Abstract
To test the hypothesis that deletion of the transient receptor potential vanilloid type 1 (TRPV1) channel exaggerates hypertension-induced renal inflammatory response, wild-type (WT) or TRPV1-null mutant (TRPV1−/−) mice were subjected to uninephrectomy and deoxycorticosterone acetate (DOCA)-salt treatment for 4 wk. Mean arterial pressure (MAP) determined by radiotelemetry increased in DOCA-salt-treated WT or TRPV1−/− mice, whereas there was no difference in MAP between two strains at the baseline or after DOCA-salt treatment. DOCA-salt treatment increased urinary excretion of albumin and 8-isoprostane in both WT and TRPV1−/− mice, and the increases were greater in magnitude in the latter strain. Periodic acid-Schiff and Mason's trichrome staining showed that kidneys of DOCA-salt-treated TRPV1−/− mice exhibited more severe glomerulosclerosis and tubulointerstitial injury compared with DOCA-salt-treated WT mice. NF-κB assay showed that DOCA-salt treatment increased renal activated NF-κB concentrations in TRPV1−/− mice compared with WT mice. Immunostaining and ELISA assay revealed that DOCA-salt-treated TRPV1−/− mice had enhanced renal infiltration of monocyte/macrophage and lymphocyte, as well as increased renal levels of proinflammatory cytokine (TNF-α, IL-6) and chemokine (MCP-1) compared with DOCA-salt-treated WT mice. Renal ICAM-1 but not VCAM-1 expression was also greater in DOCA-salt-treated TRPV1−/− than WT mice. Dexamethasone (DEXA), an immunosuppressive drug, conveyed a renoprotective effect that was greater in DOCA-salt-treated TRPV1−/− compared with WT mice. These data show that renal inflammation is exacerbated in DOCA-salt hypertension when TRPV1 gene is deleted and that the deterioration is ameliorated by DEXA treatment, indicating that TRPV1 may act as a potential regulator of the inflammatory process to lessen renal injury in DOCA-salt hypertension.
Keywords: transient receptor potential vanilloid type 1 channel, deoxycorticosterone, dexamethasone
the transient receptor potential vanilloid type 1 (TRPV1) is a nonselective cation channel of the transient receptor potential channel family (9, 27). As a polymodal receptor, it may be activated by multiple stimuli including noxious heat, low pH, and chemical irritants such as capsaicin (9, 27). TRPV1 predominantly resides in unmyelinated C-fibers and thinly myelinated Aδ-afferent nerve fibers innervating the cardiovascular system, and activation of TRPV1 expressed in these nerves causes release of a number of sensory neuropeptides including calcitonin gene-related peptide (CGRP) and substance P, which are potent vasodilators in various vascular beds (46).
In addition to stimuli indicated above, a variety of inflammatory mediators including lipoxygenase products, bradykinin, and prostaglandins may activate or sensitize TRPV1 (27, 49). As a result, the role of TRPV1 in mediating inflammatory processes and its pathophysiological relevance have drawn quite attention. It has been shown that activation of TRPV1 in various tissues elicits neurogenic inflammation and nociception (26). In contrast, studies have shown that inflammatory responses evoked by endotoxin are exaggerated in TRPV1-null mutant (TRPV1−/−) mice, indicating that TRPV1 activation may possess anti-inflammatory effects (13, 23).
Hypertension is a major cardiovascular risk factor that causes end-organ damage including end-stage renal disease (6). Recent experimental evidence shows that progression of hypertension-induced renal damage is accompanied by intrarenal leukocytic cell infiltration and inflammation (20, 35–37, 42). For example, studies in several models of experimental hypertension have shown invasion of lymphocytes and macrophages in the kidney (36, 37). Moreover, treatment with immunosuppression/anti-inflammatory drugs or with chemokine receptor antagonists to inhibit leukocyte recruitment slows progression of renal damage in hypertensive animals (20, 35, 42). Despite these interesting findings, molecular and cellular mechanisms underlying renal inflammatory responses in hypertension remain poorly characterized.
Our recent data show that ablation of TRPV1 gene exacerbates renal damage induced by deoxycorticosterone acetate (DOCA)-salt hypertension, suggesting that TRPV1 may constitute a protective mechanism against end-organ damage induced by salt-dependent hypertension (43). Based on these findings, we hypothesized that DOCA-salt-induced renal inflammatory responses characterized with disturbed production of multiple inflammatory factors are exaggerated in TRPV1−/− mice. To this end, renal function, structure, inflammatory cell infiltration, production of multiple inflammatory factors, and activity of the transcriptional NF-κB were determined in DOCA-salt-treated wild-type (WT) and TRPV1−/− mice. In addition, renal inflammatory responses to dexamethasone (DEXA), an immunosuppressive agent, were examined in DOCA-salt-hypertensive WT and TRPV1−/− mice to elucidate the role of inflammation in renal injury.
METHODS
Animals.
Ten-week-old male TRPV1−/− (B6.129S4-TRPV1tm1Jul) and control WT (C57BL/6) mice (weighing 26 to 28 g; Charles River Laboratory, Wilmington, MA) were used in this study. TRPV1−/− mice, generated by deleting an exon encoding part of the fifth and all of the sixth putative transmembrane domains (including the interconnecting p-loop) of the channel, were backcrossed to C57BL/6 mice for at least six generations (9). All of the experiments were approved by the Institutional Animals Care and Use Committee.
Telemetry blood pressure assay.
Mean arterial pressure (MAP) and heart rate (HR) were determined using the telemetry system (Data Sciences International, St. Paul, MN) according to the manufacturer's instruction. In brief, the mice were anesthetized with ketamine and xylazine (80 and 4 mg/kg sc, respectively), and the transmitter catheter was implanted into the left carotid artery with the transmitter body placed subcutaneously in the lower right hand side of the abdomen. The mice were returned to their individual cages and allowed for recovery for 3 days before starting of the radiotelemetric recording.
Experimental protocol.
