Abstract
Chronic inflammation and increased oxidative stress (OS) play an important role in diabetic nephropathy progression. Herein, we show that mesangial cells from streptozotocin-induced aging diabetic mice, a model of progressive diabetic nephropathy, exhibited increased OS and a proinflammatory phenotype characterized by elevated chemokines and ICAM-1 expression. This phenotypic change was consistent with the extensive inflammatory lesions present in aging diabetic kidneys and was not found in mesangial cells from old and young controls or young diabetic mice. Activation of the c-Jun NH2-terminal kinase (JNK) pathway was a likely contributor to the proinflammatory phenotype of aging diabetic mesangial cells since 1) phosphorylated JNK levels and JNK kinase activity were increased in these cells, 2) suppression of JNK significantly decreased monocyte chemoattractant protein-1 (MCP-1) production in these cells, and 3) activation of JNK in normal mesangial cells induced inflammation. Elevated OS in aging diabetic mesangial cells may be a cause of JNK activation and inflammation, because antioxidant treatment decreased JNK phosphorylation and MCP-1 production. Additionally, decreased expression of mitogen-activated protein kinase phosphatase 5 (MKP5) may also contribute to increased JNK and inflammation in aging diabetic mesangial cells since overexpression of MKP5 in these cells normalized phosphorylated JNK levels and reversed the proinflammatory phenotype. Moreover, knocking down of MKP5 expression in old control mesangial cells resulted in JNK activation and MCP-1 production, a phenotype seen in aging diabetic mesangial cells. Interestingly, MKP5 phosphatase activity was diminished by free radicals in vitro. Thus, OS may induce inflammation in mesangial cells by activating JNK through either a direct activation of JNK or indirectly by suppression of MKP5 activity. Proinflammatory phenotype of mesangial cells may contribute to chronic inflammatory lesions and disease progression of aging diabetic mice.
Keywords: diabetic nephropathy, mice
it has been shown that phenotypic changes occur in mesenchymal cells (13). The emphasis, however, has been on the acquisition of a profibrotic phenotype. Parenchymal cells are also actively involved in the early phases of inflammatory responses. It is unclear whether these cells could acquire a proinflammatory phenotype to prolong inflammation in diseases such as diabetic nephropathy.
The gradual expansion of extracellular matrix and chronic inflammation underlies the progression of diabetic nephropathy. A phenotypic switch of renal parenchymal cells such as mesangial cells, podocytes, and proximal tubular cells has been shown to play an important role in extracellular matrix accumulation (12, 21, 24, 33, 39). For instance, there is both in vivo and in vitro evidence that during the progression of diabetic nephropathy, some of the proximal tubular cells transdifferentiate into phenotype of mesenchymal cells that produce excessive interstitial matrix (22, 38). Epithelial-to-mesenchymal transdifferentiation is also reported in podocytes (23). Mesangial cells are major source of extracellular matrix in glomeruli (21, 24, 41, 46). Hyperglycemia, advanced glycation end products, and transforming growth factor-β (TGF-β), three major factors implicated in diabetic nephropathy, all stimulate extracellular matrix production by mesangial cells (1, 11, 36, 38, 52). A stable profibrotic phenotype was consistently observed in mesangial cell isolated from both diabetic mice and patients, suggesting that a stable phenotypic change had occurred in vivo (26, 27).
Chronic inflammation is another major contributor to progression in diabetic nephropathy (31). The extent of immune cells infiltration, especially macrophages, is directly correlated with the severity of diabetic nephropathy (6, 47). However, endothelial cells, mesangial cells, and proximal tubular cells are also actively involved in the process of chronic inflammation (29, 30, 34). In response to hyperglycemia, advanced glycation end products, and cytokines and growth factors, these intrinsic cells could initiate the recruitment of immune cells or facilitate inflammation by upregulating the expression of chemokines, adhesion molecules, and survival and growth factors (9, 32, 35). Additionally, intrinsic kidney cells could interact with immune cells to initiate a vicious cycle that could lead to a persistent inflammation (5). In latter case, a phenotypic switch of parenchymal cell from being a passive responder to an active player in inflammation may have occurred. The current report shows that mesangial cells from a model of progressive diabetic nephropathy exhibit a stable proinflammatory phenotype, partly due to excessive oxidative stress. The activation of c-Jun NH2-terminal kinase (JNK) by oxidative stress may contribute to the proinflammatory phenotype of diabetic mesangial cells.
MATERIALS AND METHODS
Animals.
Female C57B6 mice (4 and 16–17 mo of age), obtained from National Institute on Aging, were injected with streptozotocin (50 μg/g) to induce diabetes (2, 51). Mice with stable hyperglycemia (glycemia ≥250 mg/dl) at 5 mo (young) or 18 mo (old) of age were selected for study. The 18-mo-old mice were chosen because female C57B6 mice begin to have irregular, lengthened estrous cycles at ∼10 to 14 mo of age and the cycles usually cease at 18 mo of age (50). Both young and old diabetic mice were followed for 4 mo without insulin injections. At death, glomeruli were microdissected from each group of mice under a microscope at 4°C as previously described for the purpose of RNA isolation and mesangial cell culture (50). The use and all the procedures done to animals had been approved by the Institutional Animal Care and Use Committee, Mount Sinai School of Medicine.
Mesangial cell culture.
Glomeruli were seeded into fibronectin-coated wells and incubated with DMEM/F12 (3:1) medium containing 20% FBS at 37°C, 5% CO2. The outgrowing mesangial cells were characterized and propagated as previously described (10). Cells at passage 4 to 11 were used for the study. At least two lines of mesangial cells from two animals of control and diabetics were obtained.
Western blot.
Renal cortex and mesangial cells were homogenized in a lysis buffer containing protease and phosphatase inhibitors (Pierce, Rockford, IL). Ten to fifty micrograms of protein were loaded onto 10% SDS-PAGE gels. After separation, proteins were transferred to PVDF membranes. For Western blot, membrane was pretreated with a blocking buffer (Thermo Fisher Scientific, Waltham, MA) at room temperature for 30 min before incubating with the following antibodies: anti-p-IκB, anti-p-p38, and anti-p-JNK, all at 1:1,000 dilutions from Cell Signaling (Natick, MA). After overnight incubation with first antibody at 4°C, membranes were washed and incubated with a horseradish peroxidase-labeled secondary antibody and immunoreactivity was detected using the enhanced chemiluminescence assay. Membranes were washed 30 min with a stripping buffer (Thermo Fisher Scientific) before being reprobed with an anti-total-IκB, anti-total-p38, or anti-total-JNK antibody. At the end, membranes were also probed with an anti-β-actin antibody (1:5,000; Sigma, St. Louis, MO) to ensure a similar amount of protein loading.
