Abstract
Conversion of glycerol to glycerol-3-phosphate (G3P) is one of the highly conserved steps of glycerol metabolism in evolutionary diverse organisms. In plants, G3P is produced either via the glycerol kinase (GK)-mediated phosphorylation of glycerol, or via G3P dehydrogenase (G3Pdh)-mediated reduction of dihydroxyacetone phosphate (DHAP). We have recently shown that G3P levels contribute to basal resistance against the hemibiotrophic pathogen, Colletotrichum higginsianum. Since a mutation in the GLY1-encoded G3Pdh conferred more susceptibility compared to a mutation in the GLI1-encoded GK, we proposed that GLY1 is the major contributor of the total G3P pool that participates in defense against C. higginsianum.
Key words: glycerol-3-phosphate, glycerol metabolism, defense, signaling
Glycerol and its metabolites are involved in a variety of physiopathological processes in both prokaryotes and eukaryotes, most of which appear to be highly conserved,1 signifying the fundamental importance of these molecules. Glycerol-3-phosphate (G3P), an obligatory component of energy-producing reactions including glycolysis and glycerolipid biosynthesis, participates in the disease-related physiologies of many organisms. In humans, deficiencies in glycerol kinase activity (catalyzing the phosphorylation of glycerol to G3P) result in a variety of metabolic and neurological disorders, while mutations in G3P dehydrogenase (G3Pdh, catalyzing the oxidation of dihydroxyacetone phosphate, DHAP, to G3P) have been linked to sudden infant death syndrome and decreased cardiac Na2+ current resulting in ventricular arrhythmias and sudden death.2,3 Given the fact that glycerol metabolism is conserved between plants and animals, it is conceivable that glycerol and/or G3P might also participate in disease physiology of plants. However, such a role for glycerol and/or G3P remains unexplored.
Previous work from our laboratory and others has shown that GLY1-encoded G3Pdh plays an important role in plastidal oleic acid-mediated signaling4–7 and systemic acquired resistance.8 This group of enzymes also plays an important role in fungi and it was recently shown that the disruption of a G3Pdh gene in Colletotrichum gloeosporioides eliminated the ability of the mutant fungus to grow on most carbon sources in vitro, including amino acids and glucose.9 However, the G3Pdh knockout (KO) fungus grew normally in the presence of glycerol. The G3Pdh KO fungus also developed normally in its plant host (the round-leaved mallow), prompting the suggestion that glycerol, rather than glucose or sucrose, was the primary transferred source of carbon in planta. This was an unexpected finding, but direct analysis of infected host leaves revealed that their glycerol content did decrease by 40% within 48 hours of infection with C. gloeosporioides.9 Since the hemibiotroph C. gloeosporioides appears to be able to utilize glycerol for growth and conidiation in planta, it was possible that glycerol metabolism and associated pathways in the host played an important role in the establishment of infections by Colletotrichum fungi. Furthermore, it was possible that the host had evolved to sense these pathogen-mediated changes in glycerol levels and utilize them as signal(s) to initiate defense.
We tested these possibilities by characterizing the role of glycerol metabolism in the Arabidopsis—C. higginsianum interaction (Fig. 1). Infection with C. higginsianum reduced the glycerol content while concomitantly increasing the G3P content in Arabidopsis plants.10 Mutations in G3P-synthesizing genes gly1 (a G3Pdh) and gli1 (a glycerol kinase),11 resulted in enhanced susceptibility to C. higginsianum. The gly1 plants were much more susceptible than the gli1 plants, suggesting that GLY1-encoded G3Pdh played a more important role in basal resistance to C. higginsianum. Conversely, the act1 mutant, which is impaired in the acylation of G3P with oleic acid (18:1) (Fig. 1), was more resistant to the fungus. The phenotypes seen in the infected gly1 and act1 plants correlated with pathogen-induced G3P levels; C. higginsianum inoculation induced ∼2-fold higher accumulation of G3P in the act1 plants, and ∼2-fold lower G3P in the susceptible gly1 plants, as compared to wild-type plants.10 To test the hypothesis that G3P synthesized via GLY1 entered the plastidial glycerolipid pathway via the ACT1 catalyzed reaction, we generated act1 gly1 plants. The results supported the hypothesis, as act1 gly1 plants were as susceptible to C. higginsianum as gly1 plants.
