Abstract
Remorins are plant-specific proteins found associated with plasma membrane microdomains, called lipid rafts. Recently, we have shown that this lipid raft marker also accumulated at plasmodesmata, likely within the plasma membrane lining these structures. Here, we have investigated the gene expression and protein accumulation patterns of remorin at the organ and cell type levels. We show that remorin level is significantly increased in dehiscent, mature and ageing tissues, as well as in source parts of the leaves, where mature branched plasmodesmata are in majority. these results suggest that remorin predominantly associates with mature branched plasmodesmata.
Key words: plasma membrane, lipid rafts, plant protein remorin, plasmodesmata
Introduction
“At the battle of Actium, it is said, a fish of [remora's] kind stopped the prætorian ship of Antonius in its course […]. Hence it was, that the fleet of Cæsar gained the advantage in the onset” wrote Pliny the Elder in his Natural History.1 In ancient times, the remora was believed to stop a ship from sailing. In Latin remora means “delay”, while the genus name Echeneis comes from Greek echein (“to hold”) and naus (“a ship”). When Reymond and colleagues, in the mid 1990s, named the protein they were working on “remorin” (REM), they must have experienced some premonitory inspiration.2 Not only the protein binds to the inner leaflet of the membrane, like the fish to boats bark, but also, as we have recently demonstrated, it influences PVX virus cell-to-cell spreading thereby playing an potentially important role in the battle between the plant and the virus.3 The exact mechanisms of remorin actions are still unknown but data strongly suggest that REM may negatively influence viral movement by directly binding the viral movement Triple gene block 1 protein (TGBp1). This is the first role described for a REM protein, but this is likely not the only one.
The first described REM from potato belongs to a large family of proteins with conserved C-terminal domain.4 Bariola et al. detected REM by immunolocalization in most cell types of aerial tissues and roots of healthy tomato and potato plants,5 suggesting that REM “primary” role in plants is unlikely to be related to viral infection. Interestingly, Bariola et al. also described strong REM accumulation in vascular tissues, particularly around internal phloem, leaves midribs, and in root stele near the root tip.5 As for REM in tomato, its homologs from tobacco, medicago and Arabidopsis are also found enriched in plasma membrane (PM) lipid rafts (reviewed in ref. 3). We demonstrated that lipid raft REM is found in domains of ca. 70 nm diameter in PM preparation in vitro, and associates in patchy PM domains in vivo.3
In good agreement with its role in virus movement, REM was also found to be associated with plasmodesmata, PDs. Plasmodesmata (singular plasmodesma) are microscopic narrow channels that traverse the cell walls enabling transport and communication between plant cells. PDs have been implicated in cell-to-cell gating of various macromolecules including transcription factors, micro RNAs and viral ribo-nucleoproteins, possibly impacting on a wide variety of plant biology processes, for review.6 PDs can either form during cell division, at cytokinesis (primary PDs), or de novo across existing walls (secondary PDs). Primary PDs, abundant in young meristematic tissues, are randomly distributed on the wall surface and are simple rod-shaped conduits made of a central desmotubule derived from endoplasmic reticulum, a cytoplasmic sleeve and surrounded by the PM. As cells elongate and differentiate primary PDs are progressively converted to mature branched structures by the addition of secondary strands.7 These branched PD often have a central cavity,and are usually occurring in clusters in cellulose-depleted regions of the walls called pit fields.
The transition from sink to source mesophyll tissue during leaf development is a well-illustrated example of the PD maturation process. In the sink part of a leaf primary simple PD prevail whereas in the source tissues simple PD are almost absent and instead branched modified PD often grouped into pit fields are the rule.8 These structural changes are correlated with variations in the Size Exclusion Limit (SEL) or the maximum size of a molecule that can pass through PD. Simple PDs in the sink part of tobacco leaves have a very high SEL up to 50 kD. However, during the sink-source transition, the size of the molecules that can transit by diffusion decreases dramatically down. This reduction of PD SEL during tissue maturation is not restricted to the sink to source transition in leaves but rather seems to be a general rule in plant development.6,9,10 Interestingly differences between simple primary and branched PD were also observed in their capacities to interact with viral movement proteins (MPs). Hence, whereas branched PD accumulated viral MPs during leaf infection, simple PD do not.8,11,12 Altogether these data indicate that simple primary and branched mature PD behave differently with respect to their capacities to traffic macromolecules.
