Abstract
Volatile emissions from the commercial growth promoting soil bacterium Bacillus subtilis (GB03) are effective in augmenting short-term growth, photosynthetic capacity and salt tolerance in Petri-dish grown arabidopsis seedlings. In contrast, the impact sustained GB03 volatile exposure on plant growth and development has yet to be examined. here is provided physical and physiological data establishing that bacterial volatiles induce long-term growth promotion, elevated photosynthetic capacity and iron accumulation, as well as delayed albeit higher seed count compared with water-treated control plants. Plants were grown unrestricted in double Magenta boxes containing solid MS media for up to twelve weeks with GB03 volatiles introduced in separate containers within the chamber so that plant bacterial interactions were only by air-borne transmission. These results establish that GB03 volatiles induce sustained beneficial effects on Arabidopsis growth including robust and extended vegetative growth followed by elevated seed set.
Key words: Bacillus subtilis (GB03), photosynthetic efficiency, plant growth promoting rhizobacteria (PGPR), seed set, volatile organic compound (VOCs)
Introduction
Plant growth-promoting rhizobacteria (PGPR) are naturally occurring soil microorganisms that colonize roots and stimulate plant growth. Such bacteria are applied to a wide range of agricultural crops for the purpose of growth enhancement,1,2,3 with the mechanism of action an area of ongoing research. PGPR colonization has been proposed to trigger growth by bacterial synthesis of the plant hormones indole-3-acetic acid, cytokinin and gibberellins as well as by increased mineral and nitrogen availability in the soil.4,5,6,7,8 Moreover in the absence of physical contact with plant roots, blends of bacterial volatiles devoid of traditional hormones such as auxin and GA can also trigger growth promotion.9,10,11
Bacillus subtilis GB03, a commercially available soil symbiont, is one such strain that emits a complex blend of volatile components that activates plant growth promotion in Arabidopsis. A bouquet of over 25 bacterial volatile odors has been identified that trigger differential expression of approximately 600 transcripts related to cell wall modifications, primary and secondary metabolism, stress responses, hormone regulation and other expressed proteins.9,12,13 These biologically active bacterial volatiles trigger plant growth promotion by a tissue specific re-distribution of endogenous auxin.13 GB03 volatiles also augment Arabidopsis photosynthetic capacity by increasing photosynthetic efficiency and chlorophyll content.14 Mechanistic studies reveal elevation of endogenous sugar accumulation in the plant as well as suppression of classic glucose signaling responses. Overlap in sugar/ABA sensing is reported for GB03-exposed plants with suppression of ABA-biosynthetic transcripts as well as downstream ABA levels in the leaves.15 With salt stress, GB03 volatiles increase salt tolerance by concurrently down and upregulated expression of the sodium transporter HKT1 in roots and shoots, respectively, resulting in lower Na+ accumulation throughout the plant compared with controls unexposed to GB03 volatiles.16
While utilizing Petri-dish grown Arabidopsis seedlings has proven to be an effective model system to mechanistically probe early growth responses activated by volatiles from beneficial bacteria,13,14,16 an examination of long-term growth promotion by PGPR volatiles requires expanded growth conditions. Here we examine how Arabidopsis development and reproductive success is affected by uninterrupted long-term exposure to GB03 volatiles when plants are grown in unconfined Magenta boxes.
Results
Plant growth promotion with sustained GB03 exposure.
