Abstract
Maintaining a high K+/Na+ ratio in the cell cytosol, along with the transport processes implicated in the xylem and phloem loading/unloading of Na+ in plants (long-distance transport) are key aspects in plant salt tolerance. The Ca2+-dependent SOS pathway regulating Na+ and K+ homeostasis and long-distance Na+ transport has been reported in Arabidopsis. However, Arabidopsis might not be the best model to analyze the involvement of the SOS pathway in long-distance Na+ transport due to the very short stem of these plants which do not allow a precise dissection of the relative content of Na+ in stem versus leaf. This separation would be critical to assess the role of SOS1 in xylem loading/unloading, Na+ export by roots, retention in stems and the differential distribution/accumulation in old leaves. Towards this goal, tomato might represent a superior model due to its anatomical structure and agricultural significance. We recently demonstrated the key role played by the plasma membrane Na+/H+ antiporter SlSOS1 in salt tolerance in tomato by maintaining ion homeostasis under salinity stress and in the partitioning of Na+ in plant organs.
Key words: long-distance Na+ and K+ transport, plasma membrane Na+/H+ antiporter, salt tolerance, posttranscriptional gene silencing, SlSOS1, Solanum lycopersicum (tomato)
Ion homeostasis under salt stress conditions is essential in salt tolerance and involves a network of transport processes regulating uptake, extrusion through the plasma membrane, compartmentation of salts into cell vacuoles and recirculation of ions through the plant organs, thus allowing the osmotic adjustment and maintenance of high K+/Na+ ratios in the cytosol of plants.1,2 The SOS signaling pathway is essential in the salt tolerance of Arabidopsis.3 This pathway regulates Na+ and K+ homeostasis as well as long-distance Na+ transport from roots to shoots.3 In the SOS pathway, a calcium-binding protein, SOS3, senses cytosolic calcium changes elicited by salt stress.4 SOS3 physically interacts with and activates the serine/threonine protein kinase, SOS2.5 The SOS3/SOS2 kinase complex phosphorylates and activates the plasma membrane Na+/H+ exchanger encoded by the SOS1 gene.6,7 AtSOS1 expression was observed at the epidermal cells of the root tip implying a role of this transporter in extruding Na+ to the soil.8 Additionally, the preferential expression of AtSOS1 in the cells surrounding the vasculature also suggested a role of this transporter in the control of long-distance Na+ transport in plants, since this ion is transported from the root to the shoot via the xylem.8 The great significance of this Na+/H+ antiporter in salt tolerance was further recognized when overexpression of AtSOS1 restricted Na+ accumulation in plant cells and improved the salt tolerance of Arabidopsis.9
Although orthologous SOS genes have been identified in crop species like rice and wheat,10,11 the relevance of this pathway for salt tolerance in crop plants has not yet been demonstrated. Furthermore, the study of the involvement of the SOS pathway in long-distance Na+ transport by retention of salt in the stem, and the differential distribution/accumulation in old leaves, is a difficult task in Arabidopsis due to the small size of the stem. Tomato is a good alternative to study Na+ long-distance transport due to its anatomical structure, and the high genotypic diversity related to salt tolerance. The relatively more tolerant tomato species accumulate higher amounts of salt in stems and leaves while the more sensitive species accumulate salt mainly in roots.12 Physiological evidences indicate that tomato roots could determine the Na+ concentration reaching aerial parts depending on the intensity of the stress,13 probably involving the SOS pathway.
