Abstract
Flow sensing by primary cilia of the epithelial cells is involved in cystogenesis in polycystic kidney disease. We investigate whether a similar mechanism applies to the pathogenesis of cyst-like tubular dilatation induced by ureteral obstruction in mice. Robust proliferation occurs in the obstructed tubules when urine flow is interrupted as well as in the repairing tubules when urine flow is reestablished after relief of the obstruction, suggesting a urine flow-independent mechanism of proliferation. In the urothelium, proliferation is only detected above the obstruction, although urine flow ceased both above and below the obstruction. Our results support mechanical strain- rather than flow-mediated proliferation in obstructive uropathy. To understand the mechanism of cell proliferation leading to increased tubular diameter in cyst-like tubular dilatation, we examine planar cell polarity (PCP), which is necessary for oriented cell division and maintenance of tubular diameter. In dilated tubules, the orientation of cell division is randomized, atypical PKC (aPKC) is mislocalized, and the pattern of the expression of a core PCP protein, Frizzled3 (Fz3), is altered. In addition, the level of Fz3 expression is increased. These results indicate that aberrant PCP may contribute to cyst-like tubular dilatation in obstructive uropathy. Interestingly, the orientation of cell division, localization of aPKC, and Fz3 expression return to normal when obstruction is relieved, which suggest a role of normal PCP signaling in tubular repair.
Keywords: obstructive injury, orientation of cell division, mechanical strain, urine flow
obstructive uropathy and renal cystic dysplasia/hypoplasia represent the most common causes of pediatric end-stage renal disease (21, 33). In adults, urinary tract obstruction due to kidney stone or prostate hypertrophy may be associated with chronic renal insufficiency as well (26). Although molecular mechanisms underlying the pathogenesis in congenital and acquired obstruction may not overlap completely, cyst-like tubular dilation is common in both conditions.
Reversal of the obstruction results in renal structural and functional repair, indicating a regenerative ability of tubular epithelial cells (5). However, the mechanisms of cyst-like tubular dilation following ureteral obstruction and tubular repair following reversal of the obstruction are not fully understood. Studies on polycystic kidney disease (PKD), a genetic disorder characterized by cyst formation and renal failure, indicate that the primary cilium, a hair-like organelle present on the apical surface of epithelial cells, is important for development and maintenance of the normal diameter of renal tubules. It has been shown that cilia bending due to urine flow leads to an increase in intracellular Ca2+, which may act as a second messenger for several key signaling pathways required for tissue homeostasis (2, 14). Loss of cilia or mutations of ciliary proteins lead to dysregulated cell proliferation and PKD (23). It was recently shown that aberrant planar cell polarity (PCP) might be one of the mechanisms by which abnormal structure and/or function of cilia caused PKD in rodents (8). PCP is defined as polarity along a tissue plane that is perpendicular to the apical-basal axis. In addition to orienting cell division, the PCP pathway is involved in establishing epithelial polarity, convergent-extension movements, and ciliogenesis (15). Cyst-like tubular dilatation in obstructive uropathy shares some similarity with cyst formation in PKD by having an abnormally high rate of cell proliferation. We tested the hypothesis that aberrant PCP signaling and randomization of the orientation of cell division may play a similar role in cyst-like tubular dilation in obstructive uropathy.
MATERIALS AND METHODS
Unilateral ureteral obstruction and reversal of the obstruction.
C57BL/6 mice and Creksp;R26R-EYFP mice 6–8 wk old were used for the studies. Creksp;R26R-EYFP mice were generated by crossing Creksp mice expressing Cre recombinase specifically in the epithelial cells in the maturing nephron, collecting ducts, and developing urogenital tract (31) with the R26R-EYFP reporter mice generated in the laboratory of Dr. Frank Costantini (34). The bitransgenic Creksp;R26R-EYFP mice express enhanced yellow fluorescent protein (EYFP) specifically in epithelial cells of the renal tubules and urothelial cells of the ureters. For unilateral ureteral obstruction (UUO), the left ureter was obstructed by two-point ligations with silk. For reversal of the obstruction (RUUO), the midureter was completely obstructed with an atraumatic vascular clamp as described (5). The clamp was then removed after 3 days to allow reflow of the urine. The successful RUUO was confirmed at the time of kidney harvesting and histological analysis. Sham-operated mice underwent the same procedures with the exception of the obstruction of the left ureter. The kidneys were harvested and fixed with 4% paraformaldehyde before embedding in paraffin or OCT medium. Kidney paraffin sections (5 μm thick) were used for periodic acid-Schiff (PAS) stainings. Kidney cryosections (5 μm thick) were used for immunostaining. All experiments were performed with the approval of the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center at Dallas.
Immunostaining and image analysis.