One week after the radiotelemetric recording, mice were reanesthetized as described above, and the left kidney was removed. The DOCA pellet (24 mg/10 g body wt; Innovative Research of America, Sarasota, FL) was implanted subcutaneously in the neck area under anesthesia. Mice receiving DOCA were given 1% NaCl and 0.2% KCl to drink, and treatment with DOCA-salt continued for 4 wk. Control mice underwent uninephrectomy without receiving DOCA and saline. Mice were separated into four groups: 1) WT control mice receiving tap water, 2) TRPV1−/− control mice receiving tap water, 3) WT mice receiving DOCA-salt, and 4) TRPV1−/− mice receiving DOCA-salt. Each group consisted of at least seven mice. Mice were allowed to recover from anesthesia for 24 h after surgery, and radiotelemetric recording continued for an additional 4 wk.
To examine the effect of DEXA on DOCA-salt-induced renal inflammatory responses and injury, WT and TRPV1−/− mice receiving DOCA and saline were subcutaneously given vehicle or DEXA (Sigma, St. Louis, MO) at a daily dose of 0.5 mg/kg for 4 wk. DEXA was dissolved in normal saline containing ethanol (10% vol/vol) and Tween 80 (10% vol/vol). At the end of the treatment period, direct intracarotid blood pressure measurement was performed under the conscious state in DOCA-salt-treated WT and TRPV1−/− mice given vehicle or DEXA, as well as in sham-operated control WT and TRPV1−/− mice using the protocol as previously reported (44). Subsequently, the kidneys were harvested, weighed, and stored at −80°C for analysis of inflammatory mediators or fixed in 10% formaldehyde solution in phosphate buffer for histological analysis.
Urine analysis.
At the end of the 4-wk treatment, mice were placed in mouse metabolic cages for 24-h urine collection. Urinary albumin was measured with an enzyme-linked immunosorbent assay kit (Exocell, Philadelphia, PA). Urinary 8-isoprostane levels were determined using the kit from Cayman Chemical (Ann Arbor, MI).
Histological analysis.
The formaldehyde-fixed kidneys were paraffin-embedded, and sections were prepared and stained with periodic acid-Schiff (PAS) and Mason's trichrome stain. The degree of sclerosis and tubulointerstitial injury was determined in tissue sections stained with PAS and Mason's trichrome, respectively, by two examiners without knowledge of prior treatment of sections.
The degree of glomerulosclerosis was determined using a semiquantitative scoring method published previously (39). Fifty glomeruli per section were selected randomly and scored as follows: grade 0, normal glomeruli; grade 1, sclerotic area ≤25% of total glomerular area or distinct adhesion present between capillary tuft and Bowman's capsule; grade 2, sclerotic area between 25 and 50%; grade 3, sclerotic area 50 to 75%; and grade 4, sclerotic area 75 to 100% of the total glomerular area. The ultimate score was obtained by multiplying the degree of changes by the percentage of glomeruli with the same degree of injury and adding up these scores.
Tubulointerstitial injury in the renal cortex and outer medulla was defined as tubular dilation or atrophy, interstitial fibrosis, or inflammatory cell infiltration. Based on the percentage area of the damaged cortex or outer medulla, injury was graded on a scale of 0 to 4 as described previously (40): grade 0, normal; grade 0.5, small focal areas of damage; grade 1, <10%; grade 2, 10 to 25%; grade 3, 25 to 75%; and grade 4, >75% tubulointerstitial injury involvement.
Immunohistochemistry.
Renal monocyte/macrophage and lymphocyte infiltration was analyzed in tissues embedded in 3-μm-thick paraffin sections obtained 4 wk after the treatment. Sections were mounted on glass slides, deparaffinized, and rehydrated. The sections were then treated with 3% hydrogen peroxide for 5 min and blocked with 3% normal horse serum in phosphate buffer solution for 1 h. Rat anti-mouse monoclonal antibody to F4/80 (1:200; Serotec, Oxford, UK) or rabbit anti-human polyclonal antibody to CD3 (1:400; Dako) was used for overnight incubation at 4°C. Horse anti-rat IgG horseradish peroxidase or horse anti-rabbit IgG horseradish peroxidase (1:400; Vector Laboratories, Burlingame, CA) was applied and incubated for 1 h at room temperature and visualized by incubating the sections with the substrate vector fast red or 3,3′-diaminobenzidine (Vector Laboratories). Negative control experiments were performed by omitting incubation with the primary antibody.
The quantification of F4/80- and CD3-positive cells in the cortex and outer medulla was carried out in a blind fashion under ×400 magnification. For each section, 15 randomly selected cortical or medullary fields were examined. The number of positive cells was expressed as cells per square millimeter.
NF-κB transcription factor assay.
Nuclear protein was extracted from the kidney with a nuclear extract kit (Active Motif, Carlsbad, CA) based on the manufacturer's instruction. Protein concentrations of nuclear extract were determined with the use of a protein assay kit (Bio-Rad Laboratories, Hercules, CA). Transcription factor NF-κB activity in the kidney was determined with the use of the TransAM NF-κB p65 assay kit (Active Motif) following the manufacturer's protocol. In brief, nuclear proteins were added into each well coated with an unlabeled oligonucleotide containing the consensus binding site for NF-κB (5′-GGGACTTTCC-3′) and incubated for 1 h. After being washed, a primary antibody directed against the NF-κB p65 subunit was added and incubated for 1 h. Subsequently, a secondary antibody conjugated to horseradish peroxidase was applied for 1 h. A colorimetric reaction was developed with addition of a developing solution and terminated by a stop solution. The plate was read at 450 nm by an absorbance microplate reader (Molecular Devices).
Renal cytokine assay.
Immunoreactive TNF-α, IL-6, and MCP-1 were determined in renal homogenates with the use of various ELISA kits (R&D Systems, Minneapolis, MN) instructed by the manufacturers. Whole kidneys were homogenized in ice-cold phosphate buffer solution containing protease inhibitors. The total proteins were extracted using NE-PER Cytoplasmic Extraction Reagents (Pierce, Rockford, IL), following the manufacturer's instruction. The protein concentration of extract was determined using a Bio-Rad Protein Assay (Bio-Rad Laboratories). The cytokine levels in the kidney were normalized and expressed as picograms per milligram total tissue protein.
Western blot analysis.