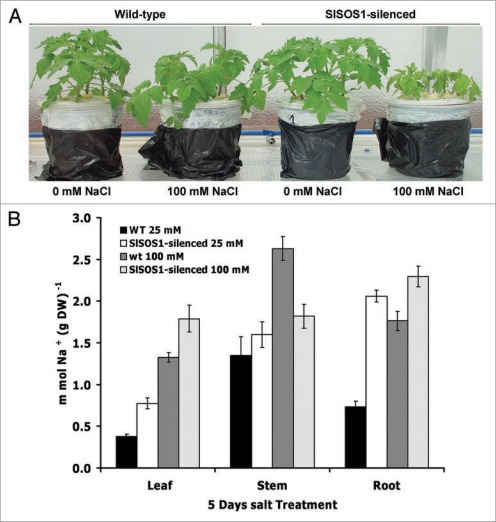
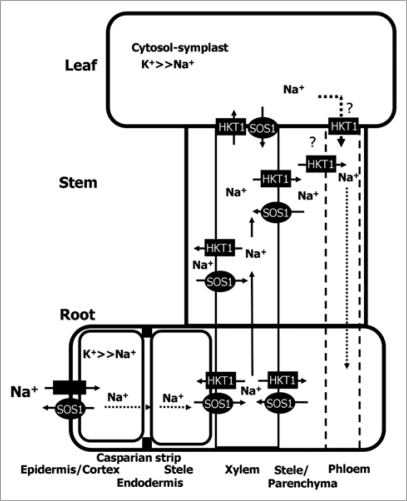
We isolated the SlSOS1 gene, encoding a Na+/H+ antiporter from tomato, and confirmed through gene silencing that it plays a crucial role in the survival of tomato plants under saline conditions.14 Silencing of SlSOS1 in tomato renders these plants more sensitive to salt stress (Fig. 1A), thus extending the observations from the model system Arabidopsis to a crop plant. Net uptake rates of Na+ in whole plants were three-fold higher in SlSOS1-silenced plants than in wild-type plants subjected to mild (25 mM NaCl) and severe salt stress (100 mM NaCl),14 indicating that the main action of SOS1 is the extrusion Na+ out of the root. As previously discussed,1 the rates of unidirectional entry of Na+ (influx) in roots are very high but they do not translate into a commensurate Na+ accumulation. The much lower rate of net Na+ uptake relative to unidirectional influx (about ten-fold) implies a substantial efflux rate of ca. 90% of the incoming Na+. Thus, even small changes in the rate of Na+ efflux, to which SOS1 is known to be critical, would have dramatic effects in the root Na+ content. However, what we would like to highlight here is the critical importance of SOS1 to control Na+ distribution among plant organs. The analyses of the fluxes of Na+ and K+ to individual organs supported an important function of this Na+/H+ antiporter in the distribution of Na+ throughout the tomato plant. Arabidopsis sos1 mutants accumulated more Na+ in shoots under severe salt stress than the wild-type, presumably because of uncontrolled Na+ uptake by roots. However, under mild stress conditions, the mutant accumulated less Na+ in the shoot than wild-type, suggesting that AtSOS1 mediated xylem loading.8 In tomato, SlSOS1-silenced plants under saline conditions accumulated more Na+ in roots and leaves than control plants under both mild and severe regimes of salt stress (Fig. 1B). By contrast, the stems of suppressed plants accumulated significantly less Na+ under severe stress (100 mM NaCl). The ability to retain Na+ in stems was clearly lost in suppressed plants, in which an acropetal gradient of Na+ was established. As most glycophytes, tomato plants exclude salts from the photosynthetic tissues in leaves by removing Na+ from the root while retaining it mainly in the stem,15,16 and SOS1 seems critical to this process. The greatest Na+ accumulation in control plants was found in stems at any time point or salt regime, whereas the suppressed plants showed preferential deposition in roots, particularly at longer times of treatment. Average Na+ contents in stems of suppressed plants remained mostly unchanged. Net Na+ fluxes carried over by xylem sap movement was much lower in suppressed plants than in controls. The greater accumulation of Na+ in the roots of suppressed plants is coherent with the known role of SOS1 in Na+ efflux in roots, but the contribution of SOS1 to the retention of Na+ in the stem of the control plants is perplexing and not immediately reconciled with the proposal that SOS1 loads Na+ into the xylem. We hypothesize that the activity of SOS1 should be coordinated with other transporters in order to control the Na+ that reaches the photosynthetic tissues (Fig. 2). A functional link and possible interplay between AtSOS1 and AtHKT1;1 has been previously suggested.17 It appears that the transport function of the SOS1 and HKT1 systems is coordinated to achieve Na+ (and K+) homeostasis18 (Fig. 2). Dysfunction of either system alters long-distance transport and adequate partition of Na+, thereby resulting in salt-sensitive phenotypes in Arabidopsis.17 Our results are coherent with a model in which SOS1 mediates the transfer of Na+ from the xylem parenchyma to xylem vessels, preferentially in roots, whereas HKT1 would mediate the reverse flux, i.e., Na+ unloading from the xylem in the stem. The coordinated actions of these transporters would ultimately determine the amount of Na+ that leaves the roots, is retained in the stem, or transferred to leaves. It remains to be shown whether the depletion of SOS1 in suppressed tomato does affect the function of the HKT1 orthologous protein in the xylem parenchyma of tomato. At the same time, SOS1 might contribute in the exclusion of Na+ from the cytosol of young leaves toward the leaf apoplast, avoiding the accumulation of intracellular Na+ in cells lacking a well-developed vacuolar system, while HKT1 might act predominantly in the fully expanded or mature leaves with a well-developed vacuolar system that can accumulate Na+. Part of apoplastic Na+ could also be retrieved by the action of HKT1 at phloem companion cells. This might contribute, to some extent, to Na+ recirculation through phloem (Fig. 2). It will be of interest to experimentally determine the expression pattern of SOS1 (and perhaps HKT1-like genes) in relation to the developmental stage of the plant organs, and also the possibility that these proteins are recruited to specific membrane domains near the xylem parenchyma-xylem vessel interface to mediate vectorial movements of Na+ in and out of the xylem sap.
Figure 1.
