Abstract
Increasing instances prove that nitric oxide (NO) plays a significant role in mediating root growth and development, and it is reported that NO acts as a messenger and mediates the auxin-induced adventitious roots (AR) developing process in cucumber explants. Compared with the current understanding of AR development in dicots, knowledge of the molecular and physiological mechanisms of crown root (CR) development in monocots is limited, and the roles of NO in CR initiation and development are still far from clear. Our recent studies demonstrate that a critical concentration of endogenous NO is indispensable for CR primordia initiation, the reduction of endogenous NO content blocks CR primordia initiation and decreases CR number in rice seedlings. In this addendum, Base on the results of our studies and previous reports, we supposed that CR formtion in monocots and AR formtion in dicots possible take part in the same NO signaling pathway, althoug in dicots, AR are formed under unusual circumstances and belong to the abnormal developmental program, and in monocot cereals, CR are genetically determined roots and belong to the normal developmental program of cereals. At last, we advanced a proposed schematic model showing the NO signaling pathway of CR emergence in monocots.
Key words: adventitious root, auxin, crown root, nitric oxide, primordia initiation, signal molecule
Nitric oxide (NO) has been reported to act as a signaling molecule in different plant tissues and to participate in a variety of physiological processes.1–4 Recently, increasing instances prove that NO plays a significant role in mediating root growth and development,5–13 and it is reported that NO acts as a messenger and mediates the auxin-induced adventitious roots (AR) developing process in cucumber explants.6–8 In dicots, AR are formed under unusual circumstances, such as wounding or hormone application at uncharacteristic sites, and belong to the abnormal developmental program. However, in monocot cereals, crown roots (CR), are genetically determined roots and belong to the normal developmental program of cereals.14 In contrast to the primary root (PR) system in dicots, monocots produce numerous CR (in some papers, CR was also called AR), which are essential for maintaining normal growth and development.15 Compared with the current understanding of root development in dicots, knowledge of the molecular and physiological mechanisms of crown root development in monocots is limited, and the roles of NO in CR initiation and development in monocots are still far from clear.
In the model monocot cereal rice, the primordia of CR originate from several ground meristem cells of the pericycle layer adjacent to the peripheral vascular cylinder in the stem.16 The development of CR was divided into 12 successive stages several years ago.17 However, based on the results of genetics and molecular biology, the process of rice CR development is now divided into seven stages,18 primordia initation and emergence are divided into different stages, which are controlled by different genes and signaling molecules.15 Application of the polar auxin transport inhibitor N-1-naphthylphalamic acid (NPA) in rice root collars (the junction between root and stem) blocked the initiation and growth of CR, and it suggested that polar auxin transport was critical for the initiation of CR in rice.19 The auxin response factors (ARFs) and the Aux/IAA repressors are the two types of transcription factor families, which are required for controlling expression of auxin response genes.20 Although an increasing number of CR deficient mutants have been proved related with abnormal auxin singaling in rice,15,21–23 the singaling pathway related with auxin in rice CR initiation and development is still far from clear.
The involvement of NO in CR development has been observed by Gouvêa et al. firstly, they found that a transient increase in NO concentration was shown to be involved in CR development induced by indole acetic acid (IAA).5 NO accumulation in response to auxin treatment also was shown by Pagnussat et al. in cucumber explants during AR formation, and auxin-inducded AR formation was prevented by the application of the specific NO scavenger cPTIO.6 In our recent publication in Planta we reported that treatment with NO scavenger cPTIO significantly decreased endogenous NO content and CR number in rice seedlings, and these decreases were recoverable with the application of sodium nitroprusside (SNP, a NO donor). Microscopic analysis of root collars revealed that treatment with cPTIO inhibited CR primordia initiation rather than emergence.4 In conclusion, our results demonstrate that a critical concentration of endogenous NO is indispensable for CR primordia initiation, the reduction of endogenous NO content blocks CR primordia initiation and decreases CR number in rice seedlings. Base on the results of our experiments and the reports that NO accumulation in response to auxin treatments, we propose that NO is an importance messenger molecule operating downstream of auxin through a linear signaling pathway during CR organogenesis in rice seedlings, just like in the other monocots and dicots. Of course, in order to prove this hypothesis, further research is required.
