Abstract
Tight regulation of the auxin hormone indole-3-acetic acid (IAA) is crucial for plant development. Newly discovered IAA antagonists are the amide-linked tryptophan conjugates of IAA and jasmonic acid (JA). JA-Trp and IAA-Trp interfered with root gravitropism in Arabidopsis, and inhibited several responses to exogenously supplied IAA. Relatively low concentrations of the inhibitors occurred in Arabidopsis, but Pisum sativum flowers contained over 300 pmole g−1 FW of JA-Trp. DihydroJA was an even more effective inhibitor than JA-Trp, suggesting that Trp conjugates with other JA derivatives may also be functional. JA-Trp and IAA-Trp add to the list of documented bioactive amide hormone conjugates. The only other example is JA-Ile, the recently discovered jasmonate signal. These examples establish that conjugation not only inactivates hormones, but in some cases creates novel compounds that function in hormone signaling.
Key words: jasmonic acid, indole-3-acetic acid, auxin, tryptophan, conjugate, plant hormone, signaling, amino acid, antagonist
Plants hold an amazing capacity to auto-regulate their growth and respond to a host of environmental challenges. Since the early discovery of the first plant hormone, indole-3-acetic acid (IAA),1 science has progressively unveiled ever more complex, and sometimes surprising, ways that plants manipulate hormones to optimize their growth and thwart their opponents. Until recently, the covalent coupling of hormones to sugars, amino acids and peptides was thought to be merely a way to dispose of excess hormone.2 The amide linkage of IAA to Asp and Glu does indeed result in IAA catabolism, while IAA-Ala and IAA-Leu are inactive stored forms of IAA.3 But the perception that all hormone conjugates are inactive changed abruptly with the discovery that the isoleucine conjugate of jasmonic acid (JA-Ile) is an active hormonal signal.
JA-Isoleucine is a Jasmonate Signal
Jasmonates are widely recognized to have critical roles in plant growth and in protecting plants from both biotic and abiotic assaults.4 Despite hints that JA conjugates might have biological activity5 the focus was almost exclusively on JA and its methyl ester (JA-Me) as the presumed signals. JA-Me was a particularly attractive candidate for an active signal because not only is it a fragrant volatile of jasmine and other flowers, it stimulates interplant responses as an airborne molecule.6 However, there is now doubt about whether either JA or JA-Me are jasmonate signals. The Arabidopsis jar1 mutant is deficient in a number of jasmonate responses and the defect was traced to the loss of an enzyme that conjugates Ile to JA.7 Only JA-Ile complements defects in the mutant and, importantly, JA-Ile promotes interaction between the presumed jasmonate receptor, COI1, and the JAZ transcriptional repressors that control jasmonate-responsive gene transcription.8,9 A few other JA-amino acid conjugates have weak activity in these assays, but JA and JA-Me do not.10 As the CO1 F-Box protein is integral to most known jasmonate responses, it is not clear how JA or JA-Me could be bona fide signals. JA-Me in particular may be a transported form of jasmonate, which is then demethylated and conjugated to Ile for activity.7,11,12 Although it is still possible that JA/JA-Me function as signals in yet unknown ways, these recent discoveries significantly alter our view of what constitutes an active hormonal signal.
Tryptophan Conjugates Inhibit Auxin in Arabidopsis
Now it turns out that JA-Ile is not the only active JA conjugate and surprisingly, the newly discovered activity is entirely different from previously recognized jasmonate functions. In Arabidopsis, the tryptophan conjugate of JA induces root agravitropism, a hallmark of disturbed auxin response.13 JA-Trp also antagonizes physiological responses to exogenous auxin, including IAA-inhibited primary root growth and IAA-promoted lateral root production. Induction of the synthetic auxin-inducible promoter DR5 fused to the β-glucuronidase reporter is also reduced by JA-Trp. Genetic analysis indicates that JA-Trp activity is independent of COI1, the apparent JA-Ile receptor, and previous evidence showed that JA-Trp does not function in COI1-mediated signaling involving JAZ transcriptional repressors.8 JA and other JA conjugates, including JA-Ile, do not interfere with root gravitropism13 and conversely, JA-Trp does not interfere with JA-Ile response. Therefore, addition of Trp to JA creates a jasmonate with novel and completely unexpected activity.