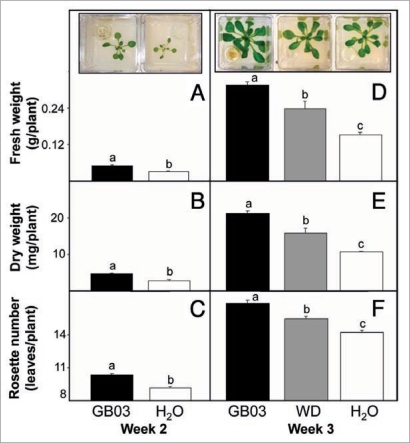
To examine how long-term GB03 exposure affects plant growth, Arabidopsis plants were treated with GB03 volatiles during the full life cycle of the plant. To increase the growing volume from previous reported I-plate Petri-dishes,9 plants were grown in double-sized Magenta boxes containing half-strength MS media with 1.5% sucrose. After two weeks of GB03 exposure plants exhibited a 58 ± 23% and 71 ± 18% increase in fresh and dry weight respectively compared to water controls (Fig. 1A and B, p < 0.05). Rosette leaf number was also greater for GB03-exposed plants compared to water controls at 10 versus 9 leaves, respectively (Fig. 1C, p < 0.05).
Figure 1.
An increase in plant growth with sustained Bacillus subtilis (GB03) exposure (black bar) compared with GB03 withdrawn (WD, grey bar) at week two and water-treated controls (white bar) as determined by fresh weight, dry weight and rosette leave number. Inset shows representative images of plant growth with specific treatments indicated on the x-axis for plant at two and three weeks old. Fresh weight [A], dry weight [B], and rosette leave number [C] for two-week old plants continuous exposed to GB03 volatiles (n = 39) or water controls (n = 25). Fresh weight [D], dry weight [E] and rosette leave number [F] for three-week old plants continuous exposed to GB03 volatiles (n = 28), GB03 withdrawn at week two (n = 15) and water controls (n = 25). Different letters indicate significant differences between treatments (ANOVA, p ≤ 0.05).
To probe whether growth promotion requires a sustained or transient GB03 signal, GB03 cultures contained in vials were or were not withdrawn (WD) after two weeks from Magenta boxes and growth measurements were taken at week three. Plant fresh weight with 2- and 3-week GB03 exposure was 57 ± 28% and 109 ± 16% greater, respectively than with water treatment alone (Fig. 1D, p < 0.05). Dry weight and rosette leaf number showed a similar pattern with abbreviated GB03-exposure time exhibiting intermediate values relative to full GB03 exposure and water controls. The dry weight of short-time exposure to GB03 volatiles was 16 mg/plant (49.5% greater than water controls, p < 0.05) compared with 21 mg/plant for full GB03 exposure (96.3% greater than water controls, p < 0.05) (Fig. 1E). At week three, GB03 short-time exposure also resulted in two less rosette leaves than GB03 full time exposure (Fig. 1F, p < 0.05).
To correlate differences in plant growth promotion with bacterial signaling, volatile emissions from plant chambers with and without bacterial cultures were measured (Table 1). Within the first week there was a mean 24-fold increase in volatile emissions when summing the five most abundant bacterial components. By week three, a mean 93-fold increase in bacterial emissions was observed. Chambers in which the bacterial culture was removed on day 14, volatile emissions dropped to levels not significantly different from water controls for week 3 and 4 time point measurements (data not shown).
Table 1.
Headspace volatile emissions from chambers containing Arabidopsis with or without Bacillus subtilis (GB03)
| Treatment | Bacterial emissions (ng 24 hrs−1)1 | |||
| Week 1 | Week 2 | Week 3 | Week 4 | |
| GB03 | 1100 ± 270a | 940 ± 300a | 2600 ± 540b | 1900 ± 430a/b |
| H2O | 47 ± 16c | 29 ± 4c | 28 ± 6c | 34 ± 2 |
Values represent the sum of the five most abundant components present from GB03 exposed plants; mean ± SD (n = 2). Data labeled with different letters indicate significant differences between time collections and treatments (ANOVA, p ≤ 0.05).