JNK kinase assay.
Two hundred micrograms of fresh cell lysate were immunoprecipitated with immobilized c-Jun fusion protein beads (Cell Signaling) overnight at 4°C. Protein beads were collected and washed afterward and incubated with ATP in a kinase buffer at 30°C for 30 min. The reaction was terminated by adding SDS loading buffer. After being denatured, reaction samples were loaded on a 10% SDS-PAGE gel and detected by Western blots using an antibody against phosphrylated c-Jun. Nonspecific controls included immunoprecipitation in the absence of protein lysate and Western blots with an irrelevant antibody.
Oxyblot.
The oxyblot protein oxidation detection kit (Chemicon International, Temecula, CA) was used for detection of overall carbonyl groups introduced into protein side chains by oxidative modification. 2,4-Dinitrophenylhydrazine (DNPH) derivatization was carried out for 15 min, following the manufacturer's instruction, on 10 μg of protein obtained from the kidney tissue and mesangial cell lysates. The DNP-derivatized protein samples were separated by 12% SDS-PAGE. Proteins were transferred to PVDF membranes, stained by Ponceau red, and then probed with an anti-dinitrophenylhydrazine antibody. Blots were developed using a chemiluminescence detection system.
Intracellular H2O2.
Mesangial cells (1×105) of old control and old diabetic were seeded in each well of six-well plates with DMEM/F12 (3:1) medium containing 20% FBS. The next morning, cells were washed with prewarmed Hank's buffer twice and incubated with 15 μM 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) at 37°C for 45 min. Fluorescence was visualized either directly under a fluorescence microscope or measured by a fluorometer, with excitation at 485 nm and emission at 530 nm.
mRNA levels.
Total RNA was isolated from glomeruli and mesangial cells using a PureYield RNA Midiprep kit (Promega, Madison, WI). Total RNA was reverse transcribed as previously described (50, 51). The levels of monocyte chemoattractant protein-1 (MCP-1), c-x-c motif ligand 1 (CXCL1), macrophage inflammatory protein 2 (MIP2), regulated on activation, normal T cell expressed and secreted (RANTES), and intracellular adhesion molecule-1 (ICAM-1) mRNA were determined by real-time PCR. The primer sequences were MCP-1, forward, 5′-AATTACCAGCAGCAAGTGTCC; reverse, 5′-GGGTCTGCACAGATCTCCTT; RANTES, forward, 5′-TTCCCTGTCATCGCTTGCTCT; reverse, 5′-CGGATGGAGATGCCGATTTT; CXCL1, forward, 5′-CTTGAAGGTGTTGCCCTCAG; reverse, 5′-AAGGGAGCTTCAGGGTCAAG; MIP2, forward, 5′-TCCAGAGCTTGAGTGTGACG; reverse, 5′-TTCAGGGTCAAGGCAAACTT; ICAM-1, forward, 5′-TGCTGCAGATGCTGTGAGAGT; reverse, 5′-AAACCCTCGACCCATGTGATC. mRNA levels were corrected by the levels of β-actin or GAPDH mRNA. The expression of mitogen-activated protein kinase phosphatase 5 (MKP5) mRNA was also determined by real-time PCR using the primer of forward, 5′-GCGACGTGGAACTGGCAGAAG; reverse, 5′-GGTACAACCCATCGGCTGGCA.
MCP-1 and RANTES.
Young and old control and young and old diabetic mesangial cells were seeded in a 24-well plate at 1 × 105 cells/well. After conditioning cells with DMEM/F12 (3:1) medium containing 0.1% FBS at 37°C for 24 h, some cells were treated with TNF-α (10 ng/ml), a JNK inhibitor SP600125 (5–20 μM; Calbiochem, San Diego, CA), 2,4-dinitro-1-chlorobenzene (DNCB; 10 μM), or N-acetyl-cysteine (NAC; 10 mM). Supernatants were collected 24 h later. MCP-1 and RANTES were measured in 1:2 or 1:4 diluted supernatants using ELISA kits from Invitrogen (Carlsbad, CA). To exclude the possibility that the effect of JNK inhibitor or NAC on MCP-1 production was simply caused by their cell toxicity, cell viability was assessed by a 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MTT at the final concentration of 250 μg/ml was added to the cells 24 h after SP600125 or NAC treatment. After further incubating cells at 37°C for 3 h, the culture medium was removed and the formazan MTT product was solubilized with dimethyl sulfoxide. The intensity of color was read with a spectrophotometer at the wavelength of 590 nm.
MKP5 stable transfection.
Old diabetic mesangial cells were transfected with a pCMV6 entry plasmid containing a full-length human MKP5 cDNA and a G418 resistant site (Origene, Rockville, MD). Stable transfected cells were obtained after selecting with G418 (1,000 μg/ml) for at least three passages. Three clones with stable MKP5 overexpression, at least threefold, were obtained and used for further experiments, including the measurement of JNK phosphorylation, chemokines, and ICAM-1 mRNA.
MKP5 siRNA.
Mouse MKP5 siRNA was purchased from Ambion (Austin, TX). RNA oligonucleotides (21 nucleotides) sequences were as follows: forward, 5′-GGC CUU UCA UGG AGU ACA ATT-3′; reverse, 5′-UUGUACUCCAUGAAAGGCCTG-3′. Cells were transfected with siRNA using Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer's protocol with slight modifications. Briefly, old control mesangial cells were seeded at 1 × 105 cells per well in six-well plates. The next day, cells were transfected with MKP5 siRNA oligonucleotides or nontarget control oligonucleotides using Lipofectamine RNAiMAX. The final concentration of siRNA in the culture medium was 25 nM. After 48 h, RNA and protein of transfected cells and supernatant were harvested to examine the MKP5 expression, the levels of phosphorylated JNK and JNK kinase activity, and MCP-1 secretion, respectively.
MKP5 phosphatase assay.
One microgram of enzymatic active recombinant human MKP5 (Biomol International, Plymouth Meeting, PA) was incubated with or without H2O2 (5 mM) in the presence or absence of 5 mM dithiothreitol (DTT) in a reaction buffer containing 100 mM Tris·HCl, pH 8.2, 40 mM NaCl, and 20% glycerol at 37°C in the dark for 30 min. Phosphatase activity was measured by adding the sample together with a substrate 3-O-methylfluorescein (500 μM; Sigma) to a 96-well plate and incubated at 30°C for 30 min. Absorbance was read at 477 nM. Negative controls included wells containing only assay buffer, DTT, H2O2, and substrate.
Statistical analysis.