Immunostaining was performed using established methods (22). The primary antibodies used were antibodies against enhanced green fluorescent protein (EGFP) that cross reacted with EYFP (fluorescein conjugated, 1:200, Rockland Immunochemicals, Gilbertsville, PA), Na-K-2Cl cotransporter (NKCC2; 1:200, Alpha Diagnostic International, San Antonio, TX), thiazide-sensitive NaCl cotransporter (TSC; 1:500, gift of Dr. Mark Knepper, National Institutes of Health, Bethesda, MD) (16), entactin (1:1,000, Chemicon International, Billerica, MA), Ki-67 (1:500; Novocastra Laboratories, Newcastle-Upon-Tyne, UK), ezrin (1:100, Upstate Cell Signaling, Lake Placid, NY), claudin 2 (1:200, Zymed Laboratories, San Francisco, CA), zonula occludens (ZO-1; 1: 500, Zymed Laboratories), ZO-1α+ (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), atypical PKC (aPKC; 1:200, Santa Cruz Biotechnology), occludin (1:500, Zymed Laboratories), and Frizzled3 (Fz3; 1:200, R&D Systems, Minneapolis, MN). Proximal tubules were stained with FITC-labeled Lotus tetragonolobus agglutinin (LTA; 1:50, Vector Laboratories, Burlingame, CA). Collecting ducts were stained with fluorescein-Dolichos biflorus agglutinin (DBA; 1:50, Vector Laboratories). Secondary antibodies included Alexa Fluor 488-conjugated goat anti-mouse IgG, Alexa Fluor 594-conjugated goat anti-rabbit IgG, or Alexa Fluor 488-conjugated goat anti-rat IgG (1:400, Molecular Probes, Eugene, OR). Tissue sections were incubated with the primary antibodies at 4°C overnight or at room temperature for 2 h after permeabilization with 0.1% Triton X-100 in PBS and blocked with 10% goat serum and 0.1% BSA. The sections were further incubated with the appropriate secondary antibodies for 1 h at room temperature. The nuclei were counterstained with DAPI. Sections were visualized with a Zeiss Axioplan 2, photographed with a digital camera, and images were analyzed with Axiovision software (Carl Zeiss International, Oberkochen, Germany). Ten randomly selected areas under ×200 magnification were used for quantification. Some images were obtained by scanning with a Zeiss laser-scanning confocal microscope (LSM 510 Meta, Oberkochen, Germany). A total of three to six mice were used at each time point of the studies.
Measurement of orientation of cell division.
The orientation of cell division was measured as described (8, 25). Briefly, kidney sections (20 μm thick) were stained with anti-phosphohistone H3 (1:1,000, Sigma-Aldrich, St. Louis, MO) to label the chromosomes of dividing cells in late anaphase and telophase. Sections were costained with anti-entactin (1:1,000, Chemicon International) to label the tubular basement membranes. Z-stack images of the medullary region were acquired using a Zeiss LSM 510 Meta confocal laser-scanning microscope (Carl Zeiss International), and three-dimensional images were reconstructed using Imaris software (Bitplane, Zurich, Switzerland) available at the UT Southwestern O'Brien Kidney Research Center. The orientation of cell division was determined by measuring the angle between the mitotic spindles of dividing cells and the longitudinal axis of the tubules.
Immunoblot analysis.
Kidneys were snap-frozen in liquid nitrogen for protein isolation. Preparation of kidney extracts and immunoblot analysis were performed as described previously (13). Immunoblots were probed with antibodies to ZO-1 (1:500, Zymed Laboratories), aPKC (1:1,000, Santa Cruz Biotechnology), and Fz3 (1:3,000, R&D Systems) followed by detection by chemiluminescence. Anti-α-tubulin antibody (1:5,000, Sigma-Aldrich) was used as a loading control. The level of protein expression was quantified by phosphoimage analysis (Alpha Innotech, San Leandro, CA).
In situ hybridization.
In situ hybridization was performed on frozen sections of the kidney using a Four jointed 1 (Fjx1) riboprobe generated by cloning the antisense into EcoRV sites of the pYX-Asc vectors (Open Biosystems, ID 5687527) and transcribing with T3 RNA polymerase. Kidneys were perfused and fixed with 4% PFA in PBS, cryoprotected in 30% sucrose, embedded in OCT, and sectioned at a thickness of 15 μm. Tissue sections were hybridized with the Fjx1 riboprobe overnight at 68°C. Digoxigenin-labeled probes were detected by incubation with alkaline phosphatase-conjugated anti-digoxigenin antibody (1:4,000, Roche) followed by application of BM-purple alkaline phosphatase substrate according to the manufacturer's directions (Roche).
Statistical analysis.
Data are shown as means ± SD. The significance of the differences between the means was calculated using Student's t-test. ANOVA was used for multiple comparisons. The Mann-Whitney U-test was used to compare the distributions of mitotic spindle orientations in the RUUO and UUO kidneys. P < 0.05 was considered statistically significant.
RESULTS
Mechanical strain rather than cessation of urine flow stimulates epithelial proliferation.
