Abstract
In Arabidopsis thaliana, ozone-induced signaling has been shown to involve the mitogen-activated protein kinases (MAPKs) MPK3 and MPK6. To identify a possible ozone-induced mitogen-activated protein kinase kinase (MAPKK) involved in the activation of these specific MAPKs, we employed RNA interference-( RNAi)-based suppression of MKK5, a known cognate MAPKK to both MPK3 and MPK6. When exposed to ozone, activation of both MPK3 and MPK6 was markedly reduced in the MKK5-suppressed plants compared to WT. Additionally, the MKK5-suppressed plants were found to be highly sensitive to ozone as determined by visible leaf damage concomitant with elevated levels of leaf-localised H2O2. Taken together, our data suggest MKK5 functions both in ozone-induced activation of MPK3 and MPK6 and in integrating ROS homeostasis during ozone stress.
Key words: Arabidopsis thaliana, ozone, MAPK, MAPKK, reactive oxygen species, RNAi, signaling
Ozone is believed to cause more damage to plants in Europe and North America than any other gaseous pollutant.1,2 The detrimental effects of this pollutant on plant life include diminished photosynthesis, growth-rate retardation, altered patterns of carbon allocation, and accelerated foliar senescence.3–6
Ozone enters the mesophyll via the stomata and diffuses through inner air spaces and cell walls to the plasmalemma7 where it is immediately converted to reactive oxygen species (ROS) such as the free radicals, superoxide anion (•O2−), hydroperoxyl radical (HO2•), the conjugate acid to the superoxide anion, and the hydroxyl radical (HO•). In addition to these free radicals, various non-radical oxygen-centered derivatives, e.g., hydrogen peroxide (H2O2) are also formed.8,9 Hydrogen peroxide is able, to some degree, to move across the plasma membrane10,11 where the potential to oxidatively modify lipids and proteins, including receptors, exists. When hydrogen peroxide encounters ferrous (Fe2+) ions, production of the very destructive hydroxyl radical is possible through what is known as the Haber-Wilstätter/Weiss cycle, via Fe-catalyzed Fenton chemistry.12–16 Since these ROS can be toxic to living cells, plants have, in addition to a basal level of antioxidant metabolites, evolved a complex, enzyme-based oxidant sensing and response system to help counteract the deleterious effects of oxidants derived from various sources, including ozone, photo-oxidation and ultra-violet radiation (UVR).
The Arabidopsis genome codes for ∼60 putative MAPKKKs, 10 MAPKKs and 20 MAPKs.17,18 MAPK cascades are highly regulated networks of phosphoproteins arranged in multiple, interconnecting, hierarchical phosphorelays, which are rapidly activated in response to a large number of external and internal stimuli including, but not limited to, ozone, UVR, hydrogen peroxide, pathogen attack, cold, drought, growth factors and cytokines,19–25 (reviewed in refs. 26 and 27).
Ozone challenge has been shown to rapidly activate MAPK signaling in both tobacco22 (WIPK and SIPK) and Arabidopsis25 (MPK3 and MPK6) through a process which requires ROS accumulation, receptor activation, calcium influx and activation of one or more upstream MAPKK(s). In Arabidopsis, it was shown that treatment of suspension-cultured cells with hydrogen peroxide also induced activation of an unidentified 44 kDa MAPK,28 while through transient heterologous expression studies in maize protoplasts, specific activation of MPK3 and MPK6 by hydrogen peroxide was demonstrated.29 Hydrogen peroxide also induces activation of an Arabidopsis MAPKKK, ANP1 and ectopic expression of constitutively active ANP129 led to activation of MPK3 and MPK6, as well as increased expression of a reporter gene under the control of the oxidant stress-responsive promoter of GST6. Recently, another upstream serine/threonine kinase (OXI1) was shown to play an important role in Arabidopsis for relaying hydrogen peroxide-induced signals to both MPK3 and MPK6.30 However, none of these studies using direct oxidant challenge addressed the identity of the intermediating MAPKK(s) involved in oxidant-induced MAPK signaling.