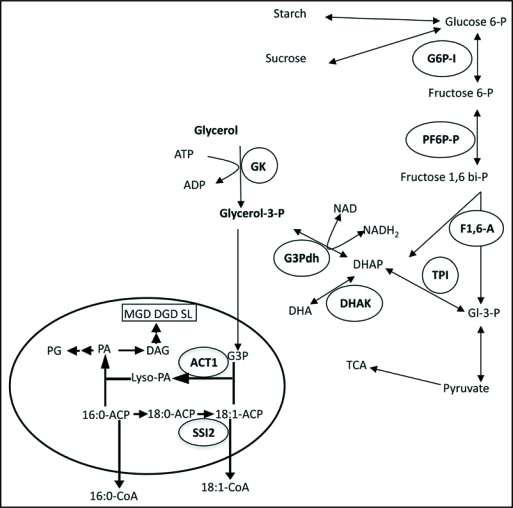
Figure 1.
A condensed scheme of glycerol metabolism in plants. Glycerol is phosphorylated to glycerol-3-phosphate (G3P) by glycerol kinase (GK; GLI1). G3P can also be generated by G3P dehydrogenase (G3Pdh) via the reduction of dihydroxyacetone phosphate (DHAP) in both the cytosol and the plastids (represented by the oval). G3P generated by this reaction can be transported between the cytosol and plastid stroma. In the plastids G3P is acylated with oleic acid (18:1) by the ACT1-encoded G3P acyltransferase. This ACT1-utilized 18:1 is derived from the stearoyl-acyl carrier protein (ACP)-desaturase (SSI2)-catalyzed desaturation of stearic acid (18:0). The 18:1-ACP generated by SSI2 either enters the prokaryotic lipid biosynthetic pathway through acylation of G3P, or is exported out of the plastids as a coenzyme A (CoA)-thioester to enter the eukaryotic lipid biosynthetic pathway. Other abbreviations used are: PA, phosphatidic acid; Lyso-PA, acyl-G3P; PG, phosphatidylglycerol; MGD, monogalactosyldiacylglycerol; DGD, digalactosyl-diacylglycerol; SL, sulfolipid; DAG, diacylglycerol; DHA, dihydroxyacetone; Gl-3-P, glyceraldehyde-3-phosphate; TCA, tricarboxylic acid cycle. Enzymes as abbreviated as: ACT1, G3P acyltransferase; SSI2, stearoyl acyl carrier protein desaturase; GK, glycerol kinase; G3Pdh, G3P dehydrogenase; TPI, triose phosphate isomerase; DHAK, dihydroxyacetone kinase; F1,6-A, fructose 1,6-biphosphate aldolase; PF6P-P, pyrophosphate fructose-6-phosphate phosphotransferase; G6P-I, glucose-6-phosphate isomerase.