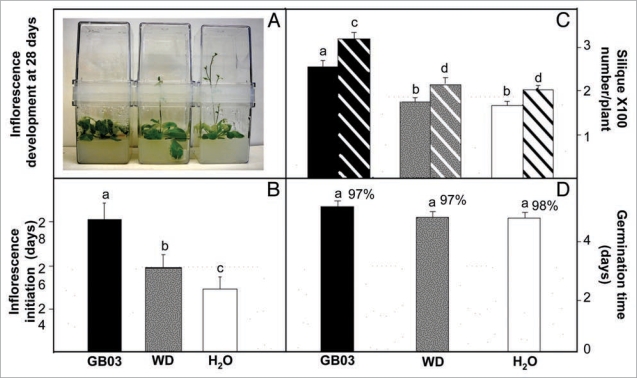
Sustained GB03 exposure elevates seed-set.
In wild type plants, there is a positive correlation between days to flowering and leaf number.17 Consistent with an increase in rosette leaf number with GB03 exposure compared to water controls, flowering time was extended with short and long exposure to GB03 volatiles (Fig. 2A and B). In addition, full GB03 exposure resulted in 263 ± 35 and 327 ± 39 siliques per plant for weeks 8 and 10 respectively, compared to average 171 ± 26 and 207 ± 32 siliques per plant in the water control plants (Fig. 2C, p < 0.05). To confirm that silique number correlates with reproductive success, seed number per silique was also measured. At 10 weeks, sustained GB03 treatment showed a non-significant increase in seed number per silique relative to short-term exposure or water control at 6.0 ± 3.1, 5.9 ± 3.0 and 5.6 ± 3.0, respectively (p > 0.05); silique dry weight also showed a non-significant difference with 9.8 ± 4.2, 10.5 ± 3.8, 10.2 ± 4.1 mg per 100 siliques for sustained GB03 exposure, short-term GB03 exposure and water control at 10 weeks, respectively (p > 0.05). Plants with an abbreviated GB03 exposure (WD) had similar silique numbers to water control plants. This increased seed production did not negatively affect germination time or viable seed percentage (Fig. 2D).
Figure 2.
An increase in silique number with sustained Bacillus subtilis (GB03) exposure (black bar) compared with GB03 withdrawn (WD, grey bar) at week two and water-treated controls (white bars). Representative images of inflorescence development with sustained (left), WD (center) and absent (right) GB03 exposures for plants 28 days after treatments are shown (A). Time of inflorescence initiation with sustained GB03 exposure (n = 16), WD (n = 16) and absent (n = 18) GB03 exposure (B). Siliques per plant collected at 8- (solid bar) and 10-weeks (diagonal bar) with GB03, WD and absence of GB03 treatment (n = 8) (C). Seed germination ratio and time after seeds planted from different treatments (sustained, n = 74; WD, n = 68; and absent GB03 exposure n = 71) (D). Different letters indicate significant differences between treatments (ANOVA, p ≤ 0.05).
Sustained GB03 exposure increases photosynthetic efficiency.
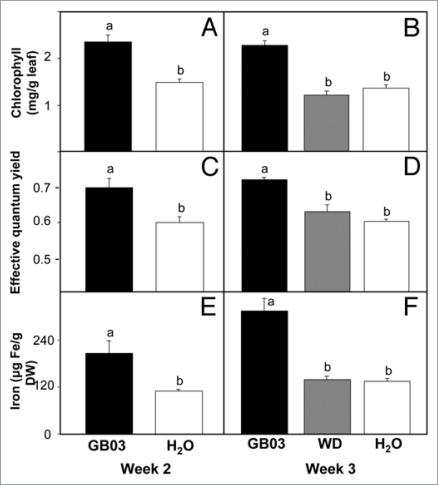
To probe how bacterial volatiles affected energy generation for seed production, parameters associated with photosynthesis were monitored. Two-week GB03-exposure increased total chlorophyll (Chl a/b) content to over 60% compared to water controls with 2.35 ± 0.14 mg Chl·g−1 FW versus 1.49 ± 0.06 mg Chl·g−1 FW, respectively (Fig. 3A, p < 0.05). Sustained GB03 exposure preserved elevated chlorophyll levels at week three with 2.3 ± 0.13 versus 1.4 ± 0.16 mg Chl·g−1 FW for water controls (Fig. 3B, p < 0.05). In contrast, an abbreviated two-week GB03 exposure lowered total chlorophyll at week three to 1.3 ± 0.15 mg Chl·g−1 FW which was not significantly different from water controls (p > 0.05).