Activation of MPK3 and MPK6 in Arabidopsis by elicitor treatment, on the other hand, has been shown to involve two closely-related MAPKKs, MKK4 or MKK5.31 A flagellin peptide (flg22) was also shown to activate an Arabidopsis signaling module consisting of MEKK1, MKK4/MKK5 and MPK3/MPK6.31 Flagellin-22, like other elicitors, elicits both rapid changes in protein phosphorylation and an oxidative burst in challenged cells, suggesting a logical link between elicitor-induced signaling and oxidant-induced signaling. Consistent with this, stable overexpression of constitutively activated forms of either MKK4 or MKK5 led to increased activation of MPK3 and MPK6, with an associated oxidative burst and lesion formation.32 While both MKK4 and MKK5 can thus serve as upstream activators of MPK3 and MPK6 in the context of elicitor response, or when ectopically expressed as constitutively active forms, it is not known whether they also control activation of these MAPKs in response to direct oxidant challenge.
Because loss-of-function alleles of MKK5 are not presently available in public depositories, we utilized post-transcriptional gene silencing (RNAi) technology to specifically manipulate the expression of MKK5 in Arabidopsis. We find that suppression of MKK5 renders the transgenic genotype highly susceptible to ozone-stress, as determined by visible leaf damage coincident with elevated levels of leaf-localised hydrogen peroxide. We also demonstrate that MKK5 is necessary for full activation of both MPK3 and MPK6, unequivocally placing MKK5 upstream of these two MAPKs in ozone-induced signaling. Our data point to a non-redundant role for MKK5 in the overall response to ozone-induced oxidant stress in Arabidopsis.
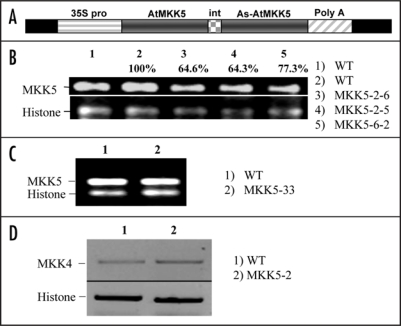
Based on antibiotic resistance selection, a transformation rate of ∼1% was achieved for the MKK5-RNAi construct (Fig. 1A). Plants which both developed and maintained green (true) leaves together with a root mass when growing on selective plates, were designated as putative transformants and subsequently transferred to soil and allowed to grow for an additional two weeks. To determine the success of MKK5 suppression, two rosette leaves were collected from a number of the T1 plants and analysed via RT-PCR using gene-specific primers. Varying levels of suppression were obtained, but in none of the recovered lines (46 plantlets) was suppression of MKK5 found to be complete (data not shown). Progeny (T2) from the parental (T1) MKK5-suppressed lines retained MKK5 suppression and were considered to be stably transformed. Plants from two MKK5-suppressed lines (MKK5-2 and MKK5-6), which were central parental lines for this study, are shown in Figure 1B. As negative controls, we used primary (T1&2) transformants (for example, line 33) that carried the MKK5-RNAi construct, but failed to show any MKK5 suppression as determined by RT-PCR (Fig. 1C), or any loss of MPK3 and MPK6 activation (Fig. 2A).
Figure 1.
MKK5-RNAi construct and loss-of-function genotype selection. (A) The MKK5-RNAi construct under the control of the 35S promoter of the Cauliflower mosaic virus; (B) PCR-positive T2 lines showing MKK5 suppression; (C) RT-PCR of a PCR-positive T2 line shows no MKK5 suppression; (D) The closely related MAPKK, MKK4, is not suppressed in the MKK5-RNAi line (MKK5-2). Relative expression (B) was quantified using imageJ analysis software. The expressions were normalized to histone levels and expressed as % expression relative to control (100%).