Kidney was homogenized in homogenization buffer containing protease inhibitors, separated on a 10% SDS-PAGE, and transferred to a polyvinylidene difluoride membrane as described previously (43). Blots were blocked 1 h at room temperature in 5% milk washing solution (50 mmol/l Tris·HCl, 100 mmol/l NaCl, and 0.1% Tween 20 at pH 7.5). Subsequently, blots were incubated overnight at 4°C with goat anti-mouse ICAM-1 polyclonal IgG or goat anti-human VCAM-1 polyclonal IgG (1:500; Santa Cruz Biotechnology, Santa Cruz, CA) in blocking solution, washed, and then incubated for 1 h with horseradish peroxidase-conjugated bovine anti-goat IgG (1:3,000; Santa Cruz Biotechnology). Detection was accomplished with enhanced chemiluminescence Western blot test (ECL, Amersham Biosciences, Piscataway, NJ). Band intensity was densitometrically determined. β-Actin was used to normalize protein loaded on blots.
Statistical analysis.
All values are expressed as means ± SE. The significance of differences between groups for blood pressure data was evaluated with an ANOVA for repeated measures followed by a Bonferroni's test. The differences among groups were analyzed using one-way ANOVA followed by a Bonferroni's adjustment for multiple comparisons. Differences were considered statistically significant at P < 0.05.
RESULTS
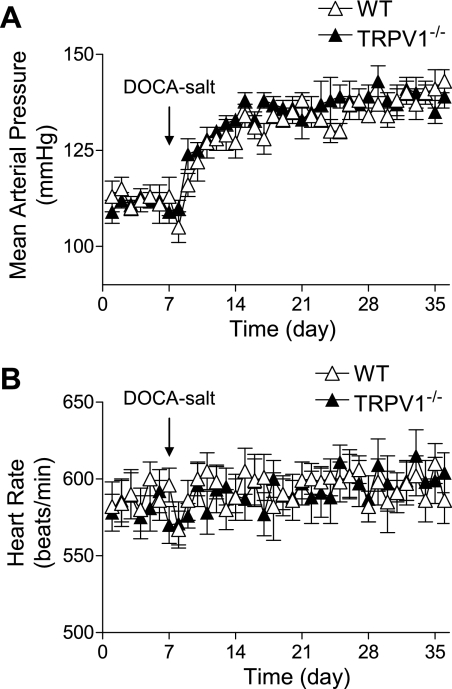
Consistent with the previous report (43), there was no significant difference between WT and TRPV1−/− mice in body weight or plasma levels of electrolytes either at the beginning or end of DOCA-salt treatment with or without DEXA (data not shown). The results of the long-term telemetric recording of MAP are shown in Fig. 1. Baseline MAP was similar in WT and TRPV1−/− mice. One week after initiation of the DOCA-salt protocol, MAP significantly increased compared with the baseline and remained at this level for 3 wk in both WT and TRPV1−/− mice with no difference between two strains (P > 0.05). In addition, DOCA-salt treatment did not affect HR in WT or TRPV1−/− mice.
Fig. 1.
Graphs show the long-term telemetric recording of blood pressure and heart rate in wild-type (WT) and transient receptor potential vanilloid type 1 (TRPV1)-null mutant (TRPV1−/−) mice with and without deoxycorticosterone acetate (DOCA)-salt treatment. Data represent daily average 24-h mean arterial pressure (A) and heart rate (B). Values are means ± SE (n = 6).
DEXA given to DOCA-salt-treated WT and TRPV1−/− mice did not affect MAP compared with DOCA-salt-treated WT and TRPV1−/− mice given vehicle (132 ± 8 vs. 135 ± 6 mmHg; 134 ± 6 vs. 138 ± 7 mmHg, P > 0.05, respectively). Similarly, DEXA treatment had no effect on HR in WT or TRPV1−/− mice (data not shown). Furthermore, there was no change in parameters including MAP, HR, as well as renal function/structure or inflammatory cells/factors between DEXA-treated sham-operated WT and TRPV1−/− mice and vehicle-treated sham-operated WT and TRPV1−/− mice, and thus sham-operated mice with or without DEXA were pooled and treated as control WT and TRPV1−/− mice.
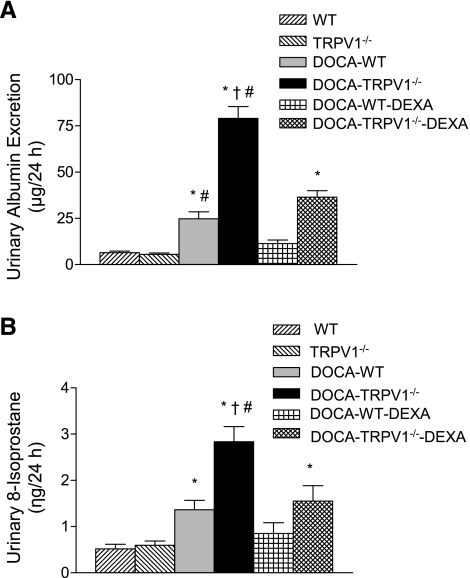
Urinary albumin excretion was used as an indicator of renal damage. As demonstrated in Fig. 2, chronic DOCA-salt treatment significantly increased urinary albumin excretion in WT mice compared with control WT or TRPV1−/− mice (P < 0.05). Deletion of TRPV1 markedly enhanced DOCA-salt-induced urinary albumin excretion compared with WT mice treated with DOCA-salt (79.0 ± 6.5 vs. 24.8 ± 3.7 μg/24 h, P < 0.05). In addition, urinary 8-isoprostane excretion was significantly increased in DOCA-salt-treated WT and TRPV1−/− mice, and the degree of the increase was greater in the latter group (1.36 ± 0.21 vs. 2.84 ± 0.33 ng/24 h, P < 0.05). Moreover, DEXA treatment prevented or reduced urinary albumin and 8-isoprostane excretion in DOCA-salt-treated WT or TRPV1−/− mice (Fig. 2).
Fig. 2.