Values were expressed as means ± SD. ANOVA or two-tailed unpaired t-test was used to evaluate the differences between the means. Significance was defined as P < 0.05.
RESULTS
Increased expression of proinflammatory genes in glomeruli and mesangial cells from old diabetic mice in vitro.
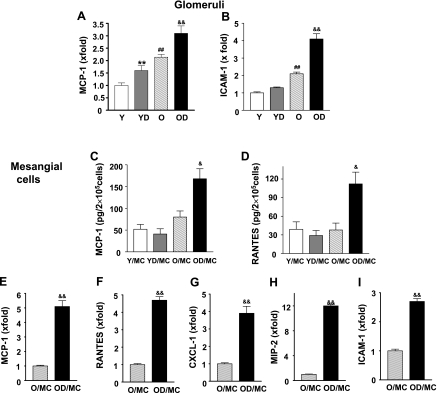
We previously showed that young C57B6 mice were resistant to diabetic nephropathy (50, 51). We found again that both albuminuria and renal lesions were relatively mild in young diabetic mice (data not shown). Glycemic levels were higher in young diabetic (397 ± 42 mg/dl, n = 7) than in old diabetic mice (301 ± 36 mg/dl, n = 11). However, aging diabetic C57B6 mice developed much more severe nephropathy and that chronic inflammation was an important contributor to disease progression. Glomerular MCP-1 mRNA levels were moderately elevated in young diabetic and old control mice and were further elevated in old diabetic mice (Fig. 1A). Moreover, glomeruli from old control mice also had higher ICAM-1 mRNA levels, and the levels were further increased in old diabetic mice (Fig. 1B). Mesangial cells isolated from old control mice showed a trend of increased MCP-1 production comparing to mesangial cells isolated from young mice (P = 0.06; Fig. 1C). A prominent proinflammatory phenotype was present in mesangial cells isolated from old diabetic mice. Both MCP-1 and RANTES production were significantly increased in old diabetic mesangial cells (Fig. 1, C and D). As shown in Fig. 1, D-I, old diabetic mesangial cells exhibited a 5-fold, 4.7-fold, 3.8-fold, 11.8-fold, and 2.6-fold, respectively, increase in MCP-1, RANTES, CXCL-1, MIP-2, and ICAM-1 mRNA expression compared with old control mesangial cells. Since no obvious increase in MCP-1 and RANTES production was found in young diabetic mesangial cells (Fig. 1, C and D, and data not shown), our study was focused on old control and old diabetic mesangial cells hereafter.
Fig. 1.
Increased proinflammatory gene expression in aging diabetic glomeruli and mesangial cells. Glomeruli were microdissected from young control (Y; n = 6), young diabetic (YD; n = 7), old control (O; n = 10), and old diabetic mice (OD; n = 10). Glomerular monocyte chemoattractant protein-1 (MCP-1; A) and intracellular adhesion molecule-1 (ICAM-1; B) mRNA levels were measured by real-time PCR and corrected for β-actin mRNA. The levels in glomeruli of young control were arbitrarily defined as 1. **P < 0.01 vs. Y. ##P < 0.01 vs. Y. &&P < 0.01 vs. O. C: MCP-1 production by mesangial cells from young control (Y/MC), young diabetic (YD/MC), old control (O/MC), and old diabetic mice (OD/MC). Mesangial cells in a 24-well plate (5 × 104/well) were placed in a serum-free condition for 24 h. Supernatants were collected from these cells for measurement of MCP-1 (n = 2 cell lines/group). Results were the average of 3 experiments with the same cell lines. &P < 0.05 vs. O/MC. D: RANTES production by mesangial cells (n = 2 cell lines/group). Results were the average of 3 experiments with the same cell lines. &P < 0.05 vs. O/MC. E–I: total RNA was extracted from mesangial cells of O and OD mice. The expression levels of MCP-1, RANTES, c-x-c motif ligand 1 (CXCL-1), macrophage inflammatory protein 2 (MIP-2), and ICAM-1 mRNA were determined by real-time PCR and corrected for β-actin mRNA levels. The levels in O/MC were arbitrarily defined as 1. Results were the average of 3 experiments with the same cell lines. &&P < 0.01 vs. O/MC.
Increased JNK phosphorylation in old diabetic mesangial cells.
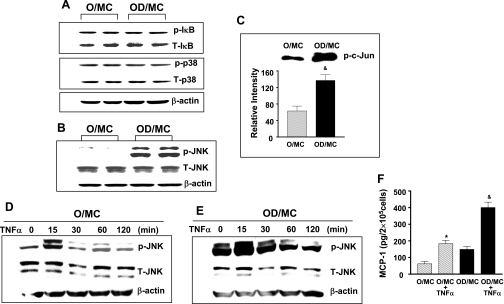
Since NF-κB and MAPK are two common pathways that cause inflammation (3, 19), we examined the levels of IκB, p38, and JNK phosphorylation in two separate mesangial cell lines from both old control and old diabetic mice. At baseline, the levels of IκB and p38 phosphorylation were similar in mesangial cells of old control and old diabetic mice (Fig. 2A). However, the levels of JNK phosphorylation were substantially elevated in mesangial cells from old diabetic mice (Fig. 2B). Kinase assay clearly demonstrated that increased JNK phosphorylation in mesangial cells from old diabetic mice was associated with increased JNK kinase activity (Fig. 2C).
Fig. 2.
Elevated phosphorylated JNK levels and JNK activity in aging diabetic mesangial cells. Two separate isolates of mesangial cells from O and OD were placed in a serum-free condition for 24 h before protein collection. A: levels of phosphorylated IκB, total IκB, phosphorylated p38, total p38, and β-actin were measured by Western blots in a same membrane probed consecutively with respective antibody. Representative gels from 3 independent measurements of 2 separate cell lines show that the levels of phosphorylated IκB and p38 were similar between O/MC and OD/MC. B: levels of phosphorylated JNK were visibly increased in OD/MC. C: kinase assay demonstrated that increased phosphorylated JNK in OD/MC was associated with increased JNK activity. Representative gel of kinase assay of O/MC and OD/MC was shown on the top. The lower bars were the relative density of bands from the measurement of 2 mesangial cell lines from each group (n = 2). &P < 0.05 vs. O/MC. D and E: serum-starved old control and diabetic mesangial cells were treated with TNF-α (10 ng/ml). Proteins were collected from cells at different time points after treatment. JNK phosphorylation was visibly increased in both O/MC and OD/MC 15 min after TNF-α stimulation. F: TNF-α (10 ng/ml) induced a significant increase in MCP-1 production in both O/MC and OD/MC (n = 2/group). *P < 0.05 vs. O/MC. &P < 0.05 vs. OD/MC.