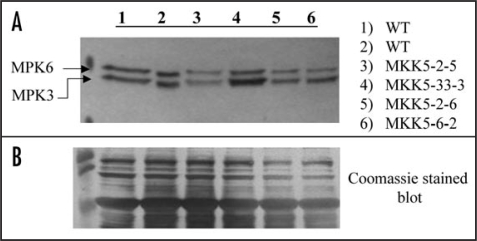
Figure 2.
Ozone-induced activation of two MAPKs is interdicted in MKK5-RNAi plants. (A) Extracted proteins (20 µg) from ozone fumigated (200 nL L−1, 10 min), five-week-old (T2) seedlings were fractionated by 10% SDS-PAGE, and transferred onto PVDF membranes. The resulting PVDF membrane was incubated using anti-phospho-ERK 1&2 antibodies, which have been shown to identify only the fully active forms of Arabidopsis MAPKs, MPK3 and MPK6. MKK5-suppressed lines show a reduction in the activation of both MPK3 (bottom band) and MPK6 (top band). Control plants (ozone) were harvested at the same time as the ozone-treated transgenic plants. (B) Coomassie stained PVDF membrane showing protein loading.
MKK4 and MKK5 are two closely related members of the Group C Arabidopsis MKKs, and share ∼78 % identity at the nucleotide level. The double-stranded RNA interference (dsRNA) construct directed at MKK5 targeted a unique N-terminal region of the gene which possesses ∼62% identity to MKK4 in the same region. To confirm that the RNAi construct specifically targeted MKK5, and not MKK4, the expression of MKK4 was analysed and found to be unaffected in the MKK5-RNAi plants (Fig 1D).
Since ozone strongly induces activation of both MPK3 and MPK6 in WT Arabidopsis,25 and MKK5 has been shown to activate both MPK3 and MPK6,32 we next evaluated the impact of MKK5 suppression on ozone-induced activation of MPK3 and MPK6. When the T2 and WT genotypes were exposed to ozone (200 nL L−1, 10 minutes), followed by western blot analysis using anti-pERK 1&2 antibodies which only recognize the fully active (doubly phosphorylated) forms of MPK3 and MPK6 in A. thaliana, a marked decrease in MPK3 and MPK6 activation was observed in the MKK5-suppressed lines (Fig. 2A), but not in the control lines (e.g., parental line 33), suggesting that MKK5 is necessary for full signal transmission to these MAPKs during ozone exposure. Here, we chose to fumigate using a lower concentration of ozone, 200 nL L−1, instead of the standard 500 nL L−1, in order to lessen the chance of killing the plants, thereby allowing these young plants to mature and set seeds. Only a limited number of transformed plant lines are represented in Figure 2.
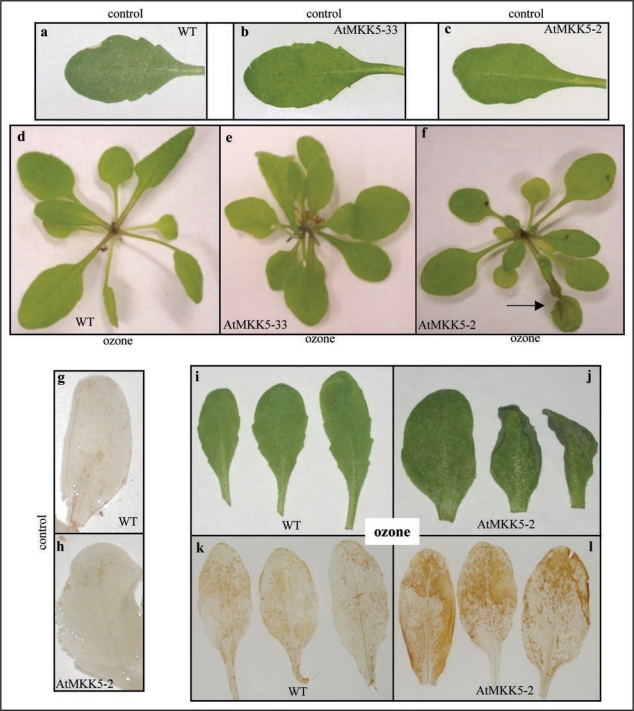
T2 MKK5-2 (RNAi) transgenic plants, along with WT and MKK5-33 plants, were exposed to either ambient air, or continuous ozone fumigation (500 nL L−1) for four hours and examined four hours post-fumigation (eight hr total time) for visible leaf damage. Leaf damage was consistently observed in leaves of MKK5-suppressed plants (Fig. 3F and J) at the eight hour time-point, whereas no damage was detected on WT (Fig. 3D and I) or MKK5-33 (Fig. 3E) leaves.
