Abstract
Nitric oxide metabolism in plant cells has a relative short history. Nitration is a chemical process which consists of introducing a nitro group (-NO2) into a chemical compound. in biological systems, this process has been found in different molecules such as proteins, lipids and nucleic acids that can affect its function. This mini-review offers an overview of this process with special emphasis on protein tyrosine nitration in plants and its involvement in the process of nitrosative stress.
Keywords: nitric oxide, nitrosative stress, 5-nitro-γ-tocopherol nitro-tyrosine, nitrolipids, peroxynitrite, post-translational modifications
Introduction
Nitric oxide (NO) is a gas that in animals participates in a broad spectrum of functions in the cardiovascular, immune and nervous systems.1,2 In higher plants, NO plays pivotal roles in controlling physiological function during plant growth and development, including seed germination, primary and lateral root growth, flowering, pollen-tube growth regulation, fruit ripening, senescence, defence response and abiotic stress, as well as being a key signaling molecule in different intracellular processes.3–8 Nitric oxide is a radical molecule, given that it has an unpaired electron in its π orbital, and this characteristic gives NO special properties. Nitric oxide can react with different macromolecules (proteins, lipids, nucleic acids, etc.) and diffuse through cell membranes. The term reactive nitrogen species (RNS) has also been introduced in the biological literature to designate nitric oxide and other NO-related molecules, such as S-nitrosothiols (RSNOs), peroxynitrite (ONOO-), dinitrogen trioxide (N2O3) and nitrogen dioxide (NO2) among others, which have relevant roles in multiple physiological processes of animal and plant cells.6,9,10 These molecules directly or indirectly are involved in post-translational modifications in cell signaling under physiological and pathological conditions including binding to metal centres, S-nitrosylation of thiol groups and nitration of tyrosine.11
Among these modifications, S-nitrosylation is the most studied in plants.12 It consists of the binding of a NO group to a cysteine residue of a protein and can alter their function.13 In animal organisms, the S-nitrosylation process is involved in a certain number of patho-physiological situations which can contribute to the generation of nitrosative stress.14 However, in plant cells much less information is available on this post-translational modification. By proteomics approaches, some putative protein targets for S-nitrosylation in plants have been identified, including cytoskeleton, metabolic, redox-related, stress-related and signaling/regulating proteins.15 For instance, in crude extracts of Arabidopsis cell cultures, glyceraldehyde-3-phosphate dehydrogenase undergoes a reversible inhibition by NO15 and similar behaviour has been described for the methionine adenosyltransferase 1.16 On the other hand, Arabidopsis nonsymbiotic hemoglobin (AHb1) scavenges NO with the production of S-nitrosohemoglobin and reduces NO emission under hypoxic stress.17 Another example is the Arabidopsis type-II metacaspase AtMC9, which blocks the autoprocessing and activation of AtMC9 zymogen through S-nitrosylation of its catalytic cysteine residue.18 It has been also shown that the S-nitrosylation in the conserved Cys53 of the transcription factor AtMYB2 inhibits its binding to DNA.19 More recently, it has been demonstrated that S-nitrosylation of Arabidopsis thaliana salicylic acid-binding protein 3 (AtSABP3) at cysteine 280, during the establishment of plant disease resistance against a virulent Pseudomonas syringae pv. tomato strain DC3000 (avrB), suppresses both the binding of the immune activator, salicylic acid (SA) and the carbonic anhydrase (CA) activity of this protein, indicating that S-nitrosylation participates in the mechanism of plant-defence response.20 Thus, this review will focus on plant nitration, which is the other post-translational mediates by RNS, with special emphasis in protein nitration.