Mesangial cells from old control and old diabetic mice were treated with TNF-α to determine whether the observed phenotypic switch in old diabetic mesangial cells affected cell response(s) to inflammatory stimuli. TNF-α induced a rapid increase in JNK, IκB, and p38 phosphorylation in old control mesangial cells (Fig. 2D, and data not shown). JNK, IκB, and p38 phosphorylation were also induced at the same time frame and to a similar extent in old diabetic mesangial cells (Fig. 2E, and data not shown). The addition of TNF-α caused an increase in MCP-1 production in mesangial cells from old control mice to levels paralleling that in old diabetic mesangial cells, but led to a further increase in mesangial cells from old diabetic mice (Fig. 2F).
JNK activation and MCP-1 production in mesangial cells.
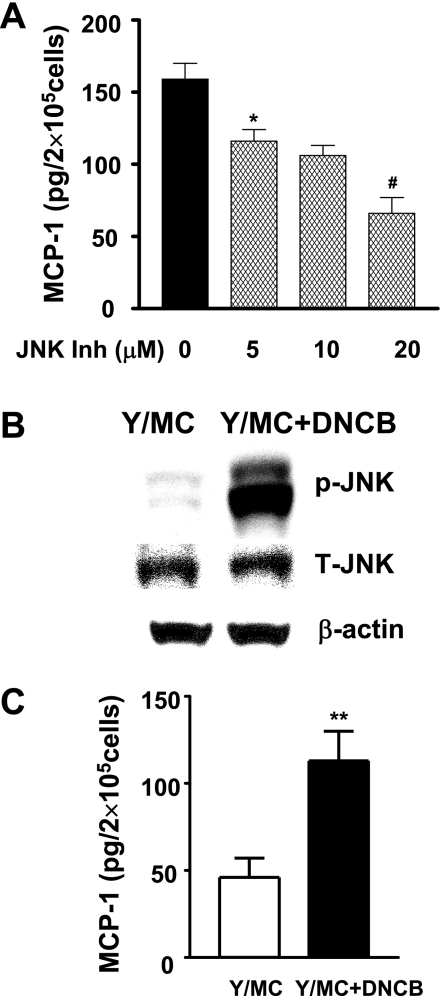
As JNK activation is actively involved in various proinflammatory responses, it might contribute to the proinflammatory phenotype of old diabetic mesangial cells (45). Indeed, SP600125, a chemical JNK inhibitor, dose dependently decreased MCP-1 production by old diabetic mesangial cells (Fig. 3A). The effect of SP600125 was unlikely caused by its direct toxicity since more than 91% of cells were alive 24 h after treating with the highest dosage of drug (20 μM) in the experiments. JNK was activated by DNCB in young mesangial cells (Fig. 3B), which was associated with a 2.2-fold increase in MCP-1 production (Fig. 3C). Similar changes were noted in mesangial cells from old mice treated with DNCB (data not shown).
Fig. 3.
JNK and mesangial cell MCP-1 production. A: OD/MC, which had elevated JNK and inflammatory phenotype, were treated with JNK inhibitor (SP600125). MCP-1 production was dose dependently decreased by JNK inhibitor. *P < 0.05 vs. untreated control. #P < 0.05 vs. cells treated with 10 μM inhibitor. Results were the average of 3 experiments with the same cell lines (n = 2). B: 2,4-dinitro-1-chlorobenzene (DNCB; 10 μM) induced JNK phosphorylation in mesangial cells from Y. C: DNCB increased MCP-1 production in Y/MC. **P < 0.01 vs. untreated cells.
Increased oxidative stress in old diabetic kidneys and their mesangial cells in vitro.
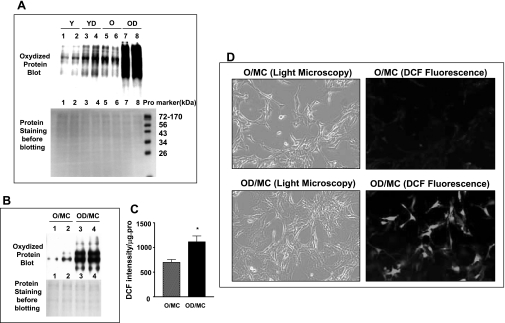
Since oxidative stress is one of the major causes of JNK activation (28, 43, 49), we examined the levels of oxidative stress in old diabetic kidneys and mesangial cells. The levels of oxidized proteins were increased in both old control and young diabetic kidneys (Fig. 4A). The extent of increase was comparable between the old controls and young diabetes. A more prominent increase in oxidized protein levels was present in old diabetic kidneys (Fig. 4A). Additionally, two mesangial cell lines isolated from old diabetic mice also exhibited elevated oxidized protein levels, compared with mesangial cells isolated from old control mice (Fig. 4B). Direct measurement of cellular H2O2 levels with DCFDA showed that diabetic mesangial cells had elevated oxidant levels, namely the increased DCF intensity (Fig. 4C). Examination of cells under a fluorescence microscope provided a direct picture of increased DCF intensity in old diabetic mesangial cells and revealed that, while most mesangial cells were strongly stained, there was some heterogeneity (Fig. 4D).
Fig. 4.
Increased oxidative stress in old diabetic kidneys and mesangial cells. A: levels of protein oxidation in the kidneys of Y (9 mo old, n = 5), YD (n = 7), O (22 mo old, n = 5), and OD (22 mo old, n = 8) mice were determined by Western blot using a specific anti-oxidized protein antibody. Representative gels of 2 animals from each group showed that, while the bands of oxidized protein are visible in samples of the kidneys from young mice (top gel, Y, lane 1, 2), stronger bands with a similar pattern of proteins were seen in kidney samples from YD and O mice (top gel, YD, lane 3, 4; O, lane 5, 6). Protein oxidation was markedly increased in kidneys from OD mice (top gel, OD, lane 7, 8). A, bottom: same gel stained with Ponceau red before Western blotting. Note that the regular protein markers shown on the bottom gel were not detected by the anti-oxidized protein antibody and therefore cannot be seen in top gel. B: oxidized protein levels were measured in 2 isolates of mesangial cells from O (lane 1, 2) and OD (lane 3, 4). Bottom gel was the staining of Ponceau red before Western blotting with the anti-oxidized protein antibody. C: H2O2 levels in O/MC and OD/MC were determined by DCF fluorescence intensity. *P < 0.05 vs. O/MC. Data were obtained from the measurements of 2 cell lines each of OD/MC and O/MC (n = 2). The experiment was repeated 3 times. D: DCF intensity was directly observed under a fluorescence microscopy. Left: image of old control and diabetic mesangial cells under light microscopy (×40). Right: same field of mesangial cells under fluorescence microscopy. An obvious increase in DCF fluorescence was shown in mesangial cells of old diabetic mice.