Figure 3.
Ozone treatment of WT and transgenic plants induces visible leaf damage and an elevated level of hydrogen peroxide. (a, d and i) = WT, (b and e) = MKK5-33, (c, f and j) = MKK5-2. Transgenic plants, together with WT Plants, were fumigated with ozone (500 nL L−1) for four hours and subsequently photographed four hours post-fumigation (eight-hours total response time). 3,3′-Diaminobenzidine staining was used to detect hydrogen peroxide accumulation in leaf tissue both before and after ozone-fumigation (g and k) = WT, (h and l) = MKK5-2. Arrow (f) points to ozone-induced leaf damage. Air controls were grown and harvested at the same time as the ozone-treated plants.
This ozone-induced leaf damage was consistently observed in all transformed plants where MKK5 suppression was observed. Even at the four hour time-point (i.e., immediately after fumigation), a number of the ozone-treated MKK5-RNAi plants started to show visible leaf damage. MKK5-suppressed plants were found to be more sensitive to ozone (500 nL L−1, 8 hr) than MPK6-suppressed plants, but less sensitive than MPK3-Deletagene-(DG) plants (data not shown). After 24 hours of continuous ozone exposure (500 nL L−1), 100% of the MKK5-RNAi test plants showed visible leaf damage, while both WT and non-MKK5-suppressed control (positive transformants) plants showed none.
Interestingly, the ozone-damaged leaves on the MKK5-suppressed plants became very dry and brittle, but maintained their green colour throughout their life span. This rapid desiccation phenotype was previously observed in MPK3-DG and MPK6-suppressed (RNAi) genotypes after ozone fumigation25 and in Arabidopsis plants stably transformed with constitutively active forms of either MKK4 or MKK5.32 Ectopic overexpression of these constitutively active forms of MKK4 and MKK5 also resulted in a prolonged activation of MPK3 and MPK6, accumulation of leaf-localized hydrogen peroxide, and cell death.32 This process of rapid dehydration appears analogous to the response of leaves displaying hypersensitive response (HR) cell death elicited by an incompatible interaction with pathogens. Rapid dehydration is thought to play an important role in limiting the multiplication of pathogens by depriving them of water.33
Since the MKK5-RNAi transgenic plants displayed increased sensitivity to ozone, we asked whether this represented an inability of the ozone-challenged tissue to control the build-up of ROS, specifically, hydrogen peroxide. To investigate this possibility, the patterns of leaf-localised hydrogen peroxide accumulation in both WT and MKK5-suppressed plants following ozone exposure, were compared.
When both the control and ozone-treated leaves from WT and the MKK5-suppressed plants were infiltrated with 3,3′-diaminobenzidine (DAB) solution, the staining patterns revealed no hydrogen peroxide accumulation in untreated leaves from either the WT or transgenic plants (Fig. 3G and H, respectively). A similar result was recently reported for plants transformed with ‘kinase inactive’ MKK5.32 However, four hours of ozone-fumigation (500 nL L−1), resulted in a marked increase in DAB staining in the MKK5-suppressed plants relative to that observed in WT plants (Fig. 3K and L, respectively), consistent with the idea that MKK5-suppressed plants were less effective at controlling the redox environment within their tissues.
This not only suggests a role (direct or indirect) for MKK5 in the plant's overall capacity to manage oxidant stress arising through ozone exposure, but also reveals that, despite the high level of sequence similarity shared by MKK5 and MKK4, MKK4 is unable to fully compensate for the partial loss of MKK5.