Bar graphs show the urinary albumin (A) and 8-isoprostane (B) excretion in WT and TRPV1−/− mice, and DOCA-salt-treated WT and TRPV1−/− mice with or without dexamethasone (DEXA) treatment. Values are means ± SE (n = 7 to 8). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with DOCA-salt-treated WT mice. #P < 0.05 compared with DOCA-salt-treated WT or TRPV1−/− mice with DEXA treatment.
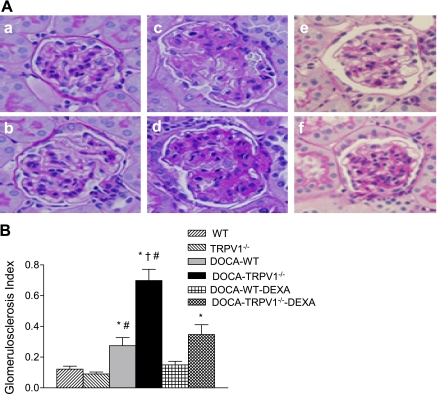
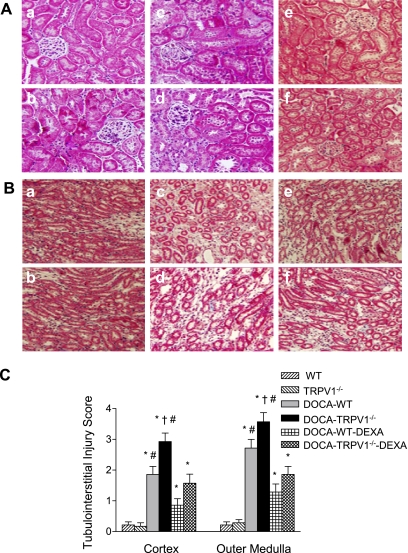
As shown in Fig. 3, more severe glomerulosclerosis was found in DOCA-salt-treated TRPV1−/− mice compared with DOCA-salt-treated WT mice. There was no difference in the glomerulosclerosis index between control WT and TRPV1−/− mice (P > 0.05), but the index was increased by DOCA-salt treatment in both WT and TRPV1−/− mice with a greater magnitude in the latter strain (0.27 ± 0.05 vs. 0.70 ± 0.07, P < 0.05). In addition, DOCA-salt treatment resulted in more severe tubular injury in the renal cortex and outer medulla of TRPV1−/− than WT mice as shown by Mason's trichrome staining (Fig. 4). Quantitative analysis showed that DOCA-salt treatment significantly increased tubular injury of the cortex and outer medulla in TRPV1−/− compared with WT mice (2.93 ± 0.28 vs. 1.86 ± 0.26, 3.57 ± 0.30 vs. 2.71 ± 0.29, P < 0.05). Renal damage was not found in control WT and TRPV1−/− mice. Treatment with DEXA prevented or significantly reduced the renal lesion including glomerulosclerosis (Fig. 3) and tubulointerstitial injury (Fig. 4) in WT and TRPV1−/− mice receiving DOCA-salt treatment with a greater effect in the latter group.
Fig. 3.
Representative light micrographs of kidney sections (A), stained with periodic acid-Schiff technique, from control WT and TRPV1−/− mice (a and b), and DOCA-salt-treated WT and TRPV1−/− mice with (e and f) or without (c and d) DEXA treatment. Bar graph (B) shows the average of glomerulosclerosis index. Values are means ± SE (n = 7 to 8). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with DOCA-salt-treated WT mice. #P < 0.05 compared with DOCA-salt-treated WT or TRPV1−/− mice with DEXA treatment.
Fig. 4.
Representative light micrographs of renal cortex (A) and outer medulla (B) sections, stained with Mason's trichrome technique, from control WT and TRPV1−/− mice (a and b), and DOCA-salt-treated WT and TRPV1−/− mice with (e and f) or without (c and d) DEXA treatment. Bar graph (C) shows the average of tubulointerstitial injury score. Values are means ± SE (n = 7 to 8). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with DOCA-salt-treated WT mice. #P < 0.05 compared with DOCA-salt-treated WT or TRPV1−/− mice with DEXA treatment.
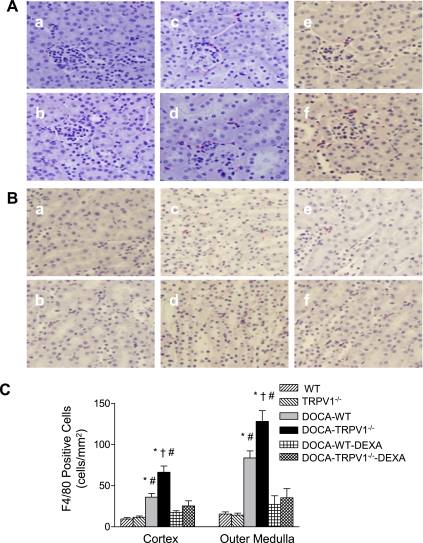
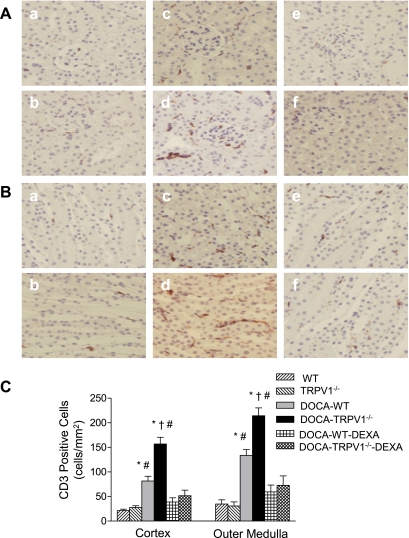
Monocyte/macrophage infiltration was determined and used as an indicator of inflammation of the kidney. The interstitial monocyte/macrophage infiltration was indicated by F4/80-positive cells that stained red. As illustrated in Fig. 5, DOCA-salt-hypertensive WT mice showed a significant increase in F4/80-positive staining in the cortex and outer medulla compared with control WT mice, and the effect of DOCA-salt treatment was enhanced in TRPV1−/− mice (cortex, 36 ± 5 vs. 66 ± 8 cells/mm2; outer medulla, 84 ± 8 vs. 128 ± 13 cells/mm2, P < 0.05). Similarly to the changes in monocyte/macrophage infiltration, DOCA-salt treatment caused a significantly increased infiltration of CD3-positive T cells in WT and TRPV1−/− mice, and the effect was greater in the latter group (Fig. 6). Moreover, the increased infiltration of monocytes/macrophages and T lymphocytes was blocked by DEXA treatment (Figs. 5 and 6).
