Abstract
A broad range of chemical plant defenses against herbivores has been studied extensively under laboratory conditions. In many of these cases there is still little understanding of their relevance in nature. In natural systems, functional analyses of plant traits are often complicated by an extreme variability, which affects the interaction with higher trophic levels. Successful analyses require consideration of the numerous sources of variation that potentially affect the plant trait of interest. In our recent study on wild lima bean (Phaseolus lunatus L.) in South Mexico, we applied an integrative approach combining analyses for quantitative correlations of cyanogenic potential (HCNp; the maximum amount of cyanide that can be released from a given tissue) and herbivory in the field with subsequent feeding trials under controlled conditions. This approach allowed us to causally explain the consequences of quantitative variation of HCNp on herbivore-plant interactions in nature and highlights the importance of combining data obtained in natural systems with analyses under controlled conditions.
Key words: natural systems, plant defensive traits, optimal defense hypothesis (ODH), cyanogenesis, lima bean, Phaseolus lunatus L., plant-herbivore interaction, plant-pathogen interaction, multiple defense syndromes
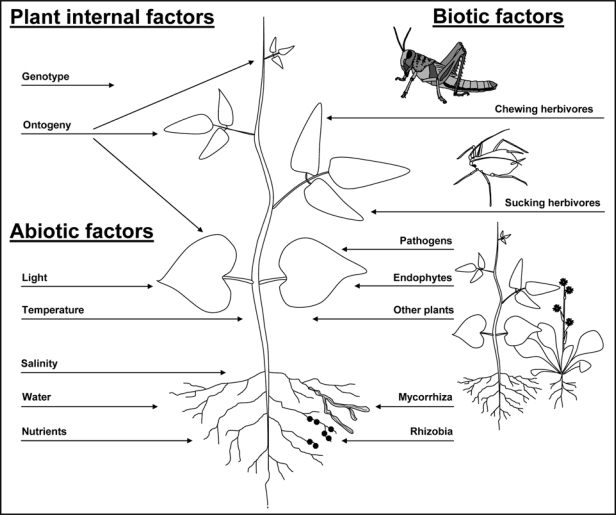
Analyzing plant defenses against herbivores in nature is often complicated by an extreme variability in multiple factors. Plant populations generally show high genetic variability resulting in substantial intraspecific variation of plant traits.1 In addition to genotypic variability, phenotypic plasticity of plants is a source of variation.2 At the level of individual plants, expression of defensive traits strongly depends on plant organ and ontogeny of plants or plant parts. Within an individual plant, it is quite common for reproductive structures and young leaves to be better chemically defended than older leaf tissues. To explain these within-plant variations of defenses, the optimal defense hypothesis (ODH) was formulated. Concerning the variability of chemical defenses of leaves, the ODH predicts that within the total foliage of a plant, young leaves make a larger contribution to plant fitness than old leaves as they have a higher potential photosynthetic value resulting from a longer expected life-time.3–5 In addition, younger leaves are often more nutritious and thus more attractive to herbivores6 and should consequently be better defended.7 In this line, the basic assumption of the ODH is that three main factors—cost of defense, risk of attack and value of the respective plant organ—determine the investment in defensive secondary metabolites.8,9 Thus, the higher the risk of a given plant tissue to be consumed by herbivores and the higher its value for plant fitness, the more energy should be allocated to its defense.10,11 Beyond genotypic and ontogenetic variability of a given defense, potential co-variation with other defensive or nutritive traits expressed by the same plant individual can strongly affect its efficiency as defense against herbivores.12,13 In addition to these endogenous sources of defensive variability, the expression of plant traits strongly depends on multiple external factors such as temperature or availability of plant nutrients, water or light (Fig. 1).14 At the same time, the outcome of herbivore-plant interactions is crucially determined by biotic interactions. Plant interactions with mutualistic microorganisms such as Rhizobia, mycorrhiza and above-ground fungal endophytes as well as tri-trophic interactions with predators and parasitoids of herbivores can all strongly impact plant fitness.15
Figure 1.
Factors influencing variability of plant defenses. Plant defensive traits are affected by various endogenous and external factors. Endogenous factors comprise plant genotype and ontogeny of plants or plant parts. External factors can be categorized as abiotic or biotic. Important abiotic factors that can influence plant defenses are light exposure, temperature, soil salinity, as well as water and nutrient availability. Biotic factors that can have an effect on plant defense are interspecific interaction with Rhizobia (in the case of legumes), mycorrhizal and endophytic fungi, pathogens as well as the interaction with conspecifics or different plant species.