Oxidative stress, JNK activation, and MCP-1 production in mesangial cells.
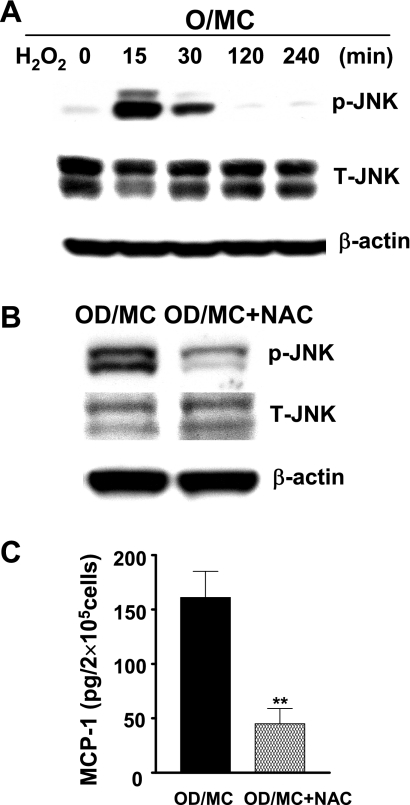
Mesangial cells from old normal mice, which had a relatively low baseline oxidant level and JNK activity compared with the cells from old diabetic mice, were treated with H2O2 (1 mM). Phosphorylated JNK levels were increased 15 min after H2O2 stimulation and persisted for 30 min (Fig. 5A). Thus, oxidative stress indeed could increase JNK phosphorylation in mesangial cells. To examine whether the inhibition of oxidative stress would decrease JNK phosphorylation and downregulate proinflammatory gene expression, old diabetic mesangial cells were treated with an anti-oxidant, NAC. The levels of JNK phosphorylation and the production of MCP-1 were decreased (∼75%) by NAC in old diabetic mesangial cells (Fig. 5, B and C). NAC treatment caused a moderate toxicity to the cells (13%). However, this toxicity alone could not account for such a large reduction of MCP-1 production by NAC.
Fig. 5.
Oxidative stress, JNK phosphorylation, and MCP-1 production. A: treatment of O/MC with H2O2 (1 mM) caused a transient increase in JNK phosphorylation. B: treatment of OD/MC with N-acetyl-cysteine (NAC), an antioxidant, decreased the levels of phosphorylated JNK. C: NAC decreased MCP-1 production in 3 lines of OD/MC. **P < 0.01 vs. untreated control. Results were the average of 3 experiments with the same cell lines (means ± SD, n = 3).
Decreased MKP5 mRNA expression in old diabetic glomeruli and mesangial cells.
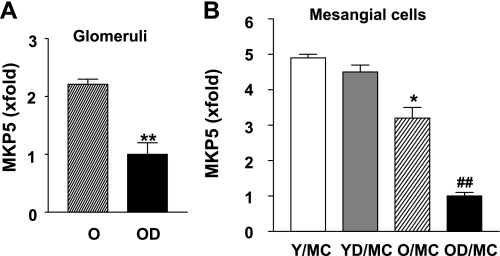
MKP are critically involved in deactivation of MAPK via removing the phosphate groups from the kinases (44, 48). Since MKP5 has been shown to specifically deactivate JNK and p38, we examined whether the activation of JNK in old diabetic mesangial cells might also be caused by decreased MKP5. MKP5 mRNA levels were significantly reduced in mesangial cells from old control mice and further decreased in mesangial cells from old diabetic mice (Fig. 6). There were no differences in MKP5 mRNA levels between mesangial cells from young control and young diabetic mice. MKP5 mRNA expression was also decreased in glomeruli from old diabetic mice.
Fig. 6.
Decreased mitogen-activated protein kinase phosphatase 5 (MKP5) mRNA expression in glomeruli and mesangial cells of OD mice. Glomeruli were microdissected from OD and O mice (n = 10/group). MKP5 mRNA expression was determined by a real-time PCR. A: glomeruli. **P < 0.01 vs. glomeruli of O mice. B: mesangial cells were isolated from 2 separate mice of Y, YD, O, and OD, respectively. *P < 0.05 vs. mesangial cells from young controls (n = 2 cell lines/age). ##P < 0.01 vs. mesangial cells from old controls (n = 2 cell lines/group).
Increasing MKP5 expression in old diabetic mesangial cells decreased JNK phosphorylation and suppressed expression of proinflammatory genes.
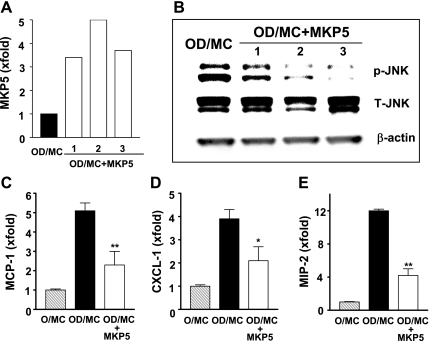
Old diabetic mesangial cells were stably transfected with a human MKP5 CDNA plasmid to determine whether decreased MKP5 expression contributed to the persistent JNK activation observed in these cells. Phosphorylated JNK levels were substantially reduced in two of three stably transfected clones of old diabetic mesangial cells that showed at least a threefold increase in MKP5 mRNA expression (Fig. 7, A and B). The reduction of phosphorylated JNK in these two clones of old diabetic mesangial cells was associated with decreased MCP-1, CXCL-1, and MIP-2 mRNA levels (Fig. 7, C, D, E).
Fig. 7.
Overexpression of MKP5 in OD/MC was associated with decreased phosphorylated JNK and inflammation. A: 3 MKP5 stably transfected OD/MC lines showed increased MKP5 mRNA expression as determined by real-time PCR. The level in untransfected OD/MC was arbitrarily defined as 1. B: phosphorylated JNK levels were decreased in 3 MKP5 stably transfected OD/MC lines (OD/MC + MKP5). The reduction was more obvious in lane 2 and 3. C–E: all 3 MKP5 stably transfected OD/MC showed decreased MCP-1, CXCL-1, and MIP2 mRNA expression compared with untransfected cells or cells transfected with an empty vector. *P < 0.05, **P < 0.01 vs. OD/MC transfected with an empty vector (n = 3).
Knocking-down of MKP5 expression in old control mesangial cells activated JNK and increased MCP-1 production.