Fig. 5.
Immunohistochemical staining of F4/80-positive cells (monocytes/macrophages in red) in renal cortex (A) and outer medulla (B) sections from control WT and TRPV1−/− mice (a and b), and DOCA-salt-treated WT and TRPV1−/− mice with (e and f) or without (c and d) DEXA treatment. Bar graph (C) shows the average of F4/80-positive cells expressed as cells per square millimeter in renal cortex and outer medulla. Values are means ± SE (n = 7 to 8). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with DOCA-salt-treated WT mice. #P < 0.05 compared with DOCA-salt-treated WT or TRPV1−/− mice with DEXA treatment.
Fig. 6.
Immunohistochemical staining of CD3-positive cells (T lymphocytes in brown) in renal cortex (A) and outer medulla (B) sections from control WT and TRPV1−/− mice (a and b), and DOCA-salt-treated WT and TRPV1−/− mice with (e and f) or without (c and d) DEXA treatment. Bar graph (C) shows the average of CD3-positive cells expressed as cells per square millimeter in renal cortex and outer medulla. Values are means ± SE (n = 7 to 8). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with DOCA-salt-treated WT mice. #P < 0.05 compared with DOCA-salt-treated WT or TRPV1−/− mice with DEXA treatment.
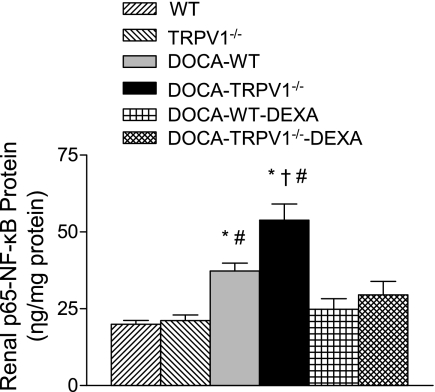
NF-κB activity in the kidneys of WT and TRPV1−/− mice subject to DOCA-salt with or without DEXA treatment was examined. As shown in Fig. 7, DOCA-salt treatment significantly enhanced NF-κB activity in WT mice compared with control WT mice (37.3 ± 2.6 vs. 19.9 ± 1.3 ng/mg protein, P < 0.05). NF-κB activity was further increased in DOCA-salt-treated TRPV1−/− mice compared with DOCA-salt-treated WT mice (53.9 ± 5.3 vs. 37.3 ± 2.6 ng/mg protein, P < 0.05). Moreover, the effect of DOCA-salt treatment on NF-κB activity was abolished by DEXA treatment in WT and TRPV1−/− mice (Fig. 7).
Fig. 7.
Bar graph shows the renal NF-κB activity in WT and TRPV1−/− mice, and DOCA-salt-treated WT and TRPV1−/− mice with or without DEXA treatment. Values are means ± SE (n = 7 to 8). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with DOCA-salt-treated WT mice. #P < 0.05 compared with DOCA-salt-treated WT or TRPV1−/− mice with DEXA treatment.
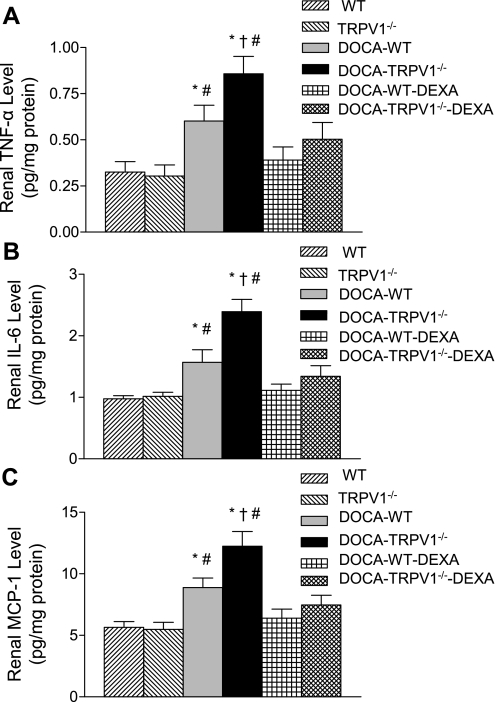
Using the ELISA assay, renal protein levels of proinflammatory cytokines/chemokines including TNF-α, IL-6, and MCP-1 were examined and the results are shown in Fig. 8. Renal TNF-α, IL-6, and MCP-1 levels were significantly increased in DOCA-salt-treated WT mice compared with control WT mice (P < 0.05). We also found that DOCA-salt treatment caused a further elevation of renal TNF-α, IL-6, and MCP-1 levels in TRPV1−/− compared with WT mice (P < 0.05). Moreover, elevated levels of proinflammatory cytokines/chemokines induced by DOCA-salt treatment were prevented by DEXA treatment in WT and TRPV1−/− mice (Fig. 8).
Fig. 8.
Bar graphs show the renal TNF-α (A), IL-6 (B), and MCP-1 (C) in WT and TRPV1−/− mice, and DOCA-salt-treated WT and TRPV1−/− mice with or without DEXA treatment. Values are means ± SE (n = 7 to 8). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with DOCA-salt-treated WT mice. #P < 0.05 compared with DOCA-salt-treated WT or TRPV1−/− mice with DEXA treatment.