UUO is a widely used animal model to study the pathogenesis of obstructive uropathy. Tubular injury was evidenced by the loss of brush border in the proximal tubules. Diffused cyst-like tubular dilation occurred in all nephron segments and the collecting ducts. To minimize irreversible injury and allow tubules to recover, we reversed the obstruction 3 days later (3). RUUO, by releasing the clamp placed in the midureter, resulted in the restoration of renal structure within 2 wk (Supplementary Fig. 1A). Cyst-like tubular dilatation and tubular repair were accompanied by profound cell proliferation in all nephron segments and collecting ducts. Three days after obstruction, proximal tubules and distal tubules exhibited higher Ki-67 expression than thick ascending limbs and collecting ducts (Supplementary Fig. 1, B and C). However, ureter uroepithelia above the site of obstruction had the highest rate of proliferation, with 31.3 ± 4.6% cells expressing Ki-67. These results suggest that higher pressure in the ureter where it is closest to the obstruction may induce stronger proliferative signals. It is also possible that different epithelial cells in the kidney and urogenital system may have different susceptibilities to injurious and proliferative signals.
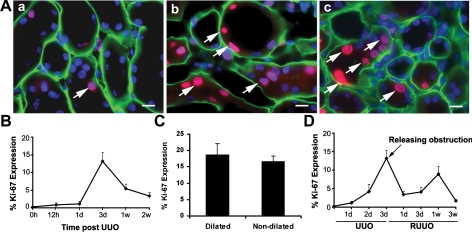
Fig. 1.
Cell proliferation in tubular epithelial cells after unilateral ureteral obstruction (UUO) and reversal of obstruction (RUUO). A: Ki-67 expression (red, arrows) in renal tubular epithelial cells in mice that underwent sham operation (a), 3 days after UUO (b), and 1 wk after RUUO (c). Tubular basement is stained with entactin (green). Nuclei are counterstained with DAPI (blue). Scale bar = 10 μM. B: quantification of Ki-67 expression in tubular epithelial cells after UUO; n = 6. C: there was no significant difference in Ki-67 expression between dilated and nondilated proximal tubules 3 days after UUO (P = 0.3, n = 4). D: quantification of Ki-67 expression in tubular epithelial cells 3 days after UUO followed by RUUO; n = 5. Values are means ± SE.
Since abnormal sensing of urine flow has been linked to cyst formation in PKD, a condition with a high rate of cell proliferation (2, 40), we investigated whether abnormal urine flow mediates cell proliferation in the kidneys with complete urinary tract obstruction (Fig. 1). In the kidneys 12 h post-UUO, 0.9 ± 0.2% of all tubular epithelial cells expressed Ki-67 (n = 5) compared with 0.3 ± 0.05% of cells in sham-operated kidneys (n = 3, P < 0.05). Ki-67 expression peaked to 16.3 ± 1.6% in all tubular epithelial cells at 3 days (n = 6) (Fig. 1B). Cell proliferation occurs in dilated tubules and tubules before dilatation. Examination of proximal tubules 3 days after UUO showed no difference in the rate of proliferation with 18.6 ± 3.6% of cells in dilated tubules and 16.7 ± 1.6% of cells in nondilated tubules expressing Ki-67 (n = 4, P = 0.3, Fig. 1C). This is likely due to the fact that both dilated and nondilated proximal tubules sustain mechanical strain because of the common obstruction in the ureter. Interestingly, RUUO allowing reflow of the urine and decreasing mechanical strain caused a significant decrease in Ki-67+ cells to 6.1 ± 1.0% 1 day after RUUO (n = 5, P < 0.05). These results suggest that cessation of urine flow and/or an increase in hydrostatic pressure stimulates epithelial proliferation, leading to cyst-like tubular dilatation. Transient obstructive injury triggered tubular repair. An increase in proliferation started at 3 days and peaked at 1 wk post-RUUO (12.0 ± 1.5% Ki-67+ cells, n = 5) (Fig. 1D), which preceded the restoration of tubular structures.
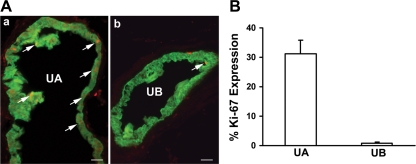
Next, we used the bitransgenic Creksp;R26R-EYFP mice that express EYFP in 100% of the uroepithelium (30, 31) to test whether urine flow or mechanical strain plays a predominate role in stimulating proliferation. The midureter was completely obstructed and the urine flow was abolished for 3 days both above and below the obstruction. Epithelial proliferation was examined both above and below the obstructive site of the ureter to address the effect of urine flow on proliferation. Dramatic epithelial proliferation was detected in EYFP+ epithelial cells above the obstruction with 31.3 ± 4.6% of cells expressing Ki-67. However, only 0.9 ± 0.4% of urothelial cells below the obstruction expressed Ki-67 (Fig. 2, A and B, n = 3), which was not different from a contralateral nonobstructed ureter (not shown). Examination of blood vessels supplying ureters below the obstruction revealed the presence of abundant red blood cells, suggesting that the low rate of proliferation below the obstruction was not due to ischemia from blood vessel occlusion. These results support mechanical strain- rather than flow-mediated proliferation following urinary tract obstruction.