Protein Nitration
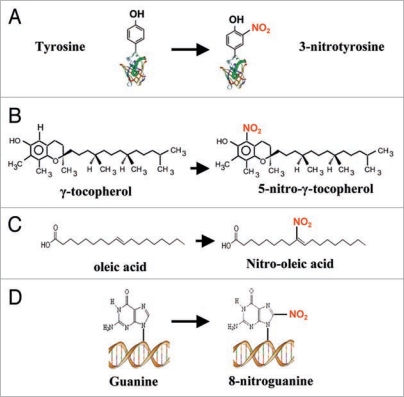
Nitration is a general chemical process for the introduction of a nitro group (-NO2) into a chemical compound. In the case of proteins, there are several amino acids which are preferentially nitrated, such as tyrosine (Y), tryptophan (W), cysteine (C) and methionine (M). However, most studies concern tyrosine nitration, which consists of the addition of a nitro group to one of the two equivalent ortho-carbons of the aromatic ring of tyrosine residues (Fig. 1A). This addition changes tyrosine into a negatively charged hydrophilic nitrotyrosine moiety and causes a marked shift of the local pKa of the hydroxyl group from 10.07 in tyrosine to 7.50 in nitrotyrosine.21 Tyrosine nitration is considered to be a selective process, and proteins usually have approximately 3 to 4 mol% of Tyr but only one or two of these tyrosines may become preferentially nitrated, this depending on several factors, such as protein structure, nitration mechanism and environment, where the protein is located.22 At present, tyrosine nitration has been shown to be capable of changing the function of a protein in several ways: (1) gain of function as well as no effect on function; and (2) inhibition of function being a much more common consequence of protein tyrosine nitration.23 However, in other cases, it has been reported that nitration of a tyrosine residue may either prevent further phosphorylation or stimulate phosphorylation.24,25
Figure 1.
Schematic reaction of nitration in different biomolecules. (A) Protein tyrosine nitration. (B) Nitration of γ-tocopherol. (C) Nitration of oleic acid. (D) Nitration of guanine.
Protein Tyrosine Nitration as a Marker of Nitrosative Stress
In mammals, protein tyrosine nitration has been detected in many tissues under normal physiological conditions26 and some of the nitrated proteins have been identified.27 Therefore, the available information indicates that low levels of tyrosine nitration may be a physiological regulator of a signaling pathway. On the other hand, tyrosine nitration is being intensively studied because it can be used as a marker of certain pathologies and nitrosative stress.21,22,28–32 Until now, several proteomic studies have identified a relatively low number of nitrated proteins. Thus, a proteomic approach together with the use of a monoclonal antibody against nitrotyrosine has identified about 40 different proteins during inflammatory challenge in rat lung and liver. The proteins identified were involved in different functions, such as oxidative stress, apoptosis, ATP production and fatty acid metabolism.33 More recently, also with the use of antibody against nitrotyrosine, another proteomic identification of tyrosine nitration targets in kidney of hypertensive rats revealed the existence of 22 differentially nitrated proteins.34 However, only catalase and glyceraldehydes-3-phosphate dehydrogenase were coincident targets in the three rat organs analysed. In any case, new studies are starting to point out the possible involvement of tyrosine nitration in signaling pathways mediated by NO.35,36
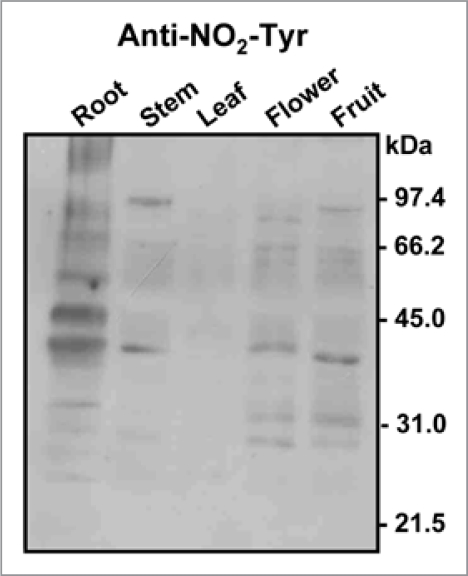
In plants, much less information is available on protein nitration under normal conditions, although previous data indicate the existence of a basal nitration present in the plant tissues analyzed. Figure 2 shows a representative immunoblot probed with an antibody against nitrotyrosine of different organs of healthy pea plants; it is possible to appreciate that the nitrated protein profiles are different (Corpas et al. unpublished results).
Figure 2.
Representative immunoblot showing the pattern of protein tyrosine nitration (NO2-Tyr) in different organs (root, stem, leaf, flower and fruit) of pea plants after 71 days of growth under optimal conditions. The numbers on the right side of the immunoblots indicate the relative molecular masses of the protein markers.