Varying levels of MKK5 suppression were obtained in the MKK5-RNAi plants, but the inability to recover fully suppressed transgenic plants suggests that MKK5 may play a role in development and/or germination.
While the MKK5-suppressed plants show no signs of spontaneous lesion formation under normal growing conditions, their overall growth rate, both in soil and on nonselective media, is consistently somewhat slower than that of WT plants. MPK6-silenced Arabidopsis plants,34 as well as tobacco (Xanthi) plants in which SIPK expression is suppressed (Samuel and Ellis, unpublished data), have both been observed to display a slower growth rate compared to that of WT plants, suggesting that both MKK5 and MPK6/SIPK are involved in growth.
The ozone-induced tissue damage (cell death) associated with MKK5-suppression resembles that elicited during the hypersensitive response (HR) as a result of an incompatible pathogen interaction. This observation is consistent with the fact that both HR- and ozone-induced cell death are known to involve the rapid production of ROS, including hydrogen peroxide. This cell death phenotype has previously been reported in MPK3-DG and MPK6-suppressed (RNAi) genotypes after ozone fumigation25 and in Arabidopsis plants stably transformed with constitutively activated forms of either MKK4 or MKK5.32 ROS accumulation in challenged tissues is a hallmark of not only pathogen attack and ozone-stress, but all abiotic stresses. Ozone is thought to behave as a volatile general elicitor of plant defense reactions,35 leading to promiscuous signaling events by mimicking the HR-related ROS burst.
It is noteworthy that the activation profiles of MPK3 and MPK6 in the MKK5-suppressed background resemble those of WT plants during ozone exposure; i.e., activation of both MAPKs is initiated at ∼15 minutes, and returns to pre-fumigation (500 nL L−1) levels around 90 minutes. Only the amplitude of activation of the two MAPKs is reduced. By contrast, suppression of MPK6 results in an ozone-sensitivity phenotype that is accompanied by a more intense and protracted activation of MPK3, while silencing of MPK3 leads to a similar ozone-sensitivity phenotype and simultaneous misregulation of MPK6.25
Recently, it was shown in aerial parts (12-day-old seedlings) of MEKK1-silenced Arabidopsis plants, that hydrogen peroxide-induced activation of both MPK3 and MPK6, as determined by in vitro kinase assays with MBP as a substrate, was greatly increased compared to WT plants, whereas, MPK4 activation was completely blocked.36 These data suggest that MEKK1 acts as a positive regulator of MPK4, but as a negative regulator of MPK3 and MPK6 during hydrogen peroxide-induced stress in aerial tissues. It would be informative to examine the activity profiles of these MAPKs, in the MEKK1-silenced background, to determine whether they are protracted or still resemble those seen in WT plants. It should be noted that these authors report that in roots, ROS-induced (hydrogen peroxide) activation of MPK4 was highly compromised in MEKK1 mutant plants, whereas MPK6 only slightly and MPK3 not at all. Clearly, a more complete understanding of these complex signaling networks and how they are affected by oxidant-related stress will require additional research.
In conclusion, the data presented here show that MKK5 plays an important and non-redundant role in the plant's overall ability to respond to ozone-induced stress and further, unequivocally places MKK5 as one of the MAPKKs involved in ozone-induced signaling to both MPK3 and MPK6. In addition, our findings suggest that the role(s) of MKK4 in the plant's overall response to ozone-induced oxidant stress and signaling to MPK3 and MPK6 deserves closer investigation.
Arabidopsis thaliana cv Columbia plants were grown under greenhouse conditions and transformed (T0 plants) using the floral-dip method37 and Agrobacterium tumefaciens (EHA105) harboring the recombinant binary vector pBIN 19/pRT 101-MKK5-RNAi. All putative transformants (kanamycin-resistant seedlings), from multiple generations, were screened and grown as previously described.27 RNA was extracted from leaf tissue of five-week-old Arabidopsis thaliana cv Columbia plants, followed by RT-PCR, as previously described.25 All plants were firstly placed onto selective plates before moving the antibiotic resistant seedlings to soil.