Fig. 2.
Mechanical strain induces proliferation in urothelium of the ureter. A: cross sections of the ureter from creksp;R26R-EYFP mice that express enhanced yellow fluorescent protein (EYFP; green) in uroepithelial cells were obtained 3 days after UUO. Increased expression of Ki-67 (red) is detected in uroepithelial cells of the ureter above the obstruction (UA; a), but not below the obstruction (UB; b). Scale bars = 50 μM. B: quantification of Ki-67 expression in uroepithelial cells above (UA) and below (UB) the obstruction. Values are means ± SE; n = 3.
The orientation of cell division is randomized in obstructed renal tubules.
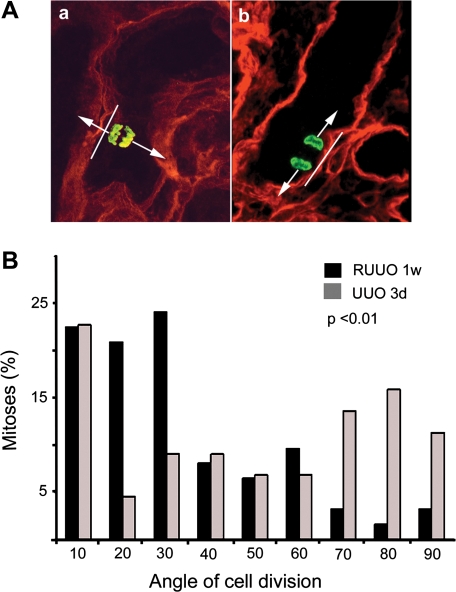
To further understand the role of mechanical strain in increasing tubular diameters, we examined the orientation of cell division in the tubules (Fig. 3A). The position of the mitotic spindle determines the orientation of cell division. A large body of evidence indicates that the orientation of the mitotic spindle is controlled by the PCP pathway (1, 4, 9). In normal kidneys, the majority of the mitotic angles are within 30° off the long axis of the tubules. Division of epithelial cells along the long axis of the tubules leads to tubular lengthening, whereas randomized division leads to increased tubular diameter and cyst formation in rodent models of PKD (8, 25, 27). We examined the orientation of cell division in the tubules 3 days after UUO and 1 wk after RUUO when tubular diameters were similar. Tubules showing straight orientation in the medulla including S3 segments of the proximal tubules, thick ascending limbs, and collecting ducts were selected for measurement of the angle of cell division because the tubular long axis could be identified on the section. At 3 days after UUO, a randomized orientation of cell division was observed, with most angles of cell division >30° in relationship to the long axis of the tubules. This is in sharp contrast to the results obtained in the tubules 1 wk post-RUUO when most cell divisions were oriented within 30° (Fig. 3). The differences in the angle of cell division are statistically significant between the groups of UUO and RUUO (P < 0.01, Mann-Whitney U-test). These results indicate that urinary tract obstruction can lead to aberrant PCP, which may contribute to cyst-like tubular dilation. Furthermore, normal PCP can be restored in mice without known genetic defects in the PCP signaling pathway once the obstruction is relieved. Our results also suggest that normal PCP may play a role in reestablishing tubular diameter during renal repair.
Fig. 3.
Orientation of cell division in the kidneys after UUO and RUUO. Tubules showing straight orientation in the medulla including S3 segments of the proximal tubules, thick ascending limbs, and collecting ducts were selected for measurement of the orientation of cell division. A: representative image showing that the orientation of cell division indicated by the localization of phosphohistone H3 (green) is randomized in the kidneys 3 days after UUO (a) and parallel to the longitudinal axis of the tubule 1 wk after RUUO (b). Tubular basement membrane is stained with entactin (red). Arrows indicate the orientation of cell division, and lines indicate the long axis of the tubules. B: distribution of the orientation of cell division in mice 3 days after UUO (gray bars) and 1 wk after RUUO (black bars). The orientation of cell division is significantly more randomized in mice 3 days after UUO and mostly parallel to the long axis of the tubule 1 wk after RUUO (P < 0.01, Mann-Whitney U-test; n = 44 for UUO and n = 62 for RUUO). The angle of cell division is determined by the orientation of mitotic spindles of the dividing cells and the longitudinal axis of the tubules.
Polarity protein aPKC is mislocalized in tubular epithelial cells.