Figure 3.
An increase in chlorophyll content, photosynthetic efficiency and iron content with sustained Bacillus subtilis (GB03) exposure (black bar) compared with GB03 withdrawn (WD, grey bar) at week two and water-treated controls (white bars). Chlorophyll content (chl. a + b) (A and B), effective photosynthetic efficiency (C and D) and iron content (E and F) (A–E, n = 4; F, n = 3). Different letters indicate significant differences between treatments (ANOVA, p ≤ 0.05).
Consistent with an increase in total chlorophyll content, GB03 exposure increased photosystem II (PSII) photosynthetic efficiency (Fig. 3C). With two weeks of GB03 exposure, the PSII effective quantum yield increased to 0.70 ± 0.03 compared with water controls at 0.60 ± 0.02 (p < 0.05) and by week three, PSII effective quantum yield was 0.72 ± 0.01 and 0.60 ± 0.01 for GB03 and water treatments respectively (p < 0.05). The abbreviated two week GB03 exposure at week three exhibited a PSII effective quantum yield of 0.63 ± 0.04 which was not significantly different than the water control (Fig. 3D, p > 0.05).
Given that iron is often a limiting metal ion in photosynthesis, iron levels were correlated with GB03 exposure (Fig. 3E). Within two weeks of GB03 exposure, total iron content was almost 100% greater than water controls at 208 ± 30 and 110 ± 5 µg·g−1 dry weight respectively (p < 0.05). By week three, total iron content of GB03 exposure plants increased to 316 ± 33 µg·g−1 dry weight that is over a 2-fold increase compared to water controls (p < 0.05). The removal of GB03 at week two resulted in no difference in iron content between treated and water control by the end of the third week (Fig. 3F, p > 0.05).
GB03 volatiles transiently increases growth-promotion gene expression.
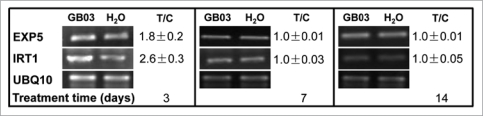
Microarray data has shown that several growth-promotion related genes were induced within 48- and 72-hours of GB03 exposure.13 To confirm that gene induction associated with growth promotion is also observed in Magenta-box-grown Arabidopsis plants, genes associated with cell wall expansion and iron assimilation, expansin 5 (EXP5) and iron-regulated transporter 1 (IRT1) respectively, were monitored. Polyubiquitin 10 (UBQ10), a ubiquitous housekeeping gene was included to ensure equal loading for the different treatments. EXP5 and IRT1 were transiently induced within three days of GB03 exposure and returned to control levels within 7- and 10-days post GB03 exposure (Fig. 4).
Figure 4.
Growth-related Arabidopsis genes including expansin 5 (EXP5) and the iron-regulated transporter 1 (IRT1) were upregulated at an early stage in plant exposed to GB03 volatiles. Polyubiquitin 10 (UBQ10), as a housekeeping gene, was used to ensure equal loading for the different treatment. Gene expression ratios (treated versus control, n = 3) were shown as mean ± SD representative RT-PCR gel images.
Discussion
Sustained exposure to Bacillus subtilis (GB03) volatile emissions triggers long-term Arabidopsis plant growth promotion compared to water controls. Sustained GB03 volatile signaling is necessary as indicated by the loss of enhanced growth when GB03 is withdrawal early in the plant development (Figs. 1–3). In addition to larger root mass16 greater rosette number likely contributes to the significantly higher fresh and dry weight measurements (Fig. 1). Although flowering time is delayed with sustained exposure to GB03 volatiles, seed number as a metric of reproductive success was significantly increased compared to water controls at the ten week harvest period.