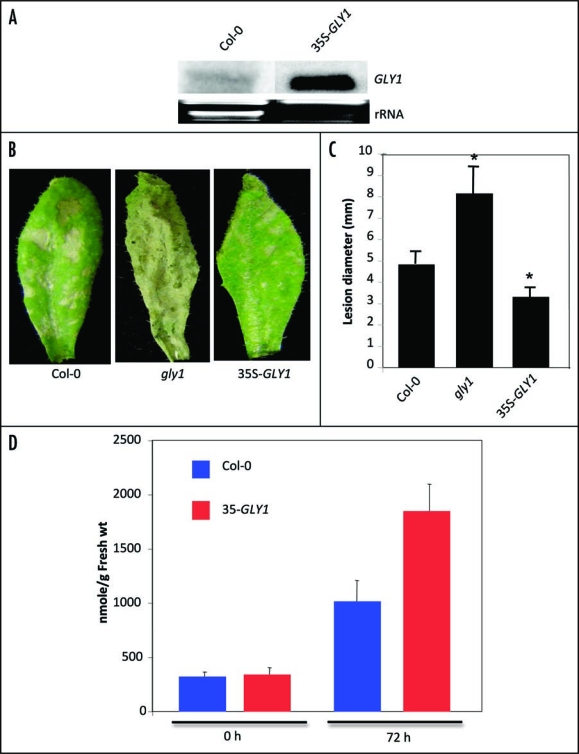
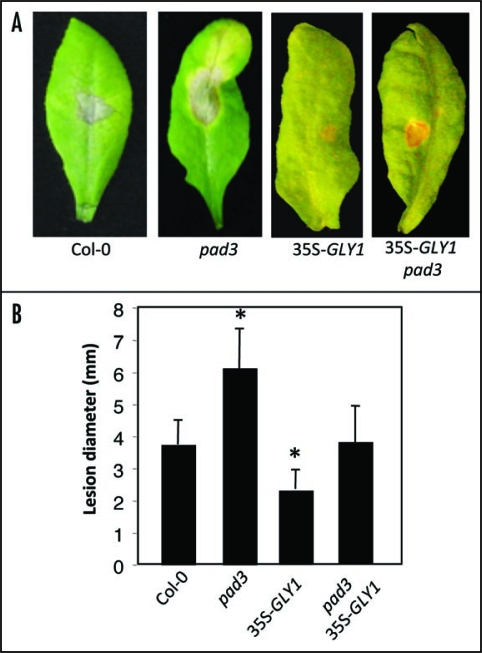
More supporting evidence for the role of G3P in defense against C. higginsianum was obtained by overexpressing GLY1 in wild-type plants (Fig. 2A). Similar to act1, overexpression of GLY1 led to a ∼2-fold increase in G3P levels after pathogen inoculation, and these plants were also more resistant to C. higginsianum (Fig. 2B–D). Furthermore, plants overexpressing GLY1 or carrying a mutation in ACT1 exhibited enhanced resistance to C. higginsianum in the pad3 mutant background (Fig. 3).10 The pad3 plants are compromised in camalexin synthesis, and are hypersusceptible to necrotrophic pathogens.
Figure 2.
Pathogen response and G3P levels in transgenic lines overexpressing GLY1. (A) Expression of the GLY1 gene in wild-type or 35S-GLY1 transgenic plant. RNA gel blot analysis was performed on ∼7 µg of total RNA. Ethidium bromide staining of rRNA was used as a loading control. (B) Disease symptoms in C. higginsianum-inoculated Col-0, gly1 or 35S-GLY1 plants at 5 dpi. The plants were spray-inoculated with 106 spores/ml of C. higginsianum. (C) Lesion size in spot-inoculated genotypes. The plants were spot-inoculated with water or 106 spores/ml and the lesion size was measured from 20–30 independent leaves at 6 dpi. Statistical significance was determined using Students t-test. Asterisks indicate data that is statistically significant from that of control (Col-0) (p < 0.05). Error bars indicate SD. (D) G3P levels in Col-0 and 35S-GLY1 plants at 0 and 72 h post-inoculation.
Figure 3.
Pathogen response in C. higginsianum-inoculated 35S-GLY1 plants in pad3 background. (A) Disease symptoms on Col-0, pad3 or 35S-GLY1 or 35S-GLY1 pad3 plants spot-inoculated with 106 spores/ml of C. higginsianum. The leaves were photographed at 7 dpi. (B) Lesion size in spot-inoculated Col-0, pad3 or 35S-GLY1 or 35S-GLY1 pad3 plants. The lesion size was measured from 20–30 independent leaves at 7 dpi. Asterisks indicate data that is statistically significant from that of control (Col-0) (p < 0.05). Error bars indicate SD.
Exogenous glycerol application increased endogenous G3P and significantly enhanced the ability of the host to resist C. higginsianum.10 Glycerol-triggered synthesis of G3P also caused a decrease in 18:1 levels, which is known to induce defense signaling, resulting in enhanced basal resistance.4–7 However, the glycerol-triggered increase in G3P precedes the reduction in 18:1 levels and confers resistance even at time points when low 18:1-mediated signaling is not induced, suggesting that the enhanced resistance after glycerol treatment was due to elevated G3P levels and not to the reduction in 18:1.