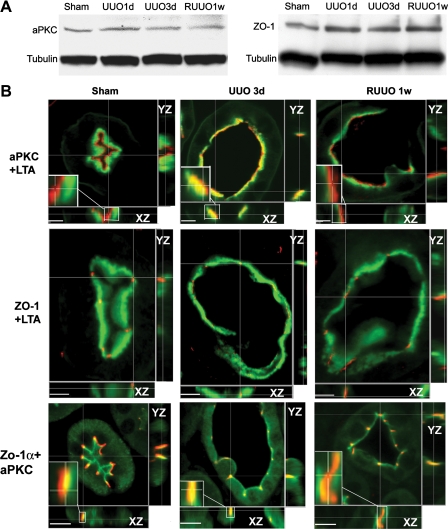
PCP and apical-basolateral polarity are interdependent. To study the abnormalities in the PCP signaling pathway, we examined the levels and subcellular localization of proteins involved in the determination of PCP and apical-basolateral polarity. aPKC and ZO-1 are constituents of the apical membrane. aPKC is involved in phosphorylation and regulation of PCP proteins (6, 28). Immunoblot analysis showed no changes in the levels of aPKC and ZO-1 during the course of transient ureteral obstruction (Fig. 4A). We examined the subcellular localization of aPKC and ZO-1 in the proximal tubules where the brush-border membrane was stained by LTA, which provided spatial reference for the changes in the localization of other apical and subapical proteins. Laser-scanning confocal microscopy examination showed a normal junctional localization of ZO-1 3 days after UUO and 1 wk after RUUO. However, aPKC was mislocalized from the apical membrane to the subapical region after obstruction. RUUO led to the relocalization of aPKC to the apical surface (Fig. 4B). To examine whether urinary tract obstruction also disrupts aPKC localization to the tight junctions, we performed coimmunostaining of aPKC and a ZO-1α+ isoform known to be expressed in renal tubules but not in glomeruli (18). In normal and RUUO kidneys, aPKC is not only expressed at the luminal surface but also colocalized with ZO-1α+ to the tight junctions. However, aPKC is more subapically localized by further merging into the ZO-1α+ domain in tubules 3 days post-UUO (Fig. 4B, bottom).
Fig. 4.
Expression and cellular localization of polarity proteins. A: immunoblot blot analysis of atypical PKC (aPKC) and zonula occludens (ZO-1) shows no changes in protein levels in the kidneys after UUO and RUUO. B: laser-scanning confocal microscopic examination of the localization of aPKC and ZO-1 in control proximal tubules, dilated proximal tubules 3 days after UUO, and recovering proximal tubules 1 wk after RUUO. Proximal tubules are stained with Lotus tetragonolobus agglutinin (LTA; green) to reveal the brush border. aPKC (red) is localized to the most apical aspect in the control tubules and the recovering tubules 1 wk after RUUO but merges into the brush-border zone in dilated tubules 3 days after UUO (top). ZO-1 (red) is localized at the apical surface in control tubules and tubules after UUO or RUUO (middle). Costaining of aPKC (green) and ZO-1α+ (red) indicates their colocalization to the tight junctions in the normal and RUUO kidneys. However, aPKC is more subapically localized by further merging into the ZO-1α+ domain in UUO 3-day tubules (bottom). Insets: higher magnification of the localization of aPKC with LTA or ZO-1α+ at the XZ-plane. Scale bars = 5 μm.
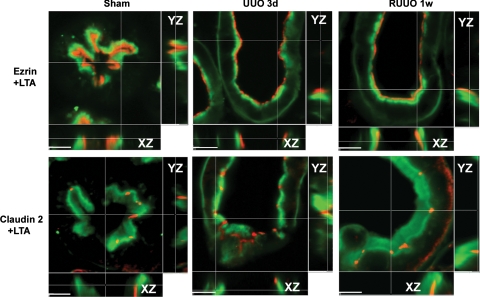
To examine whether alternation of aPKC localization from the apical surface to the subapical region represents a general defect in apical proteins, we examined subcellular localizations of additional membrane proteins that are not known to be involved in PCP signaling. Ezrin, which normally links membrane proteins to the cytoskeleton, remained at the apical surface in UUO and RUUO. Another tight junction protein, claudin 2, also remained unchanged during transient obstruction (Fig. 5). These results indicate that urinary tract obstruction leads to a specific mislocalization of aPKC, a molecule that has been shown to regulate PCP proteins. Normal protein levels yet abnormal subcellular localization of aPKC suggests a defect in recruitment of aPKC.
Fig. 5.
Coimmunostaining of apical protein ezrin (red, top) or tight junction protein claudin 2 (red, bottom) with LTA (green) in the proximal tubules shows no changes in their subcellular localizations after transient urinary tract obstruction. Scale bars = 5 μm.
Expression of PCP determinant Fz3 is altered in obstructed tubules.
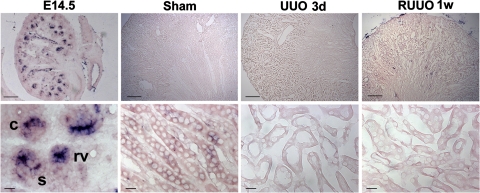
To provide evidence that a defect in aPKC recruitment may affect PCP signaling during urinary tract obstruction, we examined the expression of PCP signaling molecules Fjx1 and Fz3. In situ hybridization showed the expression of Fjx1 mRNA in the renal vesicles, comma- and S-shaped bodies in the embryonic day 14.5 kidneys. Fjx1 expression was downregulated to undetectable levels in the adult kidneys, including the kidneys with transient urinary tract obstruction (Fig. 6).
