Murer L, Addabbo F, Carmosino M, Procino G, Tamma G, Montini G, Rigamonti W, Zucchetta P, Della Vella M, Venturini A, Zacchello G, Svelto M, Valenti G. Selective decrease in urinary aquaporin 2 and increase in prostaglandin E2 excretion is associated with postobstructive polyuria in human congenital hydronephrosis. J Am Soc Nephrol 15: 2705–2712, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Nakatani Y, Hokonohara Y, Kakuta S, Sudo K, Iwakura Y, Kudo I. Knockout mice lacking cPGES/p23, a constitutively expressed PGE2 synthetic enzyme, are peri-natally lethal. Biochem Biophys Res Commun 362: 387–392, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Nally JV, Rutecki GW, Ferris TF. The acute effect of lithium on renal renin and prostaglandin E synthesis in the dog. Circ Res 46: 739–744, 1980 [DOI] [PubMed] [Google Scholar]
- 38.Nejsum LN, Zelenina M, Aperia A, Frøkiær J, Nielsen S. Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation. Am J Physiol Renal Physiol 288: F930–F938, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci USA 105: 3634–3639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nielsen J, Kwon TH, Christensen BM, Frøkiær J, Nielsen S. Dysregulation of renal aquaporins and epithelial sodium channel in lithium-induced nephrogenic diabetes insipidus. Semin Nephrol 28: 227–244, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92: 1013–1017, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA 90: 11663–11667, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norregaard R, Jensen BL, Li C, Wang W, Knepper MA, Nielsen S, Frøkiær J. COX-2 inhibition prevents downregulation of key renal water and sodium transport proteins in response to bilateral ureteral obstruction. Am J Physiol Renal Physiol 289: F322–F333, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Paliege A, Mizel D, Medina C, Pasumarthy A, Huang YG, Bachmann S, Briggs JP, Schnermann JB, Yang T. Inhibition of nNOS expression in the macula densa by COX-2-derived prostaglandin E2. Am J Physiol Renal Physiol 287: F152–F159, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 284: F253–F266, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Rao R, Hao CM, Breyer MD. Hypertonic stress activates glycogen synthase kinase 3beta-mediated apoptosis of renal medullary interstitial cells, suppressing an NFkappaB-driven cyclooxygenase-2-dependent survival pathway. J Biol Chem 279: 3949–3955, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Rao R, Zhang MZ, Zhao M, Cai H, Harris RC, Breyer MD, Hao CM. Lithium treatment inhibits renal GSK-3 activity and promotes cyclooxygenase 2-dependent polyuria. Am J Physiol Renal Physiol 288: F642–F649, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Rojek A, Nielsen J, Brooks HL, Gong H, Kim YH, Kwon TH, Frøkiær J, Nielsen S. Altered expression of selected genes in kidney of rats with lithium-induced NDI. Am J Physiol Renal Physiol 288: F1276–F1289, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Schneider A, Zhang Y, Zhang M, Lu WJ, Rao R, Fan X, Redha R, Davis L, Breyer RM, Harris R, Guan Y, Breyer MD. Membrane-associated PGE synthase-1 (mPGES-1) is coexpressed with both COX-1 and COX-2 in the kidney. Kidney Int 65: 1205–1213, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem 271: 33157–33160, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Soodvilai S, Jia Z, Wang MH, Dong Z, Yang T. mPGES-1 deletion impairs diuretic response to acute water loading. Am J Physiol Renal Physiol 296: F1129–F1135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soodvilai S, Jia Z, Yang T. Hydrogen peroxide stimulates chloride secretion in primary inner medullary collecting duct cells via mPGES-1-derived PGE2. Am J Physiol Renal Physiol 293: F1571–F1576, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Stokes JB. Effect of prostaglandin E2 on chloride transport across the rabbit thick ascending limb of Henle. Selective inhibitions of the medullary portion. J Clin Invest 64: 495–502, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stokes JB, Kokko JP. Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J Clin Invest 59: 1099–1104, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugawara M, Hashimoto K, Ota Z. Involvement of prostaglandin E2, cAMP, and vasopressin in lithium-induced polyuria. Am J Physiol Regul Integr Comp Physiol 254: R863–R869, 1988 [DOI] [PubMed] [Google Scholar]
- 56.Timmer RT, Sands JM. Lithium intoxication. J Am Soc Nephrol 10: 666–674, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, Carter D, Thomas NA, Durtschi BA, McNeish JD, Hambor JE, Jakobsson PJ, Carty TJ, Perez JR, Audoly LP. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci USA 100: 9044–9049, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker RG. Lithium nephrotoxicity. Kidney Int Suppl 42: S93–S98, 1993 [PubMed] [Google Scholar]
- 59.Xu D, Rowland SE, Clark P, Giroux A, Cote B, Guiral S, Salem M, Ducharme Y, Friesen RW, Methot N, Mancini J, Audoly L, Riendeau D. MF63 [2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)-isophthalonitrile], a selective microsomal prostaglandin E synthase-1 inhibitor, relieves pyresis and pain in preclinical models of inflammation. J Pharmacol Exp Ther 326: 754–763, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Yang T. Microsomal prostaglandin E synthase-1 and blood pressure regulation. Kidney Int 72: 274–278, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Zelenina M, Christensen BM, Palmer J, Nairn AC, Nielsen S, Aperia A. Prostaglandin E2 interaction with AVP: effects on AQP2 phosphorylation and distribution. Am J Physiol Renal Physiol 278: F388–F394, 2000 [DOI] [PubMed] [Google Scholar]