The open reading frame of MKK5 (At3g21220) was amplified using the reverse transcriptase-mediated PCR method with gene-specific primers and RNA isolated from leaves of non-treated five-week-old Arabidopsis thaliana cv Columbia plants. The double stranded RNA interference (dsRNA) construct was produced via a PCR-mediated approach using the amplification products from a unique N-terminal region (307 bp) spanning a portion of the untranslated region and coding region of the MKK5 gene. A minimal intron based on the splice junctions and flanking regions belonging to the fourth intron of MPK6 (At2g43790) was integrated into the sense strand primer. The sense strand was then amplified using a primer combination that generated a BamH1 restriction site and intron-Xba1 sequence on the opposed ends of the product, whereas the anti-sense strand was amplified using a primer combination that added BamH1 and Xba 1 restriction sites on the opposite ends of the product. These two products were then cloned into Xho1/BamH1-cut Bin19/pRT101 by means of a triple ligation to place the RNAi construct under the control of the CaMV 35S promoter in a pRT101-MKK5-RNAi plasmid. This construct model has been shown previously to effectively silence salicylate-induced protein kinase (SIPK) in tobacco38 and Arabidopsis.25
Primers used for this work are as follows: MKK5: (forward): 5′-CCC TCG AGA AAG CCA TGA AAC CGA TTC AAT CTC CTT CTG GA-3′, (reverse): 5′-GCT CTA GAC TAA GAG GCA GAA GGA AGA GGA CG-3′, RNAi (sense-forward, S1): 5′-CCC TCG AGA AAG CCA TGA AAC CGA TTC AAT CTC CTT CTG GA-3′, RNAi (sense-intron-reverse, S2): 5′GCT CTA GAC TAT GAA GCT GCA AAA ACT ACT TAC CTC CAC TTT GAG AAA AGG ACG TGA CGT-3′, RNAi (antisense forward, AS1): 5′-CGG GAT CCA AAG CCA TGA AAC CGA TTC AAT CTC CTT CTG GA-3′, RNAi (antisense reverse, AS2): 5′-GCT CTA GAC ACT TTG AGA GCG AAA GGA CGT GAC GT-3′; MKK4: (forward): 5′-GAA GAA CGA ATC AAT TTA AGC CTG-3′, (reverse): 5′-TGG GGA TAC ATG CAC CAT CAT AAG-3′; AtH1: (forward): 5′-GGT TAA AGT CAA GCT TCT TTT AAG A-3′, (reverse): 5′-GAG TGA AGA AAC CAT CAC ATT ATA-3′; 35S (forward): 5′-ATG ACG CAC AAT CCC ACT-3′.
Five-week-old Arabidopsis thaliana cv Columbia plants (WT and MKK5-RNAi) were grown either on selective plates or in soil, under environmentally controlled conditions (25/20°C, 16 hr light/8 hr dark cycle), or under greenhouse conditions. Ozone fumigation was performed using a flow-through chamber for different times as previously described for tobacco (Nicotiana tabacum) plants22 and foliage was then harvested, frozen in liquid nitrogen and stored at −80°C.
Crude protein extracts, from both control- and ozone exposed-plants, were obtained from rosette leaf tissue as previously described.25 Western blot analysis (20 or 40 µg) was performed using mammalian protein kinases (anti-pERK 1&2), which have been successfully used to detect the presence of homologous proteins in plants.23,25,38 These antibodies recognize only the doubly phosphorylated (-TxY-) epitope that confers activation of MAPKs such as MPK3 and MPK6, thus providing a convenient method to monitor the activation of ERK1&2 homologous MAPKs in plants. In Arabidopsis, the only the phosphorylated proteins recognized with anti-pERK antibodies are, MPK3 and MPK6, period.
The presence of H2O2 was visualized in situ via DAB staining as previously described.25 Arabidopsis rosette leaves were collected four hours post-ozone fumigation (500 nL L−1) from five-week-old Arabidopsis WT and MKK5-RNAi genotypes.