Fig. 6.
In situ hybridization of Four jointed 1 (Fjx1). Fjx1 is expressed in renal vesicles (rv), comma-shaped (c), and S-shaped (s) bodies in the developing nephrons in embryonic day 14.5 (E14.5) kidneys. Fjx1 expression is downregulated to undetectable levels in the adult kidneys, including the kidneys of mice with sham operation, UUO, and RUUO. Scale bars = 100 μm (top) and 10 μm (bottom).
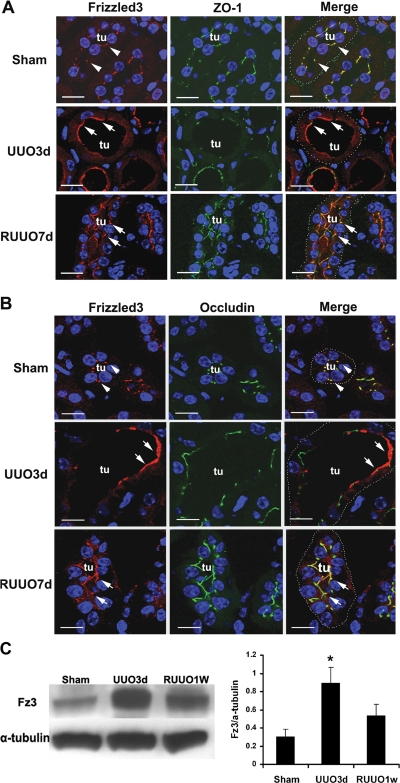
Because PCP appears to be largely regulated by the subcellular localization of a core group of proteins including members of the Frizzled (Fz) family, and because aPKC has been implicated in regulating Fz activity and maintaining normal PCP in Drosophila melanogaster eyes (6, 11), we stained kidneys with an antibody to Fz3 to examine the effect of obstruction on subcellular localization of this PCP determinant. Fz3 has been shown to control PCP by directing axonal growth and guidance, neural tube closure, and the planar orientation of hair bundles on inner ear sensory epithelial cells (43, 45). Like some other members of the Fz family, such as Fz6, Fz3 is localized to the apical region of the sensory epithelial cells (43). In normal kidneys, Fz3 was colocalized with the tight junction protein ZO-1 as punctate and linear signals. However, after transient urinary tract obstruction, its expression domain was expanded apically beyond the tight junctions and became broader in the dilated tubules (Fig. 7A). In comparison, Fz3 remained colocalized with ZO-1 in nondilated tubules (not shown). RUUO led to relocalization of Fz3 with ZO-1 (Fig. 7A). Similar alternations in Fz3 localization with another tight junction protein, occludin, were also observed during transient ureteral obstruction (Fig. 7B). Immunoblot analysis of Fz3 expression indicated a threefold increase in the obstructed kidneys and no significant change after reversal of the obstruction compared with normal control kidneys (Fig. 7C). The changes in Fz3 expression suggest that PCP signaling may be defective after urinary tract obstruction and further support the hypothesis that aberrant PCP signaling may play a role in cyst-like tubular dilatation.
Fig. 7.
Expression of Frizzled3 (Fz3) during transient ureteral obstruction. Coimmunostaining of Fz3 (red, arrowheads) with tight junction proteins ZO-1 (green, A) or occludin (green, B) shows their colocalization to the tight junctions as punctate and linear signals in normal kidneys (arrowheads). The expression of Fz3 expands beyond the tight junctions with broader apical signals in dilated tubules (tu) with UUO (arrows). Fz3 expression returns to the tight junctions in nondilated tubules after RUUO (arrows). Nuclei are counterstained with DAPI. Tubules are outlined with white dots (right). Scale bars = 10 μm. Representative immunoblot imaging and quantification (C) indicates an increased level of Fz3 expression in the UUO kidney and no significant changes in Fz3 expression following RUUO compared with control kidneys. Values are means ± SE; n = 3. *P < 0.05.
DISCUSSION
In our study, we observe a high level of proliferation in obstructed tubules and ureters following UUO and provide evidence of mechanical stretch- and pressure-mediated proliferation in obstructive uropathy. We show that cessation of urine flow does not cause uroepithelial proliferation in the ureter. In renal tubules, it remains to be examined whether cessation of urine flow per se alters tubular epithelial proliferation. Mechanical stretching and an increase in intratubular pressure due to retrograde pressure transfer are early events after obstruction (7). Mechanical force alters the activities of receptor tyrosine kinases, mitogen-activated protein kinases, and stretch-activated ion channels (41). ERK is activated 30 min after UUO and its activation precedes tubular proliferation (24), indicating that mechanical stain can be a trigger for proliferation when the urinary tract is obstructed.