The association of REM with one or both types of PDs would certainly bring insights into REM function in relation to PD biogenesis and function. Because of the strong connection that exists between tissue type and age, and PD maturation, measuring REM expression levels in different tissues may indicate a preferential association of the protein with young or branched modified PD. In this addendum, we have investigated REM gene expression and protein accumulation at the organ and cell type level using western blot and Q-RT-PCR. We detected enhanced REM level in dehiscent and aging tissues, as well as in source parts of leaves, sites where mature branched PDs prevail. Although additional evidences will be required to establish a clear correlation, these results raises the possibility that REM predominantly associates with secondary branched PDs. Implications of this finding on hypotheses about REM function are discussed.
Results
Remorin is ubiquitously expressed and accumulates throughout the plant.
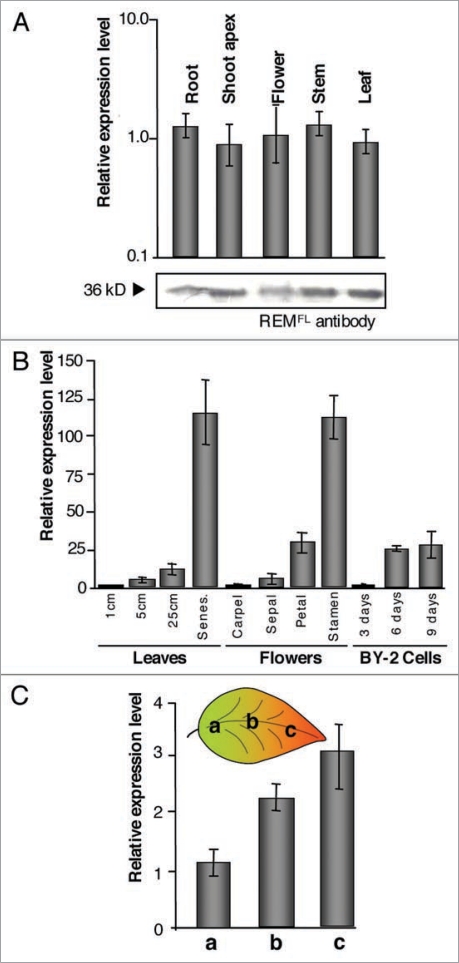
To get a general picture of REM sites of action within the plant, we first monitored NtREM1.2 gene expression and NtREM1.2 protein accumulation, in various organs of tobacco plants. For that, we collected entire organs, namely roots, shoot apices, flowers, stems and leaves, onto which we performed RNA and proteins extractions. RNAs were reverse transcribed and quantified by Q-RT-PCR, normalized to the elongation factor 1α (EF1α) gene expression level. The protein extracts from the different organs were loaded, in equal amount, on SDS-PAGE gel and NtREM1.3 protein accumulation was monitored by western blot analysis. NtREM1.2 appeared to be ubiquitously expressed at a high level (similar to EF1α) in all five organs investigated (Fig. 1A). NtREM1.2 protein was also found to accumulate at high levels in the various tissues, though slightly lower levels were measured in roots and flowers (Fig. 1A, bottom). These results indicate that the lipid raft marker REM is occurring in an ubiquitous manner throughout to the plant.
Figure 1.
REM gene expression and protein accumulation throughout tobacco plant. (A) top: NtREM1.2 gene expression in various plant organs. Bottom: Anti-REM western blot perform on protein extract from the same organs using polyclonal antibodies to REMFL as described in.3 (B) Variations of NtREM1.2 expression with leaves development, nature of flower tissues and BY-2 cells time of culture. (C) Left: REM protein accumulation monitored by western Blot does significantly vary between limb and nerve tissues of the leaf. Right: NtREM1.2 expression increases between sink (a), sink-source transition (b) and source (c) areas of the leaf. Gene expression is monitored by Q-RT-PCR, relative to eF1α gene expression in the same sample. error bars show standard deviation for 9 technical/biological replicates.
Remorin gene expression increases with organ ageing and in dehiscent tissues.