When plants are moved to unconfined Magenta boxes, sustained volatile emissions from GB03 are effective in augmenting growth, photosynthetic capacity and seed-set. When the volatile bacterial signal is withdrawn (WD), photosynthetic capacity and iron content return to untreated levels. Regulation of photosynthesis requires the integration of endogenous signals as well as environmental factors.18,19 In field studies, PGPR increases chlorophyll content and photosynthetic rate.20 In Petri-dish studies, more than 10 chloroplast associated genes have been shown to be differentially expressed when plants are exposed to GB03 volatiles at a seedling stage13 indicating that the promoting of growth may, at least in part be through an increase in photosynthetic rates. Petri-dish grown Arabidopsis exposed to GB03 VOCs also have greater chlorophyll content and higher photosynthetic efficiency.14 Since actual (effective) quantum yield of PSII photochemistry (ΦPSII) correlates well with quantum efficiency of CO2 fixation,21,22 the chlorophyll contents and effective quantum yield were measured (Fig. 3A–D). Consist with the Petri-dish data, both chlorophyll content and effective quantum yield were higher when plants were exposed continuously to GB03 VOCs. However when the GB03 producing signal was removed from the Magenta box, short-term GB03 exposed plants exhibit a less vibrant green color similar to untreated controls and paler than the rich-green color of plants with full GB03 exposure (Fig. 1D). At the same time, both the photosynthetic efficiency and chlorophyll content diminished with short-term GB03 exposure similar to untreated controls (Fig. 3B and D). The rapid chlorophyll content and photosynthetic efficiency reduction indicates that these parameters require sustained GB03 activation. The biogenesis of the photosynthetic apparatus makes heavy demands on iron availability,23 consistent with the increase of chlorophyll content and photosynthetic efficiency, total iron content increases over the two and three week time interval (Fig. 3E and F). Again a consistent GB03 signal is necessary to maintain higher iron levels since withdrawing of GB03 (WD) after two weeks results in diminished iron content to control levels within one week subsequently (Fig. 3F).
Since volatile-triggered growth promotion involves the regulation of multiple hormones including at least auxin and ABA,13,14 real-time monitoring of bacterial volatiles in parallel with metabolite monitoring may identify specific plant signals required for sustained growth promotion. Within a 48 to 72 hour interval, GB03 volatiles do trigger differential transcriptional expression associated with plant growth including cell wall loosening expansions (e.g., Exp) and iron assimilation genes (e.g., Irt1).13 The transient nature of GB03 induced gene expression observed in this study with an absence of differential gene expression for Exp5 and Irt1 at 7 and 14 days post GB03 exposure suggests that post-transcriptional mechanisms are operative for sustained growth promotion triggered by GB03 volatiles.
Materials and Methods
Plant materials and treatments.
Arabidopsis thaliana (Col-0) seeds were surface sterilized and vernalized for 2 days at 4°C in the absence of light. Seeds were then planted in closed Magenta boxes (75 × 75 × 100 mm) coupled together as pairs by a plastic collar (75 × 75 × 20 mm) and containing ca. 120 ml of half-strength Murashige et al.24 solid media (MS) with 0.7% (w/v) agar and 1.5% (w/v) sucrose. A separate glass vial (4 dr.) also containing MS media provided a restricted area within the chamber for bacterial growth. After planting, Magenta boxes were placed in a growth room set to a 14-/10-h light/dark cycle respectively using metal halide and high pressure sodium lamps with a total light intensity of 200 µmol photons m−2 s−1; temperature was set to 21 ± 4°C and relative humidity 40 ± 10%.