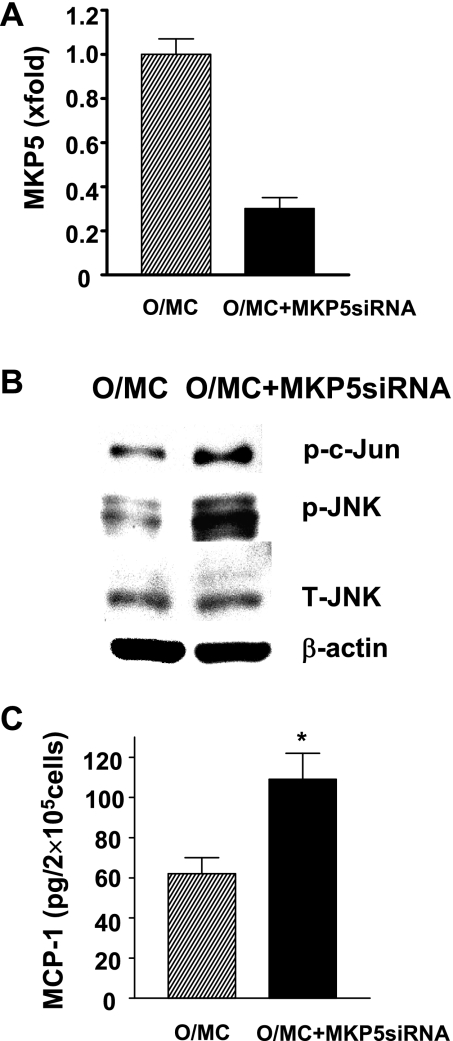
Old control mesangial cells were transfected with a MKP5 siRNA or irrelevant control siRNA to determine whether decreased MKP5 expression in these cells would result in an activation of JNK and induce a proinflammatory phenotype. Transfection with MKP5 siRNA led to a 70% reduction in MKP5 mRNA levels and resulted in an elevation in phosphorylated JNK and an increased JNK kinase activity in old control mesangial cells (Fig. 8, A, B, C). MCP-1 production was also significantly increased in MKP5 siRNA-transfected old control mesangial cells (Fig. 8D). No change in JNK activity and MCP-1 production was found in cells transfected with an irrelevant siRNA.
Fig. 8.
Knocking down of MKP5 expression in O/MC was associated with increased JNK activity and inflammation. A: 2 lines of mesangial cells from O/MC were transfected with MKP5 siRNA for 48 h. MKP5 mRNA levels were determined by real-time PCR. The level in O/MC was arbitrarily defined as 1. B: representative gel of JNK kinase activity and phosphorylated JNK level in O/MC with or without MKP5 knockdown. JNK activity, reflected by the levels of phosphorylated c-Jun on the top of the gel, was increased in O/MC with MKP5 knockdown. Knocking down of MKP5 also increased phosphorylated JNK levels in these cells while the levels of total JNK and β-actin remained similar between cells with and without MKP5 knockdown. C: MCP-1 production was increased in O/MC with MKP5 knockdown (n = 2). *P < 0.05 vs. O/MC (n = 2).
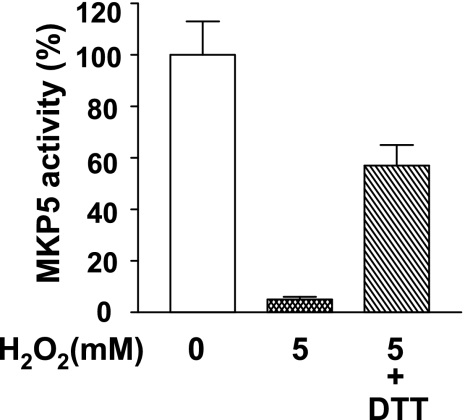
Oxidative stress decreased MKP5 enzymatic activity.
Oxidative stress decreases MKP1 and MKP3 enzymatic activity via the oxidation of cysteine residue in the highly conserved phosphatase domain of the active phophatase loop (4, 20). Among all the dual specificity MAPK phosphotases, the composition of this loop exclusively contains cysteine (7). Thus, we examined whether free radicals would modify MKP5 activity. When recombinant enzymatic active MKP5 was incubated with 5 mM H2O2 for 30 min, nearly all of the phosphatase activity was lost. The addition of DTT, together with H2O2, led to preservation of ∼60% of the enzymatic activity (Fig. 9).
Fig. 9.
H2O2 decreased MKP5 phosphatase activity. Enzymatic active recombinant MKP5 was incubated with or without 5 mM H2O2 in the presence or absence of dithiothreitol (DTT; 5 mM) for 30 min. Results shown were from 3 separate measurements of MKP5 phosphatase activity using 3-O-methylfluorescein as a substrate. The activity in untreated cells was arbitrarily defined as 100%.
DISCUSSION
Phenotypic switch of mesangial cells to profibrotic myofibroblasts, identified by markers such as the appearance of α-smooth muscle actin and the expression of interstitial extracellular matrix, has been shown to occur in various progressive glomerular diseases including diabetic nephropathy (8, 18, 37). We presented evidence here of a phenotypic switch of mesangial cells to proinflammatory. We found that in aging diabetic mice, a model of progressive diabetic nephropathy, both glomeruli and mesangial cells had a drastic increase in MCP-1 and ICAM-1 expression. Additionally, aging diabetic mesangial cells had increased RANTES, CXCL-1, and MIP-2 expression. Since the measurement of mesangial cells was performed after culturing cells out of glomeruli, the proinflammatory phenotype of aging diabetic mesangial cells seems stable. Interestingly, mesangial cells from nephropathy-resistant young C57B6 diabetic mice did not show increase in MCP-1 and RANTES, suggesting that the phenotypic switch of mesangial cells to proinflammatory may be both a marker and contributor to diabetic nephropathy progression.
NF-κB and MAPK are two important inflammatory pathways in tissues and cells (3, 19). We found that JNK, but not IκB or p38, was readily activated in the absence of major stimuli at baseline in aging diabetic mesangial cells. Importantly, elevated phosphorylated JNK levels in aging diabetic mesangial cells were associated with increased JNK kinase activity. The activation of JNK did not seem to impede the cellular response to further inflammatory stimuli since a similar degree of increase in IκB, p38, and JNK phosphorylation and MCP-1 production was observed when both aging diabetic and aging control mesangial cells were stimulated with TNF-α.
The activation of JNK has been shown to be involved in inflammation in liver and adipose tissue of diabetes and in some of proinflammatory responses of mesangial cells (14, 15, 17, 40). Since the inhibition of JNK decreased MCP-1 production in aging diabetic mesangial cells, it suggests that JNK activation may contribute to proinflammatory phenotype of aging diabetic mesangial cells. The finding that activation of JNK in old control and young mesangial cells caused a significant increase in MCP-1 expression in these cells indirectly supports a role of JNK in proinflammatory switch of mesangial cells.