To then get insight into REM function at the organ level, we performed more precise dissections of plant tissues (leaves, flowers) in which we monitored NtREM1.2 gene expression. We noticed a 10-fold increase of NtREM1.2 expression level from very young 1 cm-long leaves to 25 cm-long mature leaves, and even a 100-fold increase in senescing leaves (Fig. 1B). Dissection of flower tissues also revealed dramatic variations in NtREM1.2 expression pattern from green vegetative or reproductive tissues (sepals and carpels) to dehiscent tissues such as petals and especially stamens (Fig. 1B). To confirm the correlation between cell ageing and NtREM1.2 expression level, independently of the nature of the organ, we used BY-2 cell cultures in which we monitored NtREM1.2 expression at different time points during growth; i.e., at the beginning and the end of the exponential phase, and at the plateau of the growth curve. Although less dramatic than in plant tissues, we noticed a 25-fold increase in gene expression levels after six and nine days of culture compared to three days old cultures (Fig. 1B). These results suggest that gene expression increases with cells ageing, and that higher expression occurs in dehiscent tissues of tobacco plant.
Remorin gene expression increases in source parts of the leaves.
Leaves are the usual entry points for most viruses which exploit PD to move from cell-to-cell until they reach the leaf vasculature from which they are then transported along with the photoassimilates (i.e., from source to sink tissues). In Raffaelle et al. we have shown that altered levels of REM influence the cell-to-cell propagation of PVX, a potyvirus, in Tobacco leaves.3 This result prompted us to analyze into more details the variations of expression and accumulation of REM in leaves, especially in relation to the transition between sink and source tissues when PD are known to undergo major structural and morphological modifications. We collected samples from limb of 8 cm long leaves (Fig. 1C). In such leaves, the region near the petiole (a) has been described to correspond to sink tissue,8 whereas the tip of the leaf (c) as turned to source tissue. We also collected samples in the middle of the leaf limb (b) where the transition from sink to source occurs. We measured a two-fold increase in NtREM1.2 expression in the area of the leaf undergoing the sink-source transition compared to sink part of the leaf. The source part of the leaf showed a three times higher expression of NtREM1.2 compare to the sink part.
Discussion
Here we show that the tobacco REM is constitutively, ubiquitously and highly expressed in healthy plant organs. Remarkably, we have consistently observed higher expression levels in older tissues, namely in mature leaves, dehiscent parts of the flower, and in ageing BY-2 cell cultures. In leaves, we have measured an increase of REM expression during the sink to source transition correlating with a developmental stage where PDs are converted from simple structures randomly dispersed on the wall surface to branched ones grouped into pit fields. REM being present both in the PM sricto sensu as well as in the PM lining PD, two hypotheses can be considered regarding REM sites of action during the sink—source transition: (1) REM accumulates in mature branched PDs, (2) REM accumulates in lipid rafts of the PM (outside PD). Although present data do not allow us to distinguish unambiguously between these two hypothesizes, in situ endogenous localisation of REM (by immunofluorescence and immunogold) have already shown that REM is mainly associated with PD when compared with the PM, in young and mature leaves.3 It is therefore tempting to speculate that the observed increase of REM expression during the sink source transition is mainly due to its preferential association with PD, and that the association with the PM sricto sensu remains the same in the different tissues. Our data would then point toward a predominant association of REM with modified branched PD that prevails in mature source parts of the leaves, leading us to consider a possible role of REM in the PD maturation process. This hypothesis is further supported by data from Bariola et al.5 who have shown that the protein was accumulating around fully differentiated vascular tissues where PD are mainly branched. Beside, antheridia were shown to be sites of conversion from simple to branched PD in chara species,13 for review. Consistently, expression in flowers is at his highest in antheridia. As mentioned in the introduction modified branched PD exhibit trafficking properties that are different from the young ones in term of SEL as well as protein targeting. The identification of protein involved into this process would certainly lead to a better understanding of PD function.