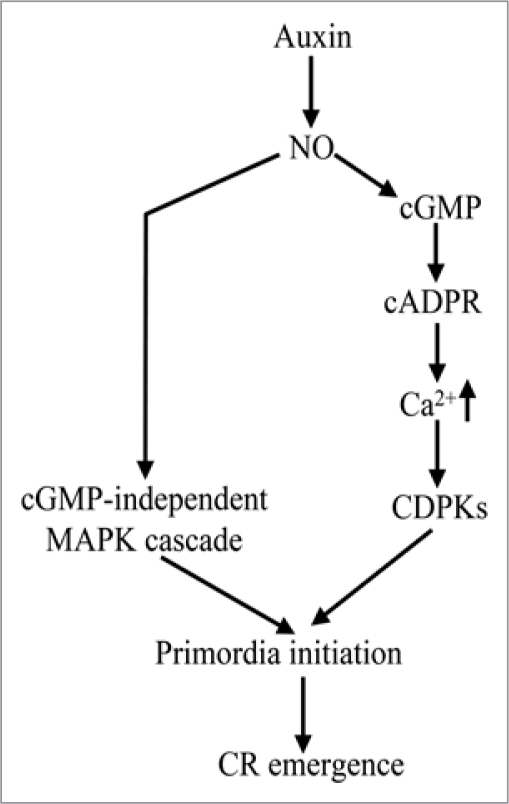
Recently, the signaling pathways induced by IAA and NO during AR formation in cucumber explants has been suggested as follow: in response to basipetal transport of IAA,6 NO accumulates in the basal region of the hypocotyls, then the accumulated NO regulates guanylate cyclase (GC) activity and elevates intracellular cGMP levels, via cADPR, cGMP induces an increase of cytosolic Ca2+ concentration through the regulation of both the release of Ca2+ from intracellular stores and the influx of Ca2+ from the extracellular space,24 as a consequence, certain CDPKs become activated contributes to the downstream responses that result in AR formation in cucumber explants.7,8 NO triggered cGMP-independent cascade and reversible protein phosphorylation also are supposed to be included during the process of NO and auxin induced AR formation.25,26
Base on the results of our studies and previous reports,4–8,19,24 we suppose that CR formtion in rice and AR formtion in cucumber explants possible take part in the same NO signaling pathway, although in dicots, AR are formed under unusual circumstances and belong to the abnormal developmental program, and in monocot cereals, CR are genetically determined roots and belong to the normal developmental program of cereals.14 Furthermore, we advanced a proposed schematic model showing the auxin/NO signaling pathway of CR emergence in monocot cereals (Fig. 1). In this model, Auxin induces an accumulation of NO, which triggers a cGMP-dependent or cGMP-independent MAPK cascade, thus leading to primordia initiation and CR emergence.
Figure 1.
Schematic illustration of a proposed model for auxin/NO signaling pathway of CR emergence in monocots. Auxin induces a accumulation of NO, which triggers a cGMP-dependent or cGMP-independent MAPK cascade, thus leading to primordia initiation and CR emergence.
We demonstrate that endogenous NO is indispensable for CR primordia initiation in rice seedlings, and in this paper we suppose that CR formation in monocots possible take part in the same NO signaling pathway as AR formtion in dicots for the first time. Although this finding may provide a new insight to understand the process of CR development in monocot cereals, further researches and molecular genetic evidences is still required to prove this suppose. Additionly, it would be interesting to know the potential relationships and regulation processes between NO and other hormones or molecules during CR emergence in monocots. Also of interest is the NO-regulated gene expression in monocots during CR primordia initiation.
Acknowledgements
This work was supported by the National Nature Science Foundation (No: 30671255), National Key Technology Research and Development Programme (No: 2006BAK02A18) and Project of National Key Basic Research and Development (No: 2002CB410804) of China.