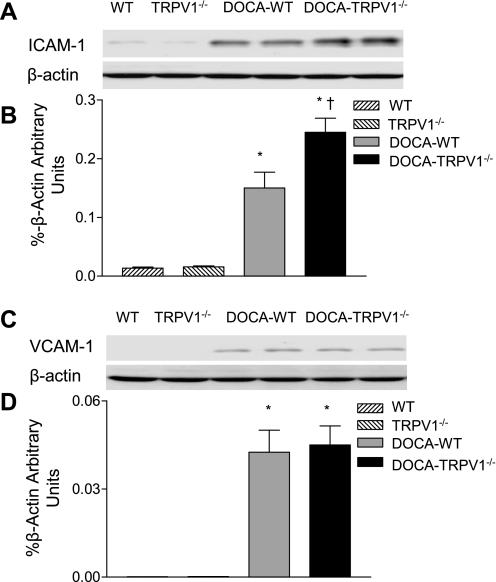
Expression of adhesion molecules including ICAM-1 and VCAM-1 was determined in WT and TRPV1−/− mice with or without DOCA-salt treatment. As demonstrated in Fig. 9, renal ICAM-1 protein expression was significantly increased in DOCA-salt-treated WT mice compared with respective controls, and ablation of TRPV1 further increased ICAM-1 protein expression induced by DOCA-salt treatment. Although DOCA- salt treatment enhanced renal VCAM-1 protein expression in WT and TRPV1−/− mice, there was no difference in VCAM-1 protein expression between the two strains (P > 0.05).
Fig. 9.
Representative Western blot of ICAM-1 (A) and VCAM-1 (C) in kidneys from control WT and TRPV1−/− mice, and WT and TRPV1−/− mice treated with DOCA-salt. Bar graphs show the relative optical density values for ICAM-1 (B) and VCAM-1 (D) in WT and TRPV1−/− mice with and without DOCA-salt treatment. Results were normalized by the corresponding β-actin. Values are means ± SE (n = 4 to 5). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with DOCA-salt-treated WT mice.
DISCUSSION
Our data show that DOCA-salt-induced renal damage, characterized with albuminuria, glomerulosclerosis, and tubulointerstitial injury, is exaggerated in TRPV1−/− mice that have similar blood pressure as WT mice. An enhanced inflammatory response is also evident in TRPV1−/− hypertensive mice induced by DOCA-salt treatment, which includes increases in renal monocyte/macrophage and lymphocyte recruitment, NF-κB activity, proinflammatory cytokine/chemokine production, and adhesion molecule expression. The degree of excessive inflammatory response is congruent with enhanced renal deterioration in DOCA-salt-treated TRPV1−/− mice. Moreover, a conventional anti-inflammatory and immunosuppressive intervention with DEXA treatment markedly ameliorated DOCA-salt-induced renal functional and morphological injury in TRPV1−/− mice without lowering blood pressure. The results provide direct evidence that the increased inflammatory response is one of the major mechanisms contributing to DOCA-salt-induced renal damage in TRPV1−/− mice. These data strongly suggest that activation of TRPV1 may attenuate renal damage via inhibiting renal inflammatory responses without lowering blood pressure.
Hypertension has been shown to be a major cause of renal inflammation and injury (6), and patients with salt-sensitive hypertension have a greater incidence of end-stage renal disease than individuals resistant to salt (38). Although the mechanistic link between salt-sensitive hypertension and inflammation is not fully understood, several plausible mechanisms may explain. Evidence shows that increased blood pressure may promote endothelial expression of cytokines and adhesion molecules, possibly due to increased shear force (11, 47). In addition to hemodynamic factors, renal inflammatory responses may be caused by hormonal or paracrine/autocrine factors including endothelin-1 (ET-1), ANG II, or reactive oxygen species (ROS) overproduced in salt-sensitive hypertension. For example, DOCA-salt hypertension is associated with overexpression of ET-1 in the vasculature and kidney, which contributes to the increase in blood pressure (18, 29). Similarly to ET-1, ANG II levels are increased in the kidney of salt-sensitive hypertensive rats (28). ET-1 and ANG II may stimulate expression of proinflammatory molecules and are considered important mediators of chronic inflammation in hypertension (8, 34). Likewise, increased ROS production in salt-sensitive hypertension may contribute to renal inflammation and damage (5). Oxidative stress has been shown to be linked to the proinflammatory state that includes upregulation of adhesion molecules and chemotactic molecules such as MCP-1 (12, 41). Our data are consistent with these findings, which show that DOCA-salt treatment increases ROS production in the kidney as demonstrated by increased urinary excretion of 8-isoprostane.
Although the mechanisms responsible for increased renal inflammatory responses in DOCA-salt-treated TRPV1−/− mice are not fully understood, several possibilities exist. Glomerular arteriolar constriction induced by hypertension and/or reactive mediators may cause glomerular ischemia that acted as an initiating factor for inflammation during hypertension. Our previous studies showed that activation of TRPV1 increases the glomerular filtration rate (GFR) leading to diuresis and natriuresis in rats (48). Moreover, activation of TRPV1 in the isolated perfused rat kidney decreases renal perfusion pressure and increases GFR (30). The fact that activation of TRPV1 decreases perfusion pressure but increases GFR while perfusion flow is constant indicates that activation of TRPV1 causes a greater vasodilation in afferent than efferent arterioles, leading to an increase in GFR despite a fall in renal perfusion pressure (30). Thus, TRPV1 dysfunction would lead to the loss of compensatory protective role of TRPV1 against the increase in renal vascular resistance, resulting in enhanced glomerular ischemia and more severe inflammation.
Compelling evidence shows that leukocytes including monocytes/macrophages and lymphocytes are the key cell population contributing to the onset or progression of renal disease (10, 15, 17). Tubulointerstitial infiltration of macrophages and T lymphocytes is a common feature of the end-stage renal diseases induced by hypertension. However, the mechanisms involved remain to be defined. Macrophages and T lymphocytes may mediate renal injury via producing proinflammatory cytokines among others or causing matrix accumulation and fibrosis (4, 16, 17). Consistently, our data show that DOCA-salt treatment results in infiltration of F4/80-positive monocytes/macrophages and CD3-positive T lymphocytes in the kidney. Moreover, deletion of TRPV1 enhances the infiltration of these cells induced by DOCA-salt treatment, which are abolished by immunosuppression with DEXA. These data indicate that TRPV1 may protect the kidney during DOCA-salt hypertension via inhibiting monocyte/macrophage and lymphocyte infiltration.