Effect of SlSOS1 silencing on growth of tomato (A) and Na+ content in different plant organs (B). Growth of whole plant of wild type and a F1 tomato (S. lycopersicum cv. Moneymaker) plant line transformed with the SlSOS1 gene silencing construct cultivated in hydroponic culture and subjected to treatment for five days with a mild (25 mM NaCl) and severe (100 mM NaCl) salt stress. Data, expressed as g DW per plant, are means ± SD of at least three independent experiments, using eight plants per line and per treatment.
Figure 2.
Schematic diagram showing Na+ fluxes mediated by SlSOS1 within the plant. The main action of SlSO1 is extruding Na+ out the root and the partitioning of Na+ in plant organs. Suppression of SlSOS1 causes a root-to-leaf gradient of Na+ distribution together with a reduced ability to accumulate Na+ in the stem. the transport function of the SOS1 and HKt systems may be coordinated to achieve Na+ and K+ homeostasis.
Our results provide evidence of the importance of the plasma membrane Na+/H+ antiporter, SOS1, in maintaining ion homeostasis in tomato, a crop plant that is a Na+ excluder with the capacity to tolerate moderate salt concentrations.12,15,16 We also show that, besides its main action in extruding Na+ out the root, SOS1 is critical for the partitioning of Na+ in plant organs and the ability of tomato plants to retain Na+ in the stems, thus preventing Na+ from reaching the photosynthetic tissues.
Acknowledgements
This work was supported by grants BIO2006-01955 (A. Belver) and BFU2006-06968/BMC (J.M. Pardo) from the Ministerio de Educación y Ciencia and by CVI 124 (A. Belver) and CVI 148 (J.M. Pardo) from the Consejería de Innovación, Ciencia y Empresa, Junta de Andalucia. R. Olías was supported by a grant of Programa Averroes from the Consejeria de Inovación, Ciencia y Empresa, Junta de Andalucia.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9679
References
- 1.Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. An Bot. 2003;391:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apse MP, Blumwald E. Sodium transport in plants. FEBS Lett. 2007;581:2247–2254. doi: 10.1016/j.febslet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Zhu JK. Salt and drought stress signal transduction in plants. Ann Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishitani M, Liu JP, Halfter U, Kim CS, Shi WM, Zhu JK. SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell. 2000;12:1667–1677. doi: 10.1105/tpc.12.9.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halfter U, Ishitani M, Zhu JK. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci USA. 2000;97:3735–3740. doi: 10.1073/pnas.040577697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA. 2002;99:8436–8441. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quintero FJ, Ohta M, Shi HZ, Zhu JK, Pardo JM. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci USA. 2002;99:9061–9066. doi: 10.1073/pnas.132092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi HZ, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H, Lee BH, Wu SJ, Zhu JK. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotech. 2003;21:81–85. doi: 10.1038/nbt766. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Atienza J, Jiang X, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, et al. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 2007;143:1001–1012. doi: 10.1104/pp.106.092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Jiang X, Zhan K, Cheng X, Chen X, Pardo JM, et al. Functional characterization of a wheat plasma membrane Na+/H+ antiporter in yeast. Arch Biochem Biophys. 2008;473:8–15. doi: 10.1016/j.abb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Cuartero J, Fernandez-Muñoz R. Tomato and salinity. Sci Hort. 1999;78:83–125. [Google Scholar]
- 13.Estañ MT, Martinez-Rodriguez MM, Perez-Alfocea F, Flowers TJ, Bolarín MC. Grafting raises the salt-tolerance of tomato through limiting the transport of sodium and chloride to the shoot. J Exp Bot. 2005;56:703–712. doi: 10.1093/jxb/eri027. [DOI] [PubMed] [Google Scholar]
- 14.Olías R, Eljakaoui Z, Li J, Marín-Manzano MC, Pardo JM, Belver A. The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environm. 2009;32:904–916. doi: 10.1111/j.1365-3040.2009.01971.x. [DOI] [PubMed] [Google Scholar]
- 15.Taleisnik E, Grunberg K. Ion balance in tomato cultivars differing in salt tolerance. I. Sodium and potassium accumulation and fluxes under moderate salinity. Physiol Plant. 1994;92:528–534. [Google Scholar]
- 16.Guerrier G. Fluxes of Na+, K+ and Cl−, and osmotic adjustment in Lycopersicon pimpinellifolium and L. esculentum during short- and long-term exposures to NaCl. Physiol Plant. 1996;97:583–591. [Google Scholar]
- 17.Rus A, Lee BH, Muñoz-Mayor A, Sharkhuu A, Miura K, Zhu JK, et al. AtHKT1 facilitates Na+ homeostasis and K+ nutrition in plants. Plant Physiol. 2004;136:2500–2511. doi: 10.1104/pp.104.042234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardo JM, Cubero B, Leidi EO, Quintero FJ. Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot. 2006;57:1181–1199. doi: 10.1093/jxb/erj114. [DOI] [PubMed] [Google Scholar]