Variability in herbivore-plant interactions can also be associated with herbivore variation. Different attackers of a particular plant species might be affected in different ways by toxins in food plants (Fig. 1). The efficiency of a specific defensive compound can also depend on the feeding mode, i.e., sucking or chewing, as well as on the degree of specialization of the herbivore to the respective plant.16 Defenses mediated by secondary plant compounds are generally believed not to affect specialist herbivores, because of their capacity to tolerate or to detoxify defensive compounds of their hosts by behavioral or physiological adaptations.17–20 In this context, the specialist herbivore paradigm predicts that adapted herbivores are less affected by a given chemical defense than generalists,21,22 although exceptions have been noted.23–25
While it is important to consider these numerous sources of variation affecting the outcome of herbivore-plant interactions when designing functional studies, a significant fraction of the variability in natural systems will always remain unidentified. Consequently, approaches combining field observations with experiments under controlled conditions provide a powerful tool to uncover functional interactions between plants and their multiple antagonists in nature.
In a recent study, we analyzed the importance of wild lima bean's cyanogenesis—i.e., the release of toxic hydrogen cyanide from preformed precursors in response to cell damage—as plant defense at a natural site in South Mexico.25 Although cyanogenesis is generally considered an efficient direct defense against herbivores, in numerous studies plant cyanide production had little or no effect on herbivores.26–28 One would like to think that most of these inconsistencies in cyanogenesis-based herbivore defense efficiency could be explained by one or more sources of variation mentioned above. Nevertheless, field studies analyzing the action of plant cyanogenesis on a quantitative basis have been scarce. In our study, a two-step approach was used to gain insight into the function of cyanogenesis in nature.25 First, cyanide concentration and herbivore damage were quantified by measuring removed leaf area of individual leaves derived from different individual plants while considering microclimate conditions. Significant negative correlations between cyanogenesis and leaf damage were observed. Second, since existing correlations do not necessarily indicate causal associations, we conducted consecutive feeding experiments under controlled conditions. To consider natural variability of lima beans' cyanogenesis observed in nature in our analysis, we prepared clones from field-grown plants with different but defined cyanogenic features. These clonal plants showed high constancy of cyanogenic traits compared to their respective mother plants and thus, could be used in comparative analyses. Every effort was made to duplicate natural conditions and so herbivore species selected for feeding trials represented those identified in the field as the most important plant consumers at the respective site (pers. observ.). Feeding trials supported our hypothesis that cyanogenesis has quantitative effects on herbivore behavior in nature and explained the negative correlation of lima bean's cyanogenesis and herbivory observed in the field.
Analytical approaches combining field observations with controlled experiments help to explain natural patterns and may represent a powerful methodological approach for functional analyses of herbivore-plant interactions.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/9088
References
- 1.Baudoin JP, Barthelemy YJ, Ndungo V. Variability of cyanide contents in the primary and secondary genepools of lima bean, Phaseolus lunatus L. FAO/IBPGR. Plant Gen Res Newsl. 1991;85:5–8. [Google Scholar]
- 2.Schlichting CD, Pigliucci M. Phenotypic evolution: A reaction norm perspective. Sunderland: Sinauer Associates; 1998. [Google Scholar]
- 3.Coley PD, Bryant JP, Chapin FS., III Resource availability and plant antiherbivore defense. Science. 1985;230:895–899. doi: 10.1126/science.230.4728.895. [DOI] [PubMed] [Google Scholar]
- 4.Coley PD. Effects of plant growth rate and leaf lifetime on the amount and type of antiherbivore defense. Oecologia. 1988;74:531–536. doi: 10.1007/BF00380050. [DOI] [PubMed] [Google Scholar]
- 5.Coley PD. Effects of leaf age and plant life history patterns on herbivory. Nature. 1980;284:545–546. [Google Scholar]
- 6.Calvo D, Molina JM. Effects of tangerine (Citrus reticulata) foliage age on Streblote panda larval development and performance. Phytoparasitica. 2005;33:450–459. [Google Scholar]