The prototype REM from Solanaceae plants is a PM lipid raft protein found in PDs that interferes with PVX virus cell-to-cell spreading presumably by direct binding to the movement protein TGBp1.3 In animal cells, the lipid raft hypothesis proposes that the recruitment and aggregation of rafts is an important driving force for virus spreading.14 As seen in mammalian, plant PM rafts could be used by viruses as platforms for viral cell-to-cell movement through PD. Considering the dynamic nature of lipid rafts, their association with PDs, and with different PD types, may be transient.
The control of SEL as a dynamic process can also be regulated by signals. Expression of virus movement proteins in plants was used to demonstrate their ability to increase PD permeability. The movement protein from TMV is able to increase the SEL in mesophyll cells by ten-fold.15,16 Comparable SEL increase is documented in when the alfalfa mosaic virus movement protein is overexpressed in Nicotiana.17 Similarly, cell-to-cell propagation of the PVX in Nicotiana requires a at least two-fold increase of PDs SEL mediated by the TGBp1 protein.18 A possible effect of restricting PD permeability is consistent with its ability to reduce PVX cell-to-cell spreading. Besides the control of PD SEL, several processes accompany cell maturation and can be correlated in increase in level. Whether other aspects of senescence and dehiscence, such as cell-wall elongation and the induced change in PD morphology, also involve remain to be investigated.
If either plays a role in conversion to mature PD and/or in restricting PD permeability more generally, how can be this function achieved? The options are very diverse considering that, as A.J. Maule wrote in a recent review “it seems unlikely that PD will be less complex than the nuclear pore complex that comprises many proteins for its structure and operation”.10 Until now, approximately thirty proteins were shown to have some association with PDs.10 Building up and functioning of PDs require cytoskeleton elements like F-actin, centrin, kinesin-like proteins, and myosin VIII that may constitute a motor for opening/dilating/closing of PDs.19 Energy to activate such motor could be provided by PD ATPases20 and regulated through calcium signaling involving calreticulin and PD specific kinases. This likely involves connections with the ER forming the desmotubule. PD functioning also relies on cell wall modifications involving notably callose synthesis, β-1,3-Glucanase, pectin methylesterase, for review see.10 Finally particular PM domains are defined within PDs that share properties with lipid rafts3 and contain and a GPI-anchored callose-binding protein21 as well as a PD-specific type I membrane receptor like protein named PDLP1.22
Although the number of actors is increasing, little is known of the maturation of PD and branching. Morphological aspects of this process have been proposed by Faulkner et al.7 that imply significant changes in all the structural components of PDs. Considering the known localization of in the inner leaflet of the PM, it is more likely to mediate connection between the PM of PDs and either element transiting through the cytoplasmic sleeve or the desmotubule and its associated proteins that could include actin and myosins. This disposition argues toward a possible role of in a cytoskeleton/desmotubule dependant reduction of the permeability of the PD cytoplasmic sleeve. Such hypothesis would be consistent with reduction of virus spreading in overexpressing plants and predominant association with secondary, less permeable, PDs. Examination of the SEL of tomato transgenic plant would provide valuable information regarding this hypothesis. Many unknowns remain before we can picture the mechanisms involved. The question of the specificity of REM-driven reduced PD permeability toward types of viruses, nature and size of molecules is one of the most urging. Possible dynamics underlying this effect, likely exploiting membrane micro-domains dynamics, may participate in assembling motor complexes for permeability regulation or alternatively sensing or receptor complexes regulating selectivity of PD conductance for instance.
Across evolution, primary PD were reported in some charophyceans that also generate a phragmoplast at cytokinesis but not in zygnematalean taxa which only exhibit a rudimental phragmoplast, by contrast secondary PD formation seems to be specific of higher plants.23 Interestingly REM belongs to a vast family of proteins that seems to have arisen with land colonization and therefore be restricted to higher plants.4 This correlation may suggest the evolution of a dedicated set of proteins related to cell-to-cell communication in higher plants. Future investigations on other members of the family will be required to test if this property can be generalized or if diversification allowed these proteins to encompass a wider variety of functions.
Material and Methods
Plant material, protein extraction and western blot.
Nicotiana tabacum cv Xanthi were grown for ten weeks in a growth chamber at 25°C under 16/8 hours day/night conditions for RNA and protein sampling. Eight-week old plants were used for agroinfiltration and confocal microscopy. Procedures for these experiments are as described in.3,4 Anti-REMFL antibodies were used for western blot detection. N. tabacum cv Bright Yellow-2 suspension cultured cells were grown in a modified Murashige and Skoog medium as described in.24
Cloning of NtREM1.2.