The mechanisms of increased tubular diameter in the obstructed kidneys are not fully understood. Studies from PKD suggest the link of abnormal ciliary structure or function to cyst formation. Verghese et al. (38) and Wang et al. (42) reported an approximate doubling in the length of cilia throughout the nephron and collecting ducts after UUO while epithelial cells maintained the arrangement of a single cilium per cell. The possibilities of stimulating proliferation by altered ciliary structure or abnormal ciliary signaling beyond flow-sensing cannot be excluded. Recent work in ciliary signaling indicates the importance of the cilium and basal body-centrosome complex in controlling PCP (32). We deleted Kif3a, a motor protein required for formation and maintenance of the cilia, in renal epithelial cells and showed that loss of the cilia resulted in aberrant PCP and cyst formation (23, 25), further supporting the role of cilia in PCP signaling.
PCP was first recognized in the insects Oncopeltus fasciatus and D. melanogaster (10, 20, 39). Fz proteins have been shown to be conserved core molecules in controlling PCP in both invertebrates and vertebrates (29). Although the Fz family is best known as receptors for Wnt signaling molecules, the Fz-mediated PCP pathway is distinct from canonical Wnt/β-catenin signaling (35, 37). In fact, Fz is believed to function in a Wnt-independent fashion in regulating PCP in Drosophila (19). Studies in Drosophila bristles and hairs that have been used as indicators for PCP demonstrate that both fz loss of function and gain of function can lead to abnormal PCP phenotypes (17, 36, 39, 46). Fz3 controls neural tube closure and PCP of vestibular and auditory sensory epithelial cells of the mouse inner ear (43). Fz3−/− mice have a curly tail and defects in several major axon tracts and typically die within 30 min of birth (44), which precludes the use of Fz3−/− mice to study the role of Fz3 in controlling PCP after UUO. Our study reveals an increase in Fz3 expression in the kidneys 3 days after UUO when randomized cell division and aberrant PCP occurs, indicating that abnormal PCP is accompanied by a high level of Fz3. It is possible that obstructive injury induces activation of Wnt signaling and Fz3 expression, whereas tubular repair requires normalization of Fz3 expression to reestablish normal PCP and correct tubular diameter. Recent studies by He et al. (12) indicate that canonical Wnt/β-catenin signaling is activated in obstructed and fibrotic kidneys. The expression of most Fz receptors, including Fz3, is increased when analyzed by RT-PCR. Their studies clearly demonstrate the important role of Wnt/β-catenin in interstitial fibrosis (12). It is important to note that our current study only indicates the coexistence of an altered Fz3 expression pattern with aberrant PCP in the obstructed renal tubule. Future studies utilizing molecular reagents, mice reporting canonical Wnt signaling activation, and kidney-specific Fz3 transgenic mice will provide useful information on whether Wnt/β-catenin activation influences the Fz-mediated PCP pathway and whether alternations of Fz3 expression underlie the mechanism of cyst-like tubular dilatation in obstructive uropathy.
The interdependency of apical-basolateral polarity and PCP is supported by the fact that aPKC is involved in the phosphorylation and regulation of a core PCP protein, Fz1, and controls microtubular organization to balance apical-basolateral and planar polarity (6, 11). Mislocalization of aPKC during urinary tract obstruction when cyst-like tubular dilatation occurs suggests that aPKC may regulate PCP signaling in mice with obstructive uropathy. This possibility is supported by the changes in the subcellular localization and the level of expression of the PCP core protein Fz3 during transient urinary tract obstruction. In mice with severe PCP defects due to a deficiency of another core PCP protein, Vangl2 (looptail mice), Fz3 protein does not localize correctly to the cell surface (43), indicating the importance of Fz3 subcellular localization in controlling normal PCP. To our knowledge, cellular localization of Fz3 in renal tubular epithelial cells has not been shown before. Our immunostaining reveals distinct Fz3 signals at the apical aspect of normal renal tubular cells. Given the similar distribution of Fz3 in sensory epithelial cells and polarized renal epithelial cells, Fz3 may play a similar role in governing PCP in renal tubules.
In summary, we show that mechanical strain induced by ureteral obstruction stimulates epithelial proliferation, and ureteral obstruction can lead to aberrant PCP. Increased proliferation and abnormal PCP signaling may contribute to cyst-like tubular dilation in concert. While proliferation increases the number of cells prerequisite for cyst formation, aberrant PCP results in misorientated cell lining, leading to an increased tubular diameter. However, unlike rodent models of PKD in which genetic abnormalities in PCP signaling pathway have been reported, C57BL/6 mice have no known genetic defects in PCP signaling. The orientation of cell division can return to normal once obstruction is relieved. Despite restoration of PCP signaling after RUUO, cell proliferation occurs during tubular repair. This result also suggests that aberrant PCP may not be the primary stimulus for epithelial proliferation after ureteral obstruction. Our results, together with a recent report of doubling in cilium length after UUO but normalization of the ciliary length after RUUO (42), suggest that normal ciliary signaling and the PCP pathway may play an important role in restoring tubular size. Although renal repair can occur after RUUO, structural and functional recovery are incomplete (5). Prolonged obstruction leads to renal fibrosis. Our study further strengthens the importance of prompt relief of urinary tract obstruction for restoration of tubular structures.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK062839, UT Southwestern O'Brien Kidney Research Center (DK079328), and funding from the Department of Pediatrics at UT Southwestern Medical Center at Dallas.