- 7.Anderson P, Agrell J. Within-plant variation in induced defence in developing leaves of cotton plants. Oecologia. 2005;144:427–434. doi: 10.1007/s00442-005-0095-3. [DOI] [PubMed] [Google Scholar]
- 8.Rhoades DF. Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH, editors. Herbivores: their interaction with secondary plant metabolites. New York and London: Academic Press; 1979. pp. 4–53. [Google Scholar]
- 9.Stamp N. Theory of plant defensive level: example of process and pitfalls in development of ecological theory. Oikos. 2003;102:672–678. [Google Scholar]
- 10.Zangerl AR, Bazzaz FA. Theory and pattern in plant defense allocation. In: Fritz RS, Simms EL, editors. Plant resistance to herbivores and pathogens: ecology, evolution and genetics. Chicago, Ilinois, USA: University of Chicago Press; 1992. pp. 363–391. [Google Scholar]
- 11.Rostas M, Eggert K. Ontogenetic and spatio-temporal patterns of induced volatiles in Glycine max in the light of the optimal defence hypothesis. Chemoecology. 2008;18:29–38. [Google Scholar]
- 12.Ballhorn DJ, Kautz S, Lion U, Heil M. Qualitative variability of lima bean's VOC bouquets and its putative consequences on plant-plant communication. Plant Signal Behav. 2008;3:1005–1007. doi: 10.4161/psb.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballhorn DJ, Kautz S, Lion U, Heil M. Trade-offs between direct and indirect defences of lima bean (Phaseolus lunatus) J Ecol. 2008;96:971–980. [Google Scholar]
- 14.Lovelock CE, Ball MC, Martin KC, Feller IC. Nutrient enrichment increases mortality of mangroves. PLoS ONE. 2009:e5600. doi: 10.1371/journal.pone.0005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer MS, Mace KC, Bernays EA. Self-medication as adaptive plasticity: Increased ingestion of plant toxins by prasitized caterpillars. PLoS ONE. 2009:e4796. doi: 10.1371/journal.pone.0004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon JM, White WLB, Sayre RT. Cyanogenesis in Cassava (Manihot esculenta Crantz) J Exp Bot. 1995;46:731–741. [Google Scholar]
- 17.Compton SG, Jones DA. An investigation of the responses of herbivores to cyanogenesis in Lotus corniculatus L. Biol J Linn Soc. 1985;26:21–38. [Google Scholar]
- 18.Nahrstedt A. Cyanogenic compounds as protecting agents for organisms. Plant Syst Evol. 1985;150:35–47. [Google Scholar]
- 19.Rausher MD. Genetic analysis of coevolution between plants and their natural enemies. Trends Genet. 1996;12:212–217. doi: 10.1016/0168-9525(96)10020-2. [DOI] [PubMed] [Google Scholar]
- 20.Zagrobelny M, Bak S, Rasmussen AV, Jorgensen B, Naumann CM, Moller BL. Cyanogenic glucosides and plant-insect interactions. Phytochemistry. 2004;65:293–306. doi: 10.1016/j.phytochem.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Van der Meijden E. Plant defence, an evolutionary dilemma: Contrasting effects of (specialist and generalist) herbivores and natural enemies. Entomol Exp Appl. 1996;80:307–310. [Google Scholar]
- 22.van Dam NM, Hadwich K, Baldwin IT. Induced responses in Nicotiana attenuata affect behavior and growth of the specialists herbivore Manduaca sexta. Oecologia. 2000;122:371–379. doi: 10.1007/s004420050043. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal AA, Kurashige NS. A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J Chem Ecol. 2003;29:1403–1415. doi: 10.1023/a:1024265420375. [DOI] [PubMed] [Google Scholar]
- 24.Ballhorn DJ, Heil M, Pietrowski A, Lieberei R. Quantitative effects of cyanogenesis on an adapted herbivore. J Chem Ecol. 2007;33:2195–2208. doi: 10.1007/s10886-007-9380-4. [DOI] [PubMed] [Google Scholar]
- 25.Ballhorn DJ, Kautz S, Heil M, Hegeman AD. Cyanogenesis of wild lima bean (Phaseolus lunatus L.) is an efficient direct defence in nature. PLoS ONE. 2009:e5450. doi: 10.1371/journal.pone.0005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scriber JM. Cyanogenic glycosides in Lotus corniculatus—their effect upon growth, energy budget and nitrogen utilization of southern armyworm, Spodoptera eridania. Oecologia. 1978;34:143–155. doi: 10.1007/BF00345163. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira C, Parra JRP, Terra WR. The effect of dietary plant glycosides on larval midgut beta-glucosidases from Spodoptera frugiperda and Diatraea saccharalis. Insect Biochem Mol Biol. 1997;27:55–59. [Google Scholar]
- 28.Glander KE, Wright PC, Seigler DS, Radrianasolo V, Randrianasolo B. Consumption of cyanogenic bamboo by a newly discovered species of bamboo lemur. A J Primatol. 1989;19:119–124. doi: 10.1002/ajp.1350190205. [DOI] [PubMed] [Google Scholar]