The full length cDNA of NtREM1.2 was cloned in pDONR221 vector from tobacco cDNAs using the primers 5′-AAA AAA GCA GGC TTA ATG GCA GAA GTA GAA GTT AAG-3′ and 5′-TTT GTA CAA GAA AGC TGG GTA TCA AAA ACA TCC AAG GAG TTT CTT-3′ that were design based on the sequence of EST KT7C.104L22 (Accession number EB449751).
Measure of gene expression by Q-RT-PCR.
Plant tissues for RNA extraction were grinded in liquid nitrogen and powder sampled as 500 mL samples. RNAs were extracted from plant tissues using RNeasy Plant mini kit (Qiagen). Purified RNA was treated with DNase I using the DNA-free kit (Ambion, Austin, TX) according to manufacturers' instructions. RNAs were quantified by spectrophotometry and checked for DNA contamination on agarose gel and by PCR of eF1α gene. cDNAs were synthesized from 200 ng total RNA using Quiagen Superscript II Reverse transcriptase. 2 uL of 1/10th diluted cDNAs were used as a template for Q-RT-PCR performed on 96-well plates with SYBR Green Master Mix kit (Bio-Rad) on a iCycler™ (Bio-Rad, Hercules, CA). Decimal dilution series of plasmids containing either eF1α or NtREM1.2 genes were included with every reaction to serve as standards. All reactions were performed as three technical replicates. Three RNA extractions were performed on three sets of plant dissections. Values shown are standard deviations for the nine technical/biological replicates. Primers for eF1α are described in,25 primers for NtREM1.2 are 5′-GGA GAG TGA GAA AGT TGT GG-3′ and 5′-CTG CTG CTT TAT CTT CGA CC-3′.
Acknowledgements
We gratefully acknowledge the French Agence Nationale de Recherche, contract ANR-05-°C°C-0082-01 for financial support for this project.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9661
References
- 1.Book VIII–XI Zoology in Naturalis Historia encyclopedia. Pliny the Elder; pp. 77–79. ca. [Google Scholar]
- 2.Reymond P, Kunz B, Paul-Pletzer K, Grimm R, Eckerskorn C, Farmer EE. Cloning of a cDNA encoding a plasma membrane-associated, uronide binding phosphoprotein with physical properties similar to viral movement proteins. Plant Cell. 1996;8:2265–2276. doi: 10.1105/tpc.8.12.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raffaele S, Bayer E, Lafarge D, Cluzet S, German Retana S, Boubekeur T, et al. Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs potato virus X movement. Plant Cell. 2009;21:1541–1555. doi: 10.1105/tpc.108.064279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raffaele S, Mongrand S, Gamas P, Niebel A, Ott T. Genome-wide annotation of remorins, a plant-specific protein family: Evolutionary and functional perspectives. Plant Physiol. 2007;145:593–600. doi: 10.1104/pp.107.108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bariola PA, Retelska D, Stasiak A, Kammerer RA, Fleming A, Hijri M, et al. Remorins form a novel family of coiled coil-forming oligomeric and filamentous proteins associated with apical, vascular and embryonic tissues in plants. Plant Mol Biol. 2004;55:579–594. doi: 10.1007/s11103-004-1520-4. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Medrano R, Xoconostle-Cazares B, Kragler F. The plasmodesmatal transport pathway for homeotic proteins, silencing signals and viruses. Curr Opin Plant Biol. 2004;7:641–650. doi: 10.1016/j.pbi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Faulkner C, Akman OE, Bell K, Jeffree C, Oparka K. Peeking into pit fields: a multiple twinning model of secondary plasmodesmata formation in tobacco. Plant Cell. 2008;20:1504–1518. doi: 10.1105/tpc.107.056903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts IM, Boevink P, Roberts AG, Sauer N, Reichel C, Oparka KJ. Dynamic changes in the frequency and architecture of plasmodesmata during the sink-source transition in tobacco leaves. Protoplasma. 2001;218:31–44. doi: 10.1007/BF01288358. [DOI] [PubMed] [Google Scholar]