Abbreviations
- cADPR
cyclic ADP-ribose
- CDPKs
calcium-dependent protein kinases
- cGMP
cyclic GMP
- CR
crown roots
- MAPK
mitogen-activated protein kinase
- NO
nitric oxide
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9715
References
- 1.Stöhr C, Stremlau S. Formation and possible roles of nitric oxide in plant roots. J Exp Bot. 2006;57:463–470. doi: 10.1093/jxb/erj058. [DOI] [PubMed] [Google Scholar]
- 2.Innocenti G, Pucciariello C, Le Gleuher M, Hopkins J, de Stefano M, Delledone M, et al. Glutathione synthesis is regulated by nitric oxide in Medicago truncatula roots. Planta. 2007;225:1597–1602. doi: 10.1007/s00425-006-0461-3. [DOI] [PubMed] [Google Scholar]
- 3.Correa-Araunde N, Lombardo C, Lamattina L. Nitric oxide: an active nitrogen molecule that modulates cellulose synthesis in tomato roots. New Phytol. 2008;179:386–396. doi: 10.1111/j.1469-8137.2008.02466.x. [DOI] [PubMed] [Google Scholar]
- 4.Xiong J, An L, Lu H, Zhu C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pection and hemicelllose content in root cell wall. Planta. 2009;230:755–765. doi: 10.1007/s00425-009-0984-5. [DOI] [PubMed] [Google Scholar]
- 5.Gouvêa CMCP, Souza JF, Magalhães CAN, Martins IS. NO-releasing substances that induce growth elongation in maize root segments. Plant Growth Regul. 1997;21:183–187. [Google Scholar]
- 6.Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L. Nitric oxide is required for root organogenesis. Plant Physiol. 2002;129:954–956. doi: 10.1104/pp.004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagnussat GC, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003;132:1241–1248. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L. Nitric oxide mediates the indole acetic acid induction activation of a mitrogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol. 2004;135:279–285. doi: 10.1104/pp.103.038554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–905. doi: 10.1007/s00425-003-1172-7. [DOI] [PubMed] [Google Scholar]
- 10.Lombardo MC, Graziano M, Polacco JC, Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav. 2006;1:28–33. doi: 10.4161/psb.1.1.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian QY, Sun DH, Zhao MG, Zhang WH. Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytol. 2007;174:322–331. doi: 10.1111/j.1469-8137.2007.02005.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao DY, Tian QY, Li YH, Zhang WH. Nitric oxide is involved in nitrate-induced inhibition of root elongation in Zea mays. Ann Bot. 2007;100:497–503. doi: 10.1093/aob/mcm142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong J, Lu H, Lu K, Duan Y, An L, Zhu C. Cadmium decreases crown root number by decreasing endogenous nitric oxide, which is indispensable for crown root primordia initiation in rice seedlings. Planta. 2009;230:599–610. doi: 10.1007/s00425-009-0970-y. [DOI] [PubMed] [Google Scholar]
- 14.Hochholdinger F, Park WJ, Sauer M, Woll K. From weeds to crops: genetic analysis of root development in cereals. Trends Plant Sci. 2004;9:42–48. doi: 10.1016/j.tplants.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Zhu L, Shou HX, Wu P. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol. 2005;46:1674–1681. doi: 10.1093/pcp/pci183. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman PB. Development of the shoot of Oryza sativa L III. Early stages in histogenesis of the stem and ontogeny of the adventitious root. Phytomorphology. 1959;9:382–404. [Google Scholar]
- 17.Kawata S, Harada J. On the development of the crown root primordial in rice plants. Proc Crop Sci Doc Jpn. 1975;45:438–457. [Google Scholar]
- 18.Itoh J, Nonomura K, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, et al. Crown rootless1, which is essential for crown root formation in rice, is a target of AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell. 2005;17:1387–1396. doi: 10.1105/tpc.105.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiefelbein JW. Cell-fate specification in the epidermis: A common patterning mechanism in the root and shoot. Curr Opin Plant Biol. 2003;6:74–78. doi: 10.1016/s136952660200002x. [DOI] [PubMed] [Google Scholar]
- 20.Guilfoyle TJ, Hagen G. Auxin response factors. Curr Opin Plant Biol. 2007;10:453–460. doi: 10.1016/j.pbi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, et al. Crown rootless1, which is essential for crown root formation in rice, is a target of an auxin response factor in auxin signaling. Plant Cell. 2005;17:1387–1396. doi: 10.1105/tpc.105.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu HJ, Wang SF, Yu XB, Yu J, He XW, Zhang SL, et al. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 2005;43:47–56. doi: 10.1111/j.1365-313X.2005.02434.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Wang J, Wang L, Wang X, Xue Y, Wu P, et al. Adventitious root formation in rice requires OsGNOM1 and is mediated by the OsPINs family. Cell Res. 2009;19:1110–1119. doi: 10.1038/cr.2009.70. [DOI] [PubMed] [Google Scholar]
- 24.Lanteri ML, Pagnussat GC, Lamattina L. Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. J Exp Bot. 2006;57:1341–1352. doi: 10.1093/jxb/erj109. [DOI] [PubMed] [Google Scholar]
- 25.Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in the plants. New Phytol. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- 26.Correa-Aragunde N, Lanteri ML, García-Mata C, ten Have A, Laxalt AM, Graziano M, et al. Nitric oxide functions as intermediate in auxin, abscisic acid and lipid signaling pathways. In: Lamattina L, Polacco JC, editors. Nitric Oxide in Plant Growth, Development and Stress Physiology. Berlin-Heidelberg: Springer-Verlag; 2007. pp. 113–129. [Google Scholar]