On the other hand, there are published data describing quantitative and qualitative changes in the profile of nitrated proteins under biotic and abiotic conditions. Some examples are: (1) in nitrite reductase antisense tobacco leaves, the induction of several tyrosine-nitrated polypeptides with molecular masses between 10- and 50-kDa has been reported;37 (2) In tobacco BY-2 suspension cells treated with a fungal elicitin, the induction of tyrosine nitration in proteins with molecular masses in the range 20–50 kDa has been also demonstrated;38 (3) In olive plants under salt stress (200 mM), a significant increase of the L-arginine-dependent NOS activity, total S-nitrosothiols (RSNO) and number of proteins that underwent tyrosine nitration in the molecular-mass range 44–60 kDa has been also reported;39 (4) In pea plants exposed to different types of environmental stress, the profile of nitrated proteins showed a significant intensification of immunoreactive bands in the range 29–59 kDa with low temperature, high temperature, high light intensity and continuous light;40 and (5) In sunflower, the infection by the pathogen Plasmopara halstedii induces an increase in proteins that undergo tyrosine nitration accompanied by a rise in RSNOs.41 All these data indicate that a boost in the number of proteins or an intensification of specific proteins resulting from tyrosine nitration could be considered as an indicator of nitrosative stress in plants,42 as has been demonstrated in animal cells. However, no nitrated proteins in plant cells are yet known.
Nitration of Other Biological Macromolecules: Vitamin E, Lipids and Nucleic Acids
The nitration process can affect the functions of other biomolecules, including vitamin E, lipids and nucleic acid. Vitamin E occurs in nature in 8 structurally related forms, including 4 tocopherols (α, β, γ and δ) and 4 tocotrienols (α, β, γ- and δ). Whereas α-tocopherol is present mainly in green tissues, γ-tocopherol is often the most prevalent form of vitamin E in plant seeds43,44 and in products derived from them, which are used for human nutrition. As a result, γ-tocopherol is the major lipid-soluble dietary antioxidant. It has been shown that γ-tocopherol can be nitrated to form 5-nitro-γ-tocopherol (Fig. 1B). In humans, the concentration of this compound rises in the plasma of people with coronary heart disease45 and in the brain of Alzheimer patients.46 In plants it has been proposed that γ-tocopherol exerts its influence on seedling development by controlling the content of nitric oxide (NO) in germinating seeds.47 More recently, Desel et al. (2007) have shown the presence of 5-nitro-γ-tocopherol,48 which it can act as scavenger of RNS when overproduced.49
Fatty acid nitration is a newly discovered mechanism that generates biologically active nitrolipids possibly involved in signaling or pathological processes.50,51 Thus, it has been demonstrated that nitrated membranes and lipids from lipoproteins can transduce NO-signaling reactions and mediate regulatory pathways for anti-inflammatory processes.52,53 For example, nitroalkene isomer derivatives of linoleic acid (LNO2) and oleic acid (OA-NO2) (Fig. 1C) have been detected in human blood. They have the capacity to regulate gene expression and PPAR (peroxisome proliferator-activated receptor) activation due to their electrophilic reactivity.32,54 Alternatively, nitroalkene can react with glutathione (GSH) to form GSH-nitroalkene adducts which represent an important mechanism in the regulation of cellullar response. More recently, in vivo and in vitro studies revealed that nitroalkenes serve as protective mediators in rat lungs by inducing the cytoprotective enzyme heme oxygenase-1.55
In addition, nucleotides within DNA and RNA can undergo nitration by various RNS (Fig. 1D). Thus, 8-nitroguanine has been found to act as a pro-oxidant to stimulate superoxide generation by NADPH cytochrome P450 reductase and nitric oxide synthases. Once formed in cells, 8-nitroguanine may impart pathophysiological consequences due to its mutagenic and prooxidant properties, as well as serving as a footprint of biological nitration.56–59
Unfortunately, research in nitrolipids or 8-nitroguanine is in an early stage of investigation and there is virtually no information available regarding these two nitration processes in plant systems, this being a new area of RNS metabolism that needs to be explored.
Conclusions and Perspective
In plant systems, the nitration process is a new area of research and the efforts should be focused on identifying and quantifying specific targets during natural plant development and under environmental conditions. Thus, protein targets of tyrosine nitration it could be expected to change among plant species, organs, developmental stages and environmental conditions. Moreover, a rise in the nitration process under stress conditions has been considered a potential marker of nitrosative stress. However, accumulated evidence indicates that under physiological conditions nitration is involved in signaling and acts as a regulatory mechanism. Therefore, the study of the function and metabolism of nitrated bio-molecules in plants is a new area of research in the metabolism of nitric oxide.