DISCLOSURES
No conflicts of interest have been declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Peter Igarashi for helpful discussions; Samuel Bradshaw, Rachel Black, and Min Han for technical assistance; and Laurel Johnson for secretarial assistance.
REFERENCES
- 1.Baena-Lopez LA, Baonza A, Garcia-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol 15: 1640–1644, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Benzing T, Walz G. Cilium-generated signaling: a cellular GPS? Curr Opin Nephrol Hypertens 15: 245–249, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Chevalier RL, Thornhill BA, Wolstenholme JT. Renal cellular response to ureteral obstruction: role of maturation and angiotensin II. Am J Physiol Renal Physiol 277: F41–F47, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439: 220–224, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cochrane AL, Kett MM, Samuel CS, Campanale NV, Anderson WP, Hume DA, Little MH, Bertram JF, Ricardo SD. Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol 16: 3623–3630, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Djiane A, Yogev S, Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell 121: 621–631, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Docherty NG, O'Sullivan OE, Healy DA, Fitzpatrick JM, Watson RW. Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Renal Physiol 290: F4–F13, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430: 689–693, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol 68: 37–57, 1982 [PubMed] [Google Scholar]
- 11.Harris TJ, Peifer M. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev Cell 12: 727–738, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiesberger T, Bai Y, Shao X, McNally BT, Sinclair AM, Tian X, Somlo S, Igarashi P. Mutation of hepatocyte nuclear factor-1beta inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest 113: 814–825, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol 13: 2384–2398, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Karner C, Wharton KA, Jr, Carroll TJ. Planar cell polarity and vertebrate organogenesis. Semin Cell Dev Biol 17: 194–203, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA 95: 14552–14557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krasnow RE, Adler PN. A single frizzled protein has a dual function in tissue polarity. Development 120: 1883–1893, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Kurihara H, Anderson JM, Farquhar MG. Diversity among tight junctions in rat kidney: glomerular slit diaphragms and endothelial junctions express only one isoform of the tight junction protein ZO-1. Proc Natl Acad Sci USA 89: 7075–7079, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence PA, Casal J, Struhl G. Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development 129: 2749–2760, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Lawrence PA, Shelton PM. The determination of polarity in the developing insect retina. J Embryol Exp Morphol 33: 471–486, 1975 [PubMed] [Google Scholar]
- 21.Lewis M, Shaw J. Report from the Paediatirc Renal Registry. In: The UK Renal Registry: The Fifth Annual Report, edited by Ansell D, Burend R, Feest T, Newman D, Roderick P, Will E, Williams AJ. London, UK: Renal Association, 2002 [Google Scholar]
- 22.Li L, Truong P, Igarashi P, Lin F. Renal and bone marrow cells fuse after renal ischemic injury. J Am Soc Nephrol 18: 3067–3077, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100: 5286–5291, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masaki T, Foti R, Hill PA, Ikezumi Y, Atkins RC, Nikolic-Paterson DJ. Activation of the ERK pathway precedes tubular proliferation in the obstructed rat kidney. Kidney Int 63: 1256–1264, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rule AD, Lieber MM, Jacobsen SJ. Is benign prostatic hyperplasia a risk factor for chronic renal failure? J Urol 173: 691–696, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Schweisguth F. Temporal regulation of planar cell polarity: insights from the Drosophila eye. Cell 121: 497–499, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet 8: 126–138, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Shao X, Johnson JE, Richardson JA, Hiesberger T, Igarashi P. A minimal Ksp-cadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1824–1836, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int 70: 854–864, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA. Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr Transplant 11: 366–373, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 1–8, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development 130: 4501–4513, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109: 371–381, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5: 367–377, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Verghese E, Weidenfeld R, Bertram JF, Ricardo SD, Deane JA. Renal cilia display length alterations following tubular injury and are present early in epithelial repair. Nephrol Dial Transplant 23: 834–831, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329: 549–551, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Vogel G. News focus: betting on cilia. Science 310: 216–218, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol 5: 1–16, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Weidenfeld R, Verghese E, Ricardo SD, Deane JA. Alterations in renal cilium length during transient complete ureteral obstruction in the mouse. J Anat 213: 79–85, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci 26: 2147–2156, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Thekdi N, Smallwood PM, Macke JP, Nathans J. Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J Neurosci 22: 8563–8573, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Zhang J, Mori S, Nathans J. Axonal growth and guidance defects in Frizzled3 knock-out mice: a comparison of diffusion tensor magnetic resonance imaging, neurofilament staining, and genetically directed cell labeling. J Neurosci 26: 355–364, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong LL, Adler PN. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J Cell Biol 123: 209–221, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]