Bacillus subtilis (GB03) solid culture was streaked onto tryptic soy agar (TSA) plates and incubated at 28°C in the absence of light for 24 h. Cells were then harvested from TSA plates in double distilled water (DDW) to yield 109 CFU mL−1, as determined by optical density and serial dilutions with plate counts. Two days after seed germination, bacterial suspension culture or DDW (50 µl) was added within the Magenta box vials for treated and control conditions, respectively. Every four weeks, vials containing GB03 bacterial culture were replaced with new GB03-inoculated media. Visual inspects of the bacterial cultures were made weekly to remove setups in which a bacterial contamination had been introduced. For treatments in which the bacteria was withdrawn (WD) two weeks after GB03 exposure, since replacing the bacteria vial with a new un-inoculated vial or leaving a gap in the media did not significantly alter subsequent plant growth, the vial was not replaced with a blank so as to minimize media contamination.
Volatile collection and analysis.
Volatiles were collected from modified Magenta boxes in which ports were added on the top of the chambers to allow for air flow in and out. Charcoal-purified air, humidified by bubbling through a supersaturated NaCl solution was allowed to enter into the chamber while volatiles were collected by a 0.5 L min−1 vacuum flow from the chamber and through Super Q adsorbent traps. For more detail on collecting bacterial volatiles (see Ryu et al.).9 Volatiles were collected over a 24 h period, extracted with dichloromethane (150 µL), and to this extract the internal standard nonyl acetate (800 ng) was added. Extracts were analyzed by capillary GC on a 15 m × 0.25 mm-i.d. fused silica column with a 0.25-µm-thick bonded [5% (w/v) phenyl] methylpolysiloxane (J&W, New Castle, DE). HP 6890 series GC parameters were set as previous described.10 The quantity of volatiles was calculated based on the peak area ratio of individual components to the internal standard. The five most abundant volatile components from GB03-treated plants (acetoin, 2,3-butanediol, methyl-3-butanal, 2-methyl-1-propanol and 3-methyl-1-butanol) were summed to provide an estimate of bacterial volatile emissions (n = 2). These same components, based on retention times were quantified for water alone and GB03 treatments.
Chlorophyll measurement.
Two and three weeks after bacterial treatment, Arabidopsis leaves were detached (one leaf per plant, four plants total) and chlorophyll amounts were determined spectrophotometrically. Approximately 10 mg tissue was ground with 1 mL 80% acetone (20% water) in glass grinder (Fisher Scientific), followed by centrifugation at 13,000 g for 5 min. The supernatant was used for spectrometric measurements at wavelengths of 470, 646.8 and 663.2 nm. Total chlorophyll content was calculated using the formula as reported by Lichtentthaler25: total chlorophyll = (7.15 * A663.2 + 18.71 * A646.8)/1000/(fresh weight of leaves); calculate values were reported as mg Chl per g FW (n = 4).
Chlorophyll fluorescence measurements.
Chlorophyll A fluorescence emissions from attached leaves were measured with a pulse amplitude-modulated fluorometer (PAM 101/103; Heinz Walz GmbH, Effeltrich, Germany). Experimental protocol and nomenclature was based on descriptions by Maxwell et al.26 Plants (n = 4) were removed from the Magenta box with media still associated with the roots and placed in Petri dishes during photosynthesis measurements. F0 (minimal fluorescence level for the dark-adapted state) was measured with leaves previously kept in the dark for at least 30 min. After this, the maximal level of fluorescence in dark-adapted state (Fm) was measured by means of application of a saturating flash. Saturating light 1-sec pulses were provided by a KL 1500 light source (Schott, Wiesbaden, Germany). The ratio Fv/Fm (Fv = Fm − Fo) was used to estimate the potential quantum yield of PSII photochemistry. Leaves were then illuminated with an actinic light (250 µmol photons m−2sec−1) in order to activate photosynthetic reactions. Preliminary experiments showed that illumination for 10 min at room temperature was enough to obtain steady-state fluorescence. Once leaves were adapted to the light, a saturating flash was applied to obtain the maximal level of fluorescence for the light-adapted state (Fm'). Immediately after the flash, the actinic light was switched off and F0' measurements were performed following short-term application of low-intensity far-red light. The quantum efficiency of electron transport through PSII complexes (ΦPSII = (Fm' − F)/Fm') was then calculated.