Proinflammatory cytokines play a vital role in the pathogenesis of hypertension and end-stage renal disease caused by hypertension (14, 19). TNF-α, an inflammatory cytokine, is synthesized primarily by monocytes and macrophages. TNF-α levels have been shown to be elevated in end-stage renal diseases in salt-sensitive hypertension (19, 22). Moreover, suppression of TNF-α activity with neutralizing antibodies or receptor blockers attenuates renal damage in several animal models (19, 24). Our data show that DOCA-salt treatment leads to increased levels of renal TNF-α as well as IL-6 in the absence of elevated serum cytokines (data not shown) and that the induction of both TNF-α and IL-6 in the kidney is enhanced when TRPV1 gene is deleted. These data indicate that TRPV1 activation may inhibit the renal production of injurious proinflammatory cytokines including TNF-α and IL-6 to protect against DOCA salt-induced renal damage. It must also be acknowledged that inhibition of other cytokines may occur and contribute to renal protection induced by TRPV1 activation.
Blood leukocyte trafficking to the site of inflammation involves a complex cascade of events progressing from rolling and activation of leukocytes to adhesion and transmigration. Leukocyte infiltration requires orderly expression of cellular adhesion molecules such as ICAM-1 and VCAM-1 (1). In addition, chemokines are critically involved in leukocyte recruitment under inflammation conditions (3). Thus, we examined renal expression of ICAM-1 and VCAM-1 as well as renal MCP-1 levels as a potent chemotactic factor of monocyte/macrophages. Consistent with previous findings showing that adhesion molecules and MCP-1 are increased in kidney of DOCA-salt-hypertensive rats (19), our results show that DOCA-salt treatment increases renal ICAM-1 expression and MCP-1 levels in WT mice, and these increases are further enhanced in TRPV1−/− mice. In contrast, while DOCA-salt treatment increases VCAM-1 expression in the kidney, there is no significant difference in VCAM-1 expression between DOCA-salt-treated WT and TRPV1−/− mice. These data suggest activation of TRPV1 may attenuate monocyte/macrophage infiltration via inhibition of ICAM-1 expression and MCP-1 production.
Inflammatory responses are regulated at the level of transcription by the transcription factor NF-κB (32). Activated NF-κB induces numerous proinflammatory gene expression, including those encoded for cytokines, chemoattractant factors, and cell adhesion molecules, leading to inflammatory responses (32). To determine whether deletion of TRPV1 aggravates renal inflammation through a possible NF-κB-dependent mechanism, renal NF-κB activity was examined. Our data show that renal NF-κB activity is increased in DOCA-salt-treated WT mice, and importantly it is further elevated in the kidneys of DOCA-salt-treated TRPV1−/− mice. These data suggest that inhibition of NF-κB activity may mediate TRPV1 action of anti-inflammation and tissue protection.
To further elucidate the role of inflammatory responses in DOCA-salt-induced renal injury when TRPV1 is genetically deleted, the effect of DEXA was examined. DEXA, a potent synthetic form of glucocorticoids with its known anti-inflammatory properties, activates the cytoplasmic glucocorticoid α-receptor that subsequently binds to activated NF-κB and prevents the transcription factor from binding to κB sites on the genes important for inflammation. Treatment of hypertensive models with DEXA has been shown to be an effective way to reduce renal immune cell infiltration and to ameliorate renal injury. For example, DEXA attenuates renal immunocompetent cell infiltration and reduces renal damage in double-transgenic rats harboring both human renin and angiotensinogen genes (35). Consistently, our data show that treatment of TRPV1−/− mice with DEXA markedly decreases DOCA-salt-induced renal damage and that the renal protective effect by DEXA is accompanied with the striking decrease in inflammatory responses. The data indicate that exacerbated renal damage seen in DOCA-salt-treated TRPV1−/− mice is attributed to enhanced inflammatory responses due to the loss of the beneficial anti-inflammatory protection of TRPV1.
Given the complexity of inflammatory responses, mechanisms underlying TRPV1 anti-inflammatory effects may involve pathways beyond suppression of NF-κB activity. TRPV1 is known to be mainly expressed in primary sensory nerves. Pretreatment with capsaicin, a selective TRPV1 agonist, in a rodent model of allergic inflammation leads to increased TNF-α levels, suggesting that loss of TRPV1-positive sensory nerves enhances TNF-α production (21). In addition, further increased cytokine levels are found in peritoneal lavage fluid of TRPV1−/− mice treated with endotoxin, supporting the notion that TRPV1 protects against acute immune response in sepsis (13). We previously showed that TRPV1 is activated by high-salt intake, leading to release of neurotransmitters including CGRP (45). In addition to its well-recognized cardiovascular effects, CGRP has been shown to modulate antigen presentation, phagocytosis, and production of cytokines induced by endotoxin (2, 25, 33). Indeed, inflammatory responses are worsened in CGRP-null mutant mice (7). Further studies are required to clarify the mechanisms responsible for anti-inflammatory action of TRPV1.
In summary, our results show that DOCA-salt treatment causes enhanced renal inflammation, more severe deterioration in renal function, and exaggerated renal tissue damage when TRPV1 gene is deleted. Suppression of inflammatory responses with DEXA reduces renal functional and morphological damage in DOCA-salt-treated TRPV1−/− mice. Taken together, these findings suggest that the enhanced inflammatory responses may be one of the key mechanisms contributing to renal damage induced by DOCA-salt treatment in TRPV1−/− mice.
Perspectives
Patients with salt-sensitive hypertension are much more likely to experience end-stage renal disease (38). However, mechanisms responsible for the progression of end-stage renal disease in salt-sensitive hypertension are ill defined. Impairment in renal sensory nerves and TRPV1 function occurs in Dahl salt-sensitive rats, leading to decreased GFR and renal excretory function in this strain in the face of salt challenge (31, 45). The data in the present study using the DOCA-salt-hypertensive model provide additional evidence indicating that TRPV1-mediated protection may serve as a general protecting mechanism in glomerular and tubular injury associated with inflammation and hypertension. These findings suggest that activation of TRPV1 may be a potential therapeutic strategy for the prevention of renal inflammation and end-stage organ damage in salt-sensitive hypertension.