Oxidative stress is the most notable JNK activator (28, 43, 49). Not surprisingly, aging diabetic kidneys had elevated oxidative stress as evidenced by a prominent increase in oxidized protein levels and the increase in lipid peroxidation products (data not shown). Consistent with the in vivo data, we found that mesangial cells from aging diabetic mice also exhibited higher oxidant levels compared with mesangial cells from aging control mice. Since oxidative stress was indeed able to induce JNK phosphorylation in mesangial cells and furthermore, the inhibition of free radicals by anti-oxidant NAC substantially decreased JNK phosphorylation and inflammation in aging diabetic mesangial cells, these data suggest a causative role of oxidative stress in JNK activation and inflammatory phenotypic switch of mesangial cells. The levels of oxidative stress were also elevated in kidneys of both young diabetic and old control mice. However, the level of elevation was much less than in kidneys of aging diabetic mice. Since neither an obvious inflammatory lesion of kidneys nor a proinflammatory phenotype of mesangial cells was present in young diabetic and old control mice, a higher degree of oxidative stress may be required to induce these changes.
MKP is a family of dual specificity phosphatase that dephosphorylates both threonine and tyrosine residues in the active loop of MAPK (7). MKP5 as a member of MKP family selectively inactivates JNK and p38, but not ERK1/2 (7, 44). MKP5 has been implicated in immune function regulation as MKP5-deficient mice exhibited elevated innate immune responses with an increased JNK but not p38 activity. mRNA expression of MKP5 was decreased in aging diabetic glomeruli as well as in mesangial cells. Thus, the increase in JNK activity in aging diabetic kidneys and mesangial cells may also be partly related to decreased MKP5 expression. The cause(s) of decreased MKP5 expression in aging diabetic kidneys and mesangial cells are unknown. MKP5 mRNA levels were comparable in mesangial cells between young control and young diabetic mice and were higher in these mesangial cells than in mesangial cells of old control and old diabetic mice. This may contribute partly to less JNK activity and noninflammatory phenotype of mesangial cells in young control and young diabetic mice. Interestingly, free radicals have been shown to cause oxidation of a critical cysteine residue in active loop of MKPs, resulting in a loss of phosphatase activity and enhanced protein degradation (7). We found that this was also in the case of MKP5. A modest concentration of H2O2 could completely destroy the phosphatase activity of MKP5 in half an hour. The presence of reducing agent DTT could partially preserve MKP5 enzymatic activity when coincubating with H2O2. Thus, oxidative stress could activate JNK through both a direct increase in JNK phosphorylation and an indirect decrease in MKP5 activity in aging diabetic mesangial cells. Since restoration of MKP5 expression in aging diabetic mesangial cells reduced both phosphorylated JNK and inflammation, and furthermore, knocking down of MKP5 expression in old control mesangial cells induced JNK activation and inflammation, MKP5 obviously plays an important role in inflammatory phenotypic switch in mesangial cells.
JNK pathway is critically involved in cell life and death decision and in inflammation in liver and some of other cells (43). The role of JNK activation in kidney diseases especially in diabetic nephropathy is unknown. The inhibition of JNK showed partial protection against fibrosis in obstructed kidney model (25). Ijaz et al. (16) reported that JNK was chronically activated in glomeruli of db/db mice, although the significance of its activation was not clear since the inhibition of JNK did not seem to be beneficial. However, the models used in their study did not have progressive disease and there were few if any of renal inflammatory lesions.
It is unclear why aging diabetic mice developed inflammatory renal lesions and proinflammatory phenotype of mesangial cells while young diabetic mice did not. One of the discrepancies between old and young diabetic mice was that the levels of oxidative stress were much higher in old diabetic kidneys. Since the levels of oxidative stress were comparable between young diabetic and old control kidneys and old control kidneys also did not have inflammatory lesions and proinflammatory phenotype of mesangial cells, these data suggest that a higher level of oxidative stress may be required for chronic inflammation to occur. Additionally, since MKP5 mRNA levels remained unchanged in young diabetic mesangial cells but were decreased in old control and further decreased in old diabetic mesangial cells, the levels of MKP5 may also be a factor in determining phenotype of mesangial cells.
GRANTS
This work is supported by National Institutes of Health Grant 5R01AG027628–03 to F. Zheng.
REFERENCES
- 1.Abe H, Matsubara T, Iehara N, Nagai K, Takahashi T, Arai H, Kita T, Doi T. Type IV collagen is transcriptionally regulated by Smad1 under advanced glycation end product (AGE) stimulation. J Biol Chem 279: 14201–14206, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Authors online. http://www.amdcc.org/shared/protocols.