Understanding the precise roles of G3P will require in-depth analysis of real-time alterations in its levels on a cellular level during pathogenesis. This is complicated by the presence of multiple isoforms of G3Pdh that contribute to the total G3P pool, and by the lack of appropriate tools for monitoring precise changes in intracellular G3P. Systematic analysis of various G3Pdh mutants, in combination with each other and with gli1, should yield novel insights into pathway(s) and steps regulating levels of G3P in the cell.
Acknowledgements
This work was supported by grants from the USDA-NRI (200403287, 2006-01854), NSF (MCB#0421914, IOS#0749731) and the Kentucky Science and Engineering Foundation (622RDE-006).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/9111
References
- 1.Brisson D, Vohl M-C, St-Pierre J, Hudson T, Gaudet D. Glycerol: a neglected variable in metabolic processes? Bioessays. 2001;23:534–542. doi: 10.1002/bies.1073. [DOI] [PubMed] [Google Scholar]
- 2.Van Norstrand DW, Valdivia CR, Tester DJ, Ueda K, London B, Makielski JC, Ackerman MJ. Molecular and functional characterization of novel glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) mutations in sudden infant death syndrome. Circulation. 2007;116:2253–2259. doi: 10.1161/CIRCULATIONAHA.107.704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, et al. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116:2260–2268. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kachroo A, Venugopal SC, Lapchyk L, Falcone D, Hildebrand D, Kachroo P. Oleic acid levels regulated by glycerolipid metabolism modulate defense gene expression in Arabidopsis. Proc Natl Acad Sci USA. 2004;101:5152–5157. doi: 10.1073/pnas.0401315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kachroo P, Venugopal SC, Navarre DA, Lapchyk L, Kachroo A. Role of salicylic acid and fatty acid desaturation pathways in ssi2-mediated signaling. Plant Physiol. 2005;139:1717–1735. doi: 10.1104/pp.105.071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra-Shekara AC, Venugopal SC, Barman SR, Kachroo A, Kachroo P. Plastidial fatty acid levels regulate resistance gene-dependent defense signaling in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:7277–7282. doi: 10.1073/pnas.0609259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia Y, Gao Q-M, Yu K, Navarre D, Hildebrand D, Kachroo A, Kachroo P. An intact cuticle in distal tissues is essential for the induction of systemic acquired resistance in plants. Cell Host & Microbe. 2009;5:155–165. doi: 10.1016/j.chom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Nandi A, Welti R, Shah J. The Arabidopsis thaliana dihydroxyacetone phosphate reductase gene SUPPRESSSOR OF FATTY ACID DESATURASE DEFICIENCY1 is required for glycerolipid metabolism and for the activation of systemic acquired resistance. Plant Cell. 2004;16:465–477. doi: 10.1105/tpc.016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Y, Shen W, Dauk M, Wang F, Selvaraj G, Zou J. Targeted gene disruption of glycerol-3-phosphate dehydrogenase in Colletotrichum gloeosporioides reveals evidence that glycerol is a significant transferred nutrient from host plant to fungal pathogen. J Biol Chem. 2004;279:429–435. doi: 10.1074/jbc.M308363200. [DOI] [PubMed] [Google Scholar]
- 10.Chanda B, Venugopal SC, Kulshrestha S, Navarre D, Downie B, Vaillancourt L, et al. Glycerol-3-phosphate levels are associated with basal resistance to the hemibiotrophic fungus Colletotrichum higginsianum in Arabidopsis. Plant Physiol. 2008;147:2017–2029. doi: 10.1104/pp.108.121335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang L, Li J, Zhao T, Xiao F, Tang X, Thilmony R, et al. Interplay of the Arabidopsis nonhost resistance gene NHO1 with bacterial virulence. Proc Natl Acad Sci USA. 2003;100:3915–3924. doi: 10.1073/pnas.0637377100. [DOI] [PMC free article] [PubMed] [Google Scholar]