Acknowledgements
The Natural Sciences and Engineering Research Council of Canada provided funding for this research. At the time this work was completed, G.P.M. and M.A.S. held graduate fellowships from the University of British Columbia.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/9298
References
- 1.Koch JR, Scherzer AJ, Eshita SM, Davis KR. Ozone sensitivity in hybrid poplar is correlated with the lack of defense-gene activation. Plant Physiol. 1998;118:1243–1252. doi: 10.1104/pp.118.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langebartels C, Heller W, Fuhrer G, Lippert M, Simons S, Sandermann H., Jr Memory effects in the action of ozone on conifers. Ecotoxicol Environ Saf. 1998;41:62–72. doi: 10.1006/eesa.1998.1668. [DOI] [PubMed] [Google Scholar]
- 3.Reich PB, Amundson RG. Ambient levels of O3 reduce net photosynthesis in tree and crop species. Science. 1985;230:566–570. doi: 10.1126/science.230.4725.566. [DOI] [PubMed] [Google Scholar]
- 4.Coleman MD, Dickerson RE, Isebrands JG, Karnosky DF. Carbon utilization allocation and partitioning in aspen clones varying in sensitivity to tropospheric ozone. Tree Physiol. 1995;15:593–604. doi: 10.1093/treephys/15.9.593. [DOI] [PubMed] [Google Scholar]
- 5.Brendley BW, Pell EJ. Ozone-induced changes in biosynthesis of Rubisco and associated compensation to stress in foliage of hybrid poplar. Tree Physiol. 1998;18:81–90. doi: 10.1093/treephys/18.2.81. [DOI] [PubMed] [Google Scholar]
- 6.Guidi L, Nali C, Lorenzini G, Filippi F, Soldatini GF. Effect of chronic ozone fumigation on the photosynthetic process of poplar clones showing different sensitivity. Environ Pollut. 2001;113:245–254. doi: 10.1016/s0269-7491(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 7.Sharma YK, Davis KR. The effects of ozone on antioxidant responses in plants. Free Radic Biol Med. 1997;23:480–488. doi: 10.1016/s0891-5849(97)00108-1. [DOI] [PubMed] [Google Scholar]
- 8.Runeckles VC, Vaartnou M. EPR evidence for superoxide anion formation in leaves during exposure to low levels of ozone. Plant Cell Environ. 1997;20:306–314. [Google Scholar]
- 9.Pellinen R, Palva T, Kangasjärvi J. Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J. 1999;20:349–356. doi: 10.1046/j.1365-313x.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- 10.Finkel F. Oxygen radicals and signalling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 11.Branco MR, Marinho HS, Cyrne L, Antunes F. Decrease of H2O2 Plasma Membrane Permeability during Adaptation to H2O2 in Saccharomyces cervisiae. J Biol Chem. 2004;279:6501–6506. doi: 10.1074/jbc.M311818200. [DOI] [PubMed] [Google Scholar]
- 12.Fenton HJH. The oxidation of tartaric acid in the presence of iron. J Chem Soc Proc. 1894;10:157–158. [Google Scholar]
- 13.Haber F, Willstätter R. Unpaarigheit und radikalketten im reaktion-mechanismus organischer und enzymatischer Vorgänge. Chem Ber. 1931;64:2844–2856. (Ger). [Google Scholar]
- 14.Haber F, Weiss J. Über die katalyse des hydroperoxydes. Naturwiss. 1932;51:948–950. (Ger). [Google Scholar]
- 15.Grimes HD, Perkins KK, Boss WF. Ozone degrades into hydroxyl radicals under physiological conditions. Plant Physiol. 1983;72:1016–1020. doi: 10.1104/pp.72.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storz G, Tartaglia LA, Ames BN. Transcriptional regulator of oxidative stress inducible genes: direct activation by oxidation. Science. 1990;248:189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- 17.Hardie DG. Plant protein serine/threonine kinases: classification and function. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:97–131. doi: 10.1146/annurev.arplant.50.1.97. [DOI] [PubMed] [Google Scholar]