- 3.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336: 1066–1071, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Boivin B, Zhang S, Arbiser JL, Zhang ZY, Tonks NK. A modified cysteinyl-labeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells. Proc Natl Acad Sci USA 105: 9959–9964, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown HJ, Lock HR, Sacks SH, Robson MG. TLR2 stimulation of intrinsic renal cells in the induction of immune-mediated glomerulonephritis. J Immunol 177: 1925–1931, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int 69: 73–80, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal 16: 769–779, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Floege J, Burns MW, Alpers CE, Yoshimura A, Pritzl P, Gordon K, Seifert RA, Bowen-Pope DF, Couser WG, Johnson RJ. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int 41: 297–309, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Fornoni A, Ijaz A, Tejada T, Lenz O. Role of inflammation in diabetic nephropathy. Curr Diabetes Rev 4: 10–17, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Fornoni A, Lenz O, Tack I, Potier M, Elliot SJ, Striker LJ, Striker GE. Matrix accumulation in mesangial cells exposed to cyclosporine A requires a permissive genetic background. Transplantation 70: 587–593, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Fornoni A, Striker LJ, Zheng F, Striker GE. Reversibility of glucose-induced changes in mesangial cell extracellular matrix depends on the genetic background. Diabetes 51: 499–505, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Gruden G, Perin PC, Camussi G. Insight on the pathogenesis of diabetic nephropathy from the study of podocyte and mesangial cell biology. Curr Diabetes Rev 1: 27–40, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807–1816, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Huang S, Konieczkowski M, Schelling JR, Sedor JR. Interleukin-1 stimulates Jun N-terminal/stress-activated protein kinase by an arachidonate-dependent mechanism in mesangial cells. Kidney Int 55: 1740–1749, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Ijaz A, Tejada T, Catanuto P, Xia X, Elliot SJ, Lenz O, Jauregui A, Saenz MO, Molano RD, Pileggi A, Ricordi C, Fornoni A. Inhibition of C-jun N-terminal kinase improves insulin sensitivity but worsens albuminuria in experimental diabetes. Kidney Int 75: 381–388, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, Yan W, Xu H. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes 58: 104–115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson RJ, Floege J, Yoshimura A, Iida H, Couser WG, Alpers CE. The activated mesangial cell: a glomerular “myofibroblast”? J Am Soc Nephrol 2: S190–S197, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy–from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 1754: 253–262, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Song MC, Kwak IH, Park TJ, Lim IK. Constitutive induction of p-Erk1/2 accompanied by reduced activities of protein phosphatases 1 and 2A and MKP3 due to reactive oxygen species during cellular senescence. J Biol Chem 278: 37497–37510, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Lenz O, Striker LJ, Jacot TA, Elliot SJ, Killen PD, Striker GE. Glomerular endothelial cells synthesize collagens but little gelatinase A and B. J Am Soc Nephrol 9: 2040–2047, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, Lan HY. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol 164: 1389–1397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol 172: 299–308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lupia E, Elliot SJ, Lenz O, Zheng F, Hattori M, Striker GE, Striker LJ. IGF-1 decreases collagen degradation in diabetic NOD mesangial cells: implications for diabetic nephropathy. Diabetes 48: 1638–1644, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Ma FY, Flanc RS, Tesch GH, Han Y, Atkins RC, Bennett BL, Friedman GC, Fan JH, Nikolic-Paterson DJ. A pathogenic role for c-Jun amino-terminal kinase signaling in renal fibrosis and tubular cell apoptosis. J Am Soc Nephrol 18: 472–484, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Makino H, Kashihara N, Sugiyama H, Kanao K, Sekikawa T, Shikata K, Nagai R, Ota Z. Phenotypic changes of the mesangium in diabetic nephropathy. J Diabetes Complications 9: 282–284, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Makino H, Kashihara N, Sugiyama H, Kanao K, Sekikawa T, Okamoto K, Maeshima Y, Ota Z, Nagai R. Phenotypic modulation of the mesangium reflected by contractile proteins in diabetes. Diabetes 45: 488–495, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta 1780: 1325–1336, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Morii T, Fujita H, Narita T, Shimotomai T, Fujishima H, Yoshioka N, Imai H, Kakei M, Ito S. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complications 17: 11–15, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Münzel D, Lehle K, Haubner F, Schmid C, Birnbaum DE, Preuner JG. Impact of diabetic serum on endothelial cells: an in vitro analysis of endothelial dysfunction in diabetes mellitus type 2. Biochem Biophys Res Commun 362: 238–244, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19: 433–442, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Park J, Ryu DR, Li JJ, Jung DS, Kwak SJ, Lee SH, Yoo TH, Han SH, Lee JE, Kim DK, Moon SJ, Kim K, Han DS, Kang SW. MCP-1/CCR2 system is involved in high glucose-induced fibronectin and type IV collagen expression in cultured mesangial cells. Am J Physiol Renal Physiol 295: F749–F757, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Phillips A, Janssen U, Floege J. Progression of diabetic nephropathy. Insights from cell culture studies and animal models. Kidney Blood Press Res 22: 81–97, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Radeke HH, Resch K. The inflammatory function of renal glomerular mesangial cells and their interaction with the cellular immune system. Clin Investig 70: 825–842, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Sato W, Kosugi T, Zhang L, Roncal CA, Heinig M, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Grant MB, Croker BP, Nakagawa T. The pivotal role of VEGF on glomerular macrophage infiltration in advanced diabetic nephropathy. Lab Invest 88: 949–961, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol 16: S30–S33, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Schnaper HW, Kopp JB, Poncelet AC, Hubchak SC, Stetler-Stevenson WG, Klotman PE, Kleinman HK. Increased expression of extracellular matrix proteins and decreased expression of matrix proteases after serial passage of glomerular mesangial cells. J Cell Sci 109: 2521–2528, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Simonson MS. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int 71: 846–854, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Skill NJ, Johnson TS, Coutts IG, Saint RE, Fisher M, Huang L, El Nahas AM, Collighan RJ, Griffin M. Inhibition of transglutaminase activity reduces extracellular matrix accumulation induced by high glucose levels in proximal tubular epithelial cells. J Biol Chem 279: 47754–47762, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 6: 386–397, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Sterzel RB, Schulze-Lohoff E, Marx M. Cytokines and mesangial cells. Kidney Int Suppl 39: S26–S31, 1993 [PubMed] [Google Scholar]
- 42.Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb MJ. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J 15: 4629–4642, 1996 [PMC free article] [PubMed] [Google Scholar]
- 43.Temkin V, Karin M. From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: the receptor-interacting protein 1 odyssey. Immunol Rev 220: 8–21, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Theodosiou A, Smith A, Gillieron C, Arkinstall S, Ashworth A. MKP5, a new member of the MAP kinase phosphatase family, which selectively dephosphorylates stress-activated kinases. Oncogene 18: 6981–6988, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ. c-Jun NH2-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol Cell Biol 23: 2871–2882, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia L, Wang H, Munk S, Kwan J, Goldberg HJ, Fantus IG, Whiteside CI. High glucose activates PKC-zeta and NADPH oxidase through autocrine TGF-β1 signaling in mesangial cells. Am J Physiol Renal Physiol 295: F1705–F1714, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Yonemoto S, Machiguchi T, Nomura K, Minakata T, Nanno M, Yoshida H. Correlations of tissue macrophages and cytoskeletal protein expression with renal fibrosis in patients with diabetes mellitus. Clin Exp Nephrol 10: 186–192, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Blattman JN, Kennedy NJ, Duong J, Nguyen T, Wang Y, Davis RJ, Greenberg PD, Flavell RA, Dong C. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature 430: 793–797, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Chen F. Reactive oxygen species (ROS), troublemakers between nuclear factor-kappaB (NF-kappaB) and c-Jun NH2-terminal kinase (JNK). Cancer Res 64: 1902–1905, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Zheng F, Plati AR, Potier M, Schulman Y, Berho M, Banerjee A, Leclercq B, Zisman A, Striker LJ, Striker GE. Resistance to glomerulosclerosis in B6 mice disappears after menopause. Am J Pathol 162: 1339–1348, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng F, Striker GE, Esposito C, Lupia E, Striker LJ. Strain differences rather than hyperglycemia determine the severity of glomerulosclerosis in mice. Kidney Int 54: 1999–2007, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Ziyadeh FN. Mediators of diabetic renal disease: the case for TGF-β as the major mediator. J Am Soc Nephrol 15: S55–S57, 2004 [DOI] [PubMed] [Google Scholar]