Endogenous iron measurements.
The iron concentration in leaves was determined as described previously.27 Tissue of 100–200 mg was ground with liquid N2 and mineralized according to Beinert.28 Iron concentration was measured by absorbance of Fe2+-O-phenanthroline at 510 nm at pH 6.0 using thioglycolic acid as a reducing agent. Samples (n = 4) were weighed and dry weights (with a 100°C desiccation) were calculated based on a dry weight:wet weight conversion factor.
Flowering time determination.
Flowering time was determined based on the day in which the main stem bolted 1 cm.
Silique seed counting.
At ten weeks after germination, siliques were collected and counted for seed number per silique. For each treatment, 8 plants were selected and 20 siliques were randomly selected from each plant for seed counting (for each treatment, 160 siliques were used for seeds/silique counting). Sets of 100 siliques (n = 3) were dried to determine silique dry weight.
Seed germination rate.
Seeds were prepared as previously described and counted as germinated when the radicle penetrated the seed coat.
Reverse-transcriptase PCR (RT-PCR).
First strand cDNA was synthesized from 5 µg total RNA using MuMLV-RT (Fisher Scientific, Houston, TX). The following primers were generated (5′ to 3′) based on Zhang et al.14,29: [UBQ10], CGA TTA CTC TTG AGG TGG AG and AGA CCA AGT GAA GTG TGG AC; [EXP5], TAG TAA TCT CGC TTC TCG TG and CGT TGA TCG TAA CCT TAT C; [IRT1], GCA ATC TCT CCA GCA ACT TC and TCT TGC TGG TGT ATA GGC TC. Agarose gel electrophoresis images were taken by Kodak Gel Logic 100 Imaging System (Fisher Scientific, Houston, TX, USA) and quantified by using Image J 1.33u (http://rsb.info.nih.gov/ij/, National Institute of Health, USA).
Statistical analysis.
Data were analyzed by GLM procedure using SAS statistical software version 8 (SAS Institute, Cary, NC). Treatment differences were separated using Duncan's multiple range test at the 5% level. Significant treatment effects were investigated when analysis of variance was significant (p < 0.05).
Acknowledgements
This research was supported in part by the Robert Welch Foundation (D-1478) and Hersch Foundation/ACS for Chemical Research.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9709
References
- 1.Kloepper JW, Leong J, Teintze M, Schroth MN. Enhanced plant growth by siderophores produced by plant growth promoting rhizobacteria. Nature. 1980;286:885–886.. [Google Scholar]
- 2.Kloepper JW, Zablotowicz RM, Tipping EM, Lifshitz R. In: The Rhizosphere and Plant Growth. Keister KL, Cregan PB, editors. Kluwer: Dordrecht, The Netherlands; 1991. pp. 315–326. [Google Scholar]
- 3.Kloepper JW, Rodriguez-Kabana R, Zehnder GW, Murphy J, Sikora E, Fernandez C. Plant root-bacterial interactions in biological control of soil borne diseases and potential extension to systemic and foliar diseases. Aust J Plant Pathol. 1999;28:27–33. [Google Scholar]
- 4.Lin W, Okon Y, Hardy RWF. Enhanced mineral uptake by Zea mays and Sorghum bicolor roots inoculated with Azospirillum brasilense. Appl Environ Microbiol. 1983;45:1775–1779. doi: 10.1128/aem.45.6.1775-1779.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loper JE, Schroth MN. Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet. Phytopath. 1986;76:386–389. [Google Scholar]
- 6.MacDonald EMS, Powell GK, Regier DA, Glass NL, Roberto F, Kosuge T, Morris RO. Secretion of zwatin, Ribosylzeatin and ribosyl-1″-methylzeatin by Pseudomonas savastanoi plasmidcoded cytokinin biosynthesis. Plant Physiol. 1986;82:742–747. doi: 10.1104/pp.82.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmusk S, Nicander B, Granhall U, Tillberg E. Cytokinin production by Paenibacillus polymyxa. Soil Biol Biochem. 1999;31:1847–1852. [Google Scholar]