Acknowledgements
This work was supported by grants from the Ministry of Education and Science (BIO2006-14949-C02-01 and BIO200614949-C02-02) and Junta de Andalucía (groups BIO 192 and BIO 286).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9466
References
- 1.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R Soc Med. 1999;92:164–169. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez-Ruiz A, Lamas S. Two decades of new concepts in nitric oxide signaling: from the discovery of a gas messenger to the mediation of nonenzymatic posttranslational modifications. IUBMB Life. 2009;1:91–98. doi: 10.1002/iub.144. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AD. Nitric oxide signaling in plants. Vitam Horm. 2005;72:339–398. doi: 10.1016/S0083-6729(05)72010-0. [DOI] [PubMed] [Google Scholar]
- 4.Corpas FJ, Barroso JB, Carreras A, Quirós M, León AM, Romero-Puertas MC, et al. Cellular and subcellular localization of endogenous nitric oxide in young and senescent pea plants. Plant Physiol. 2004;136:2722–2733. doi: 10.1104/pp.104.042812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corpas FJ, Barroso JB, Carreras A, Valderrama R, Palma JM, León AM, et al. Constitutive argininedependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta. 2006;224:246–254. doi: 10.1007/s00425-005-0205-9. [DOI] [PubMed] [Google Scholar]
- 6.Corpas FJ, Carreras A, Valderrama R, Chaki M, Palma JM, del Río LA, et al. Reactive nitrogen species and nitrosative stress in plants. Plant Stress. 2007;1:37–41. [Google Scholar]
- 7.Lamattina L, García-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- 8.Neill S, Bright J, Desikan R, Hancock J, Harrison J, Wilson I. Nitric oxide evolution and perception. J Exp Bot. 2008;59:25–35. doi: 10.1093/jxb/erm218. [DOI] [PubMed] [Google Scholar]
- 9.Durzan DJ, Pedroso MC. Nitric oxide and reactive nitrogen oxide species in plants. Biotechnol Genet Eng Rev. 2002;19:293–337. doi: 10.1080/02648725.2002.10648032. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th edn. Oxford: Oxford University Press; 2007. [Google Scholar]
- 11.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004;287:262–268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 12.Corpas FJ, del Río LA, Barroso JB. Post-translational modifications mediated by reactive nitrogen species: nitrosative stress responses or components of signal transduction pathways? Plant Signal Behav. 2008;3:301–303. doi: 10.4161/psb.3.5.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun J, Steenbergen C, Murphy E. S-Nitrosylation: NO related redox signaling to protect against oxidative stress. Antioxid Redox Signal. 2006;8:1693–1705. doi: 10.1089/ars.2006.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benhar M, Forrester MT, Stamler JS. Nitrosative stress in the ER: a new role for S-nitrosylation in neurodegenerative diseases. ACS Chem Biol. 2006;1:355–358. doi: 10.1021/cb600244c. [DOI] [PubMed] [Google Scholar]
- 15.Lindermayr C, Saalbach G, Durner J. Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol. 2005;137:921–930. doi: 10.1104/pp.104.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindermayr C, Saalbach G, Bahnweg G, Durner J. Differential inhibition of Arabidopsis methionine adenosyltransferases by protein S-nitrosylation. J Biol Chem. 2006;281:4285–4291. doi: 10.1074/jbc.M511635200. [DOI] [PubMed] [Google Scholar]
- 17.Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M, et al. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell. 2004;16:2785–2794. doi: 10.1105/tpc.104.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belenghi B, Romero-Puertas MC, Vercammen D, Brackenier A, Inzé D, Delledonne M, Van Breusegem F. Metacaspase activity of Arabidopsis thaliana is regulated by S-nitrosylation of a critical cysteine residue. J Biol Chem. 2007;282:1352–1358. doi: 10.1074/jbc.M608931200. [DOI] [PubMed] [Google Scholar]
- 19.Serpa V, Vernal J, Lamattina L, Grotewold E, Cassia R, Terenzi H. Inhibition of AtMYB2 DNA-binding by nitric oxide involves cysteine S-nitrosylation. Bochem Biophys Res Commun. 2007;361:1048–1053. doi: 10.1016/j.bbrc.2007.07.133. [DOI] [PubMed] [Google Scholar]
- 20.Wang YQ, Feechan A, Yun BW, Shafiei R, Hofmann A, Taylor P, et al. S-nitrosylation of AtSABP3 antagonizes the expression of plant immunity. J Biol Chem. 2009;284:2131–2137. doi: 10.1074/jbc.M806782200. [DOI] [PubMed] [Google Scholar]