GRANTS
This work was supported in part by National Institutes of Health Grants HL-57853, HL-73287, and DK-67620 and a grant from Michigan Economic Development.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J 8: 504–512, 1994 [PubMed] [Google Scholar]
- 2.Asahina A, Moro O, Hosoi J, Lerner EA, Xu S, Takashima A, Granstein RD. Specific induction of cAMP in Langerhans cells by calcitonin gene-related peptide: relevance to functional effects. Proc Natl Acad Sci USA 92: 8323–8327, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol 15: 675–705, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest 101: 364–371, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory responase. Hypertension 37: 781–786, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 44: 595–601, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bowers MC, Katki KA, Rao A, Koehler M, Patel P, Spiekerman A, DiPette DJ, Supowit SC. Role of calcitonin gene-related peptide in hypertension-induced renal damage. Hypertension 46: 51–57, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Callera GE, Montezano AC, Touyz RM, Zorn TM, Carvalho MH, Fortes ZB, Nigro D, Schiffrin EL, Tostes RC. ETA receptor mediates altered leukocyte-endothelial cell interaction and adhesion molecules expression in DOCA-salt rats. Hypertension 43: 872–879, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Cattell V. Macrophages in acute glomerular inflammation. Kidney Int 45: 945–952, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Cheng JJ, Wung BS, Chao YJ, Wang DL. Cyclic strain enhances adhesion of monocytes to endothelial cells by increasing intercellular adhesion molecule-1 expression. Hypertension 28: 386–391, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, Kunsch C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol 290: H1862–H1870, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Clark N, Keeble J, Fernandes ES, Starr A, Liang L, Sugden D, de Winter P, Brain SD. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J 21: 3747–3755, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O'Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol 171: 315–325, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 295: F515–F524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eardley KS, Zehnder D, Quinkler M, Lepenies J, Bates RL, Savage CO, Howie AJ, Adu D, Cockwell P. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int 69: 1189–1197, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Eddy AA. Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol 5: 1273–1287, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Elmarakby AA, Morsing P, Pollock JS, Pollock DM. Omapatrilat increases renal endothelin in deoxycorticosterone acetate-salt hypertensive rats. Vascul Pharmacol 40: 253–259, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD. Chemokine receptor 2b inhibition provides renal protection in angiotensin II-salt hypertension. Hypertension 50: 1069–1076, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franco-Penteado CF, De Souza IA, Camargo EA, Teixeira SA, Muscara MN, De Nucci G, Antunes E. Mechanisms involved in the enhancement of allergic airways neutrophil influx by permanent C-fiber degeneration in rats. J Pharmacol Exp Ther 313: 440–448, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Gu JW, Tian N, Shparago M, Tan W, Bailey AP, Manning RD., Jr Renal NF-κB activation and TNF-α upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 291: R1817–R1824, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Helyes Z, Elekes K, Németh J, Pozsgai G, Sándor K, Kereskai L, Börzsei R, Pintér E, Szabó A, Szolcsányi J. Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol 292: L1173–L1181, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Hruby ZW, Shirota K, Jothy S, Lowry RP. Antiserum against tumor necrosis factor-α and a protease inhibitor reduce immune glomerular injury. Kidney Int 40: 43–51, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Ichinose M, Sawada M. Enhancement of phagocytosis by calcitonin gene-related peptide (CGRP) in cultured mouse peritoneal macrophages. Peptides 17: 1405–1414, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Jancsó N, Jancsó-Gábor A, Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother 31: 138–151, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 413: 203–210, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592–597, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larivière R, Thibault G, Schiffrin EL. Increased endothelin-1 content in blood vessels of deoxycorticosterone acetate-salt hypertensive but not in spontaneously hypertensive rats. Hypertension 21: 294–300, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Li JP, Wang DH. Increased GFR and renal excretory function by activation of TRPV1 in the isolated perfused kidney. Pharmacol Res 57: 239–246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li JP, Wang DH. Role of TRPV1 channels in renal haemodynamics and function in Dahl salt-sensitive hypertensive rats. Exp Physiol 93.8: 945–953, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290: L622–L645, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Monneret G, Pachot A, Laroche B, Picollet J, Bienvenu J. Procalcitonin and calcitonin gene-related peptide decrease LPS-induced TNF production by human circulating blood cells. Cytokine 12: 762–764, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-κB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension 35: 193–201, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chávez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 282: F191–F201, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Rostand SG, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for end stage renal disease. N Engl J Med 306: 1276–1279, 1982 [DOI] [PubMed] [Google Scholar]
- 39.Saito T, Sumithran E, Glasgow EF, Atkins RC. The enhancement of aminonucleoside nephrosis by the coadministration of protamine. Kidney Int 32: 691–699, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Shih W, Hines WH, Neilson EG. Effects of cyclosporine A on the development of immune-mediated interstitial nephritis. Kidney Int 33: 1113–1118, 1988 [DOI] [PubMed] [Google Scholar]
- 41.Tanaka M, Mokhtari GK, Terry RD, Balsam LB, Lee KH, Kofidis T, Tsao PS, Robbins RC. Overexpression of human copper/zinc superoxide dismutase (SOD1) suppresses ischemia-reperfusion injury and subsequent development of graft coronary artery disease in murine cardiac grafts. Circulation 110: II200–II206, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD., Jr Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 292: H1018–H1025, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Babánková D, Huang J, Swain GM, Wang DH. Deletion of TRPV1 receptors exaggerates renal damage in DOCA-salt hypertension. Hypertension 52: 264–270, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Wang DH. Prevention of endothelin-1-induced increases in blood pressure: role of endogenous CGRP. Am J Physiol Heart Circ Physiol 287: H1868–H1874, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension 47: 609–614, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev 17: 533–585, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Wung BS, Cheng JJ, Chao YJ, Lin J, Shyy YJ, Wang DL. Cyclical strain increases monocyte chemotactic protein-1 secretion in human endothelial cells. Am J Physiol Heart Circ Physiol 270: H1462–H1468, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Zhu Y, Wang DH. Segmental regulation of sodium and water excretion by TRPV1 activation in the kidney. J Cardiovasc Pharmacol 51: 437–442, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, DiMarzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457, 1999 [DOI] [PubMed] [Google Scholar]