- 8.Glick BR, Patten CN, Holguin G, Penrose DM. Biochemical and genetic mechanisms used by plant growth promotion bacteria. London: Imperial College Press; 1999. pp. 1–13. [Google Scholar]
- 9.Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Paré PW, Kloepper JW. Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA. 2003;100:4927–4932. doi: 10.1073/pnas.0730845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004;134:1017–1026. doi: 10.1104/pp.103.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paré PW, Farag M, Krishnamachari V, Zhang H, Ryu CM, Kloepper JW. Elicitors and priming agents initiate plant defense responses. Photosyn Res. 2005;85:149–159. doi: 10.1007/s11120-005-1001-x. [DOI] [PubMed] [Google Scholar]
- 12.Farag MA, Ryu CM, Sumner LW, Paré PW. GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochem. 2006;67:2262–2268. doi: 10.1016/j.phytochem.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M, et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta. 2007;226:839–851. doi: 10.1007/s00425-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, Paré PW. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008;56:264–273. doi: 10.1111/j.1365-313X.2008.03593.x. [DOI] [PubMed] [Google Scholar]
- 15.Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Kim MS, Sun Y, Dowd SE, Shi H, Paré PW. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant Microbe Interaction. 2008;21:737–744. doi: 10.1094/MPMI-21-6-0737. [DOI] [PubMed] [Google Scholar]
- 17.Koornneef M, Hanhart CJ, Van Der Veen JH. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- 18.Ku SB, Eewards GE, Tanner CB. Effects of light, carbon dioxide, and temperature on photosynthesis, oxygen inhibition of photosynthesis, and transpiration in Solanum tuberosum. Plant Physiol. 1977;59:868–872. doi: 10.1104/pp.59.5.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leister D. Genomics-based dissection of the cross-talk of chloroplasts with the nucleus and mitochondria in Arabidopsis. Gene. 2005;354:110–116. doi: 10.1016/j.gene.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 20.Han HS, Lee KD. Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res J Agricult Biol Sci. 2005;1:210–215. [Google Scholar]
- 21.Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–90. [Google Scholar]
- 22.Edwards GE, Baker NR. Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynth Res. 1993;37:89–102. doi: 10.1007/BF02187468. [DOI] [PubMed] [Google Scholar]
- 23.Spiller S, Terry N. Limiting factors in photosynthesis II. Iron stress diminishes photochemical capacity by reducing the number of photosynthetic units. Plant Physiol. 1980;65:121–125. doi: 10.1104/pp.65.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 25.Lichtentthaler HK. Chlorophyll and carotenoids: Pigments of photosynthetic membranes. Meth Enzymol. 1987;148:350–382. [Google Scholar]
- 26.Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. J Exp Bot. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- 27.Lobreaux S, Briat JF. Ferritin accumulation and degradation in different organs of pea (Pisum sativum) during development. Biochem J. 1991;274:601–606. doi: 10.1042/bj2740601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beinert H. Micro methods for quantitative determination of iron and copper in biological material. Methods Enzymol. 1978;54:435–445. doi: 10.1016/s0076-6879(78)54027-5. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Sun Y, Xie X, Kim M-S, Dowd SE, Paré PW. A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 2009;58:568–577. doi: 10.1111/j.1365-313X.2009.03803.x. [DOI] [PubMed] [Google Scholar]