- 21.Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54:619–634. doi: 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- 22.Bartesaghi S, Ferrer-Sueta G, Peluffo G, Valez V, Zhang H, Kalyanaraman B, et al. Protein tyrosine nitration in hydrophilic and hydrophobic environments. Amino Acids. 2007;32:501–515. doi: 10.1007/s00726-006-0425-8. [DOI] [PubMed] [Google Scholar]
- 23.Radi R. Nitric oxide, oxidants and protein tyrosine nitration. Proc Natl Acad Sci USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi W-Q, Cai H, Xu D-D, Su X-Y, Lei P, Zhao Y-F, et al. Tyrosine phosphorylation/dephosphorylation regulates peroxynitrite-mediated peptide nitration. Regul Pept. 2007;144:1–5. doi: 10.1016/j.regpep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Rayala SK, Martin E, Sharina IG, Molli PR, Wang X, Jacobson R, et al. Dynamic interplay between nitration and phosphorylation of tubulin cofactor B in the control of microtubule dynamics. Proc Natl Acad Sci USA. 2007;104:19470–19475. doi: 10.1073/pnas.0705149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenacre SA, Ischiropoulos H. Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radical Res. 2001;34:541–581. doi: 10.1080/10715760100300471. [DOI] [PubMed] [Google Scholar]
- 27.Souza JM, Peluffo G, Radi R. Protein tyrosine nitration—functional alteration or just a biomarker? Free Radic Biol Med. 2008;45:357–366. doi: 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Inoue H, Hisamatsu K, Ando K, Ajisaka R, Kumagai N. Determination of nitrotyrosine and related compounds in biological specimens by competitive enzyme immunoassay. Nitric Oxide. 2002;7:11–17. doi: 10.1016/s1089-8603(02)00005-8. [DOI] [PubMed] [Google Scholar]
- 29.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003;305:776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 30.Schopfer FJ, Baker PR, Freeman BA. NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response? Trends Biochem Sci. 2003;28:646–654. doi: 10.1016/j.tibs.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Tsikas D, Mitschke A, Suchy MT, Gutzki FM, Stichtenoth DO. Determination of 3-nitrotyrosine in human urine at the basal state by gas chromatography- tandem mass spectrometry and evaluation of the excretion after oral intake. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:146–156. doi: 10.1016/j.jchromb.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 32.Rubbo H, Radi R. Protein and lipid nitration: role in redox signaling and injury. Biochim Biophys Acta. 2008;1780:1318–1324. doi: 10.1016/j.bbagen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Aulak KS, Miyagi M, Yan L, West KA, Massillon D, Crabb JW, et al. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc Natl Acad Sci USA. 2001;98:12056–12061. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyther R, Ahmeda A, Johns E, Sheehan D. Proteomic identification of tyrosine nitration targets in kidney of spontaneously hypertensive rats. Proteomics. 2007;7:4555–4564. doi: 10.1002/pmic.200700503. [DOI] [PubMed] [Google Scholar]
- 35.Tedeschi G, Cappelletti G, Nonnis S, Taverna F, Negri A, Ronchi C, et al. Tyrosine nitration is a novel post-translational modification occurring on the neural intermediate filament protein peripherin. Neurochem Res. 2007;32:433–441. doi: 10.1007/s11064-006-9244-2. [DOI] [PubMed] [Google Scholar]
- 36.Ischiropoulos H. Protein tyrosine nitration—an update. Arch Biochem Biophys. 2008;484:117–121. doi: 10.1016/j.abb.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 37.Morot-Gaudry-Talarmain Y, Rockel P, Moureaux T, Quillere I, Leydecker MT, Kaiser WM, et al. Nitrite accumulation and nitric oxide emission in relation to cellular signaling in nitrite reductase antisense tobacco. Planta. 2002;215:708–715. doi: 10.1007/s00425-002-0816-3. [DOI] [PubMed] [Google Scholar]
- 38.Saito S, Yamamoto-Katou A, Yoshioka H, Doke N, Kawakita K. Peroxynitrite generation and tyrosine nitration in defense responses in tobacco BY-2 cells. Plant Cell Physiol. 2006;47:689–697. doi: 10.1093/pcp/pcj038. [DOI] [PubMed] [Google Scholar]
- 39.Valderrama R, Corpas FJ, Carreras A, Fernández-Ocaña A, Chaki M, Luque F, et al. Nitrosative stress in plants. FEBS Letts. 2007;581:453–461. doi: 10.1016/j.febslet.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Corpas FJ, Chaki M, Fernández-Ocaña A, Valderrama R, Palma JM, Carreras A, et al. Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol. 2008;49:1711–1722. doi: 10.1093/pcp/pcn144. [DOI] [PubMed] [Google Scholar]