- 9.Kim I, Kobayashi K, Cho E, Zambryski PC. Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis. Proc Natl Acad Sci USA. 2005;102:11945–11950. doi: 10.1073/pnas.0505622102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maule AJ. Plasmodesmata: structure, function and biogenesis. Curr Opin Plant Biol. 2008;11:680–686. doi: 10.1016/j.pbi.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Ding B, Haudenshield JS, Hull RJ, Wolf S, Beachy RN, Lucas WJ. Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell. 1992;4:915–928. doi: 10.1105/tpc.4.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itaya A, Woo YM, Masuta C, Bao Y, Nelson RS, Ding B. Developmental regulation of intercellular protein trafficking through plasmodesmata in tobacco leaf epidermis. Plant Physiol. 1998;118:373–385. doi: 10.1104/pp.118.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwiatkowska M. Plasmodesmal changes are related to different developmental stages of antheridia of Chara species. Protoplasma. 2003;222:1–11. doi: 10.1007/s00709-003-0001-y. [DOI] [PubMed] [Google Scholar]
- 14.Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta. 2005;1746:234–251. doi: 10.1016/j.bbamcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Wolf S, Deom CM, Beachy RN, Lucas WJ. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science. 1989;246:377–379. doi: 10.1126/science.246.4928.377. [DOI] [PubMed] [Google Scholar]
- 16.Waigmann E, Lucas WJ, Citovsky V, Zambryski P. Direct functional assay for tobacco mosaic virus cell-to-cell movement protein and identification of a domain involved in increasing plasmodesmal permeability. Proc Natl Acad Sci USA. 1994;91:1433–1437. doi: 10.1073/pnas.91.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poirson A, Turner AP, Giovane C, Berna A, Roberts K, Godefroy-Colburn T. Effect of the alfalfa mosaic virus movement protein expressed in transgenic plants on the permeability of plasmodesmata. J Gen Virol. 1993;74:2459–2461. doi: 10.1099/0022-1317-74-11-2459. [DOI] [PubMed] [Google Scholar]
- 18.Angell SM, Davies C, Baulcombe DC. Cell-to-cell movement of potato virus X is associated with a change in the size-exclusion limit of plasmodesmata in trichome cells of Nicotiana clevelandii. Virology. 1996;216:197–201. doi: 10.1006/viro.1996.0046. [DOI] [PubMed] [Google Scholar]
- 19.Baluska F, Cvrcková F, Kendrick-Jones J, Volkmann D. Sink plasmodesmata as gateways for phloem unloading. Myosin VIII and calreticulin as molecular determinants of sink strength? Plant Physiol. 2001;126:39–46. doi: 10.1104/pp.126.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleland RE, Fujiwara T, Lucas W. Plasmodesmal-mediated cell-to-cell transport in wheat roots is modulated by anaerobic stress. Protoplasma. 1994;178:81–85. doi: 10.1007/BF01404123. [DOI] [PubMed] [Google Scholar]
- 21.Simpson C, Thomas C, Findlay K, Bayer E, Maule AJ. An Arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell. 2009;21:581–594. doi: 10.1105/tpc.108.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas CL, Bayer EM, Ritzenthaler C, Fernandez-Calvino L, Maule AJ. Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol. 2008;6:180–190. doi: 10.1371/journal.pbio.0060007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham LE, Cook ME, Busse JS. The origin of plants: body plan changes contributing to a major evolutionary radiation. Proc Natl Acad Sci USA. 2000;97:4535–4540. doi: 10.1073/pnas.97.9.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couchy I, Bolte S, Crosnier MT, Brown S, Satiat-Jeunemaitre BJ. Identification and localization of a beta-COP-like protein involved in the morphodynamics of the plant Golgi apparatus. J Exp Bot. 2003;54:2053–2063. doi: 10.1093/jxb/erg230. [DOI] [PubMed] [Google Scholar]
- 25.Lochman J, Mikes V. Ergosterol treatment leads to the expression of a specific set of defence-related genes in tobacco. Plant Mol Biol. 2006;62:43–51. doi: 10.1007/s11103-006-9002-5. [DOI] [PubMed] [Google Scholar]