- 18.Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, et al. Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci. 2002;7:301–308. doi: 10.1016/s1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- 19.Boulton TG, Nye SH, Robbins DG, Ip NY, Radziejewska E, Morgenbesser SD, et al. ERKs: a family of serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 20.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;1:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 21.Schenk PW, Snaar-Jagalska BE. Signal perception and transduction: the role of protein kinases. Biochim Biophys Acta. 1999;1449:1–24. doi: 10.1016/s0167-4889(98)00178-5. [DOI] [PubMed] [Google Scholar]
- 22.Samuel MA, Miles GP, Ellis BE. Ozone treatment rapidly activates MAP kinase signalling in plants. Plant J. 2000;22:367–376. doi: 10.1046/j.1365-313x.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- 23.Miles GP, Samuel MA, Ellis BE. Suramin inhibits oxidant-induced MAPK signalling in plants. Plant Cell Environ. 2002;25:521–527. [Google Scholar]
- 24.Miles GP, Samuel MA, Jones AM, Ellis BE. Mastoparan rapidly activates plant MAPkinase signalling independent of heterotrimeric G proteins. Plant Physiol. 2004;134:1332–1336. doi: 10.1104/pp.103.037275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miles GP, Samuel MA, Zhang Y, Ellis BE. RNA interference-based (RNAi) suppression of MPK6, an Arabidopsis mitogen-activated protein kinase, results in hypersensitivity to ozone and misregulation of MPK3. Environ Pollut. 2005;138:230–237. doi: 10.1016/j.envpol.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Pitzschke A, Hirt H. Mitogen-activated kinases and reactive oxygen species signalling in plants. Plant Physiol. 2006;141:351–356. doi: 10.1104/pp.106.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colcombet J, Hirt H. Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J. 2008;413:217–226. doi: 10.1042/BJ20080625. [DOI] [PubMed] [Google Scholar]
- 28.Desikan R, Clarke A, Atherfold P, Hancock JT, Neill SJ. H2O2 activates a MAPkinase-like enzyme in Arabidopsis thaliana suspension cultures. J Exp Bot. 1999;50:1863–1866. [Google Scholar]
- 29.Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. PNAS. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rentel MC, Lecourleux D, Ouaked F, Usher SL, Peterson L, Okamoto H, et al. OXl1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature. 2004;427:858–861. doi: 10.1038/nature02353. [DOI] [PubMed] [Google Scholar]
- 31.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 32.Ren D, Yang Y, Zhang S. Cell death is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem. 2002;277:559–565. doi: 10.1074/jbc.M109495200. [DOI] [PubMed] [Google Scholar]
- 33.Goomatchtig S, Capoen W, James EK, Holsters M. Switch from intracellular to intercellular invasion during water stress-tolerant legume nodulation. PNAS. 2004;101:6303–6308. doi: 10.1073/pnas.0401540101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menke FLH, van Pelt JA, Pieterse CMJ, Klessig DF. Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell. 2004;16:897–907. doi: 10.1105/tpc.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandermann H, Ernst D, Heller W, Langerbartels C. Ozone: an abiotic elicitor of plant defence reactions. Trends Plant Sci. 1998;3:47–50. [Google Scholar]
- 36.Nakagami H, Soukupova H, Schikora A, Zarsky V, Hirt H. A Mitogen-Activated protein Kinase Kinase Kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem. 2006;281:38697–38704. doi: 10.1074/jbc.M605293200. [DOI] [PubMed] [Google Scholar]
- 37.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 38.Samuel MA, Ellis BE. Double jeopardy: both overexpression and suppression of a redoxactive plant Mitogen-Activated Protein Kinase render Tobacco plants ozone sensitive. Plant Cell. 2002;14:2059–2069. doi: 10.1105/tpc.002337. [DOI] [PMC free article] [PubMed] [Google Scholar]