- 41.Chaki M, Fernández-Ocaña AM, Valderrama R, Carreras A, Esteban FJ, Luque F, et al. Involvement of reactive nitrogen and oxygen species (RNS and ROS) in sunflower-mildew interaction. Plant Cell Physiol. 2009;50:265–279. doi: 10.1093/pcp/pcn196. [DOI] [PubMed] [Google Scholar]
- 42.Corpas FJ, del Río LA, Barroso JB. Need of biomarkers of nitrosative stress in plants. Trends Plant Sci. 2007;12:436–438. doi: 10.1016/j.tplants.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Munné-Bosch S, Alegre L. The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci. 2002;21:31–57. [Google Scholar]
- 44.Szymanska R, Kruk J. Gamma-tocopherol dominates in young leaves of runner bean (Phaseolus coccineus) under a variety of growing conditions: the possible functions of gamma-tocopherol. Phytochemistry. 2008;69:2142–2148. doi: 10.1016/j.phytochem.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Morton LW, Ward NC, Croft KD, Puddey IB. Evidence for the nitration of gamma-tocopherol in vivo: 5-nitro-gamma-tocopherol is elevated in the plasma of subjects with coronary heart disease. Biochem J. 2002;364:625–628. doi: 10.1042/BJ20020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williamson KS, Gabbita SP, Mou S, West M, Pye QN, Markesbery WR, et al. The nitration product 5-nitro-gamma-tocopherol is increased in the Alzheimer brain. Nitric Oxide. 2002;6:221–227. doi: 10.1006/niox.2001.0399. [DOI] [PubMed] [Google Scholar]
- 47.Desel C, Krupinska K. The impact of tocochromanols on early seedling development and NO release. J Plant Physiol. 2005;162:771–776. doi: 10.1016/j.jplph.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Desel C, Hubbermann EM, Schwarz K, Krupinska K. Nitration of gamma-tocopherol in plant tissues. Planta. 2007;226:1311–1322. doi: 10.1007/s00425-007-0552-9. [DOI] [PubMed] [Google Scholar]
- 49.Abbasi AR, Hajirezaei M, Hofius D, Sonnewald U, Voll LM. Specific roles of alpha- and gamma-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007;143:1720–1738. doi: 10.1104/pp.106.094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trostchansky A, Rubbo H. Nitrated fatty acids: mechanisms of formation, chemical characterization and biological properties. Free Radic Biol Med. 2008;44:1887–1896. doi: 10.1016/j.freeradbiomed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Lima ES, Bonini MG, Augusto O, Barbeiro HV, Souza HP, Abdalla DS. Nitrated lipids decompose to nitric oxide and lipid radicals and cause vasorelaxation. Free Radic Biol Med. 2005;39:532–539. doi: 10.1016/j.freeradbiomed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, et al. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jain K, Siddam A, Marathi A, Roy U, Falck JR, Balazy M. The mechanism of oleic acid nitration by ·NO2. Free Radic Biol Med. 2008;45:269–283. doi: 10.1016/j.freeradbiomed.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Iles KE, Wright MM, Cole MP, Welty NE, Ware LB, Matthay MA, et al. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid mediates protective effects through regulation of the ERK pathway. Free Radic Biol Med. 2009;46:866–875. doi: 10.1016/j.freeradbiomed.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akuta T, Zaki MH, Yoshitake J, Okamoto T, Akaike T. Nitrative stress through formation of 8-nitroguanosine: insights into microbial pathogenesis. Nitric Oxide. 2006;14:101–108. doi: 10.1016/j.niox.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Terasaki Y, Akuta T, Terasaki M, Sawa T, Mori T, Okamoto T, et al. Guanine nitration in idiopathic pulmonary fibrosis and its implication for carcinogenesis. Am J Respir Crit Care Med. 2006;174:665–673. doi: 10.1164/rccm.200510-1580OC. [DOI] [PubMed] [Google Scholar]
- 58.Hoki Y, Murata M, Hiraku Y, Ma N, Matsumine A, Uchida A, et al. 8-Nitroguanine as a potential biomarker for progression of malignant fibrous histiocytoma, a model of inflammation-related cancer. Oncol Rep. 2007;18:1165–1169. [PubMed] [Google Scholar]
- 59.Kaneko K, Akuta T, Sawa T, Kim HW, Fujii S, Okamoto T, et al. Mutagenicity of 8-nitroguanosine, a product of nitrative nucleoside modification by reactive nitrogen oxides, in mammalian cells. Cancer Lett. 2008;262:239–247. doi: 10.1016/j.canlet.2007.12.007. [DOI] [PubMed] [Google Scholar]