Equally surprising, the Trp conjugate of IAA has very similar properties to JA-Trp. This activity is not a general feature of Trp-containing compounds however, as the Trp conjugates with benzoic and t-cinnamic acids are inactive. Free Trp also did not interfere with root gravitropism, but it did suppress root inhibition by IAA. Apparently this is because IAA-Trp is synthesized from the IAA and Trp assimilated from the medium. This is the first clear evidence that a conjugate of IAA has biological activity, and that activity is opposite from the function of IAA itself.
Relatively low levels of JA-Trp and IAA-Trp (<10 pmole g−1 FW) were found in various Arabidopsis organs13 and similarly low levels were present in tomato, rice and soybean (data not shown). This does not necessarily indicate functional irrelevance, however. The values were comparable to the basal levels of JA, JA-Ile and IAA in several tissues. Furthermore, quantitation was done on whole organs, such as roots, so it is possible that Trp conjugates are higher in specific cell types where their regulatory activity is important. Root growth in Arabidopsis mutants devoid of JA, hence lacking JA-Trp, was more sensitive to exogenous auxin while the coi1 jasmonate signaling mutant was not. This would be expected if endogenous JA-Trp functions in auxin response by a mechanism distinct from JA-Ile.
Other Species Contain JA-Trp and Related Conjugates may be Active
At least one plant species accumulates JA-Trp much more abundantly in some tissues. Table 1 shows that although undetectable in leaves, over 300 pmole g−1 FW of JA-Trp was found in Pisum sativum flowers, with lesser amounts in developing seeds. IAA-Trp, on the other hand, was comparable in amount to that of Arabidopsis. A second independent derivatization method (methylation followed by acetylation) yielding different masses for the conjugates was also used to prepare extracts for quantitation by GC/MS. This procedure gave similar results, confirming that the high values for JA-Trp were not an artifact from another compound with an identical Rt on GC/MS.
Table 1.
Trp conjugates in Pisum sativum
| Tissue | JA-Trp | IAA-Trp | IAA (pmole g−1 FW) | JA | JA-Ile |
| Leaf | nd | nd | 59.3 (8.0) | 30.3 (1.0) | 3.7 (0.8) |
| Seed | 42.9 (2.2) | 7.4 (2.5) | 43.5 (0.7) | 298.5 (30.5) | 72.5 (3.9) |
| Flower | 313.8 (26.3) | nd | 66.3 (1.5) | 58.5 (12.4) | 5.4 (1.0) |
Quantitation was done as described previously.13 Detection limit of 0.1 and 0.5 pmole g−1 FW for JA-Trp and IAA-Trp, respectively. nd, not detected.
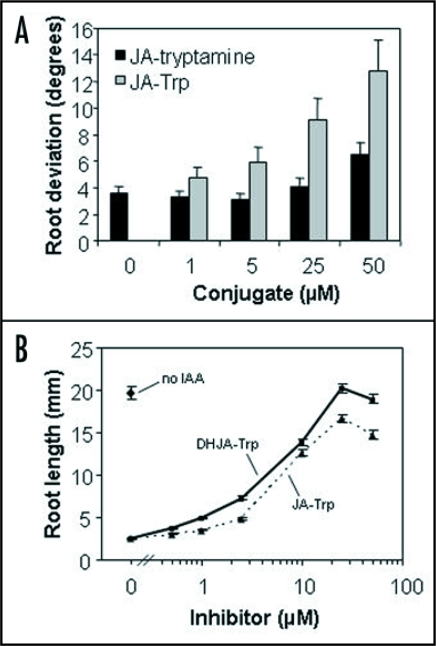
Why P. sativum accumulates high levels of JA-Trp in reproductive tissues is unclear, but it might be informative to evaluate additional leguminous species. JA-Trp also occurs in flowers of broad bean (Vicia faba), although the quantities were not previously determined.14 JA conjugates with another aromatic amino acid, tyrosine, and conjugates with the related amines and amino acids tyramine, dopa and dopamine were also reported.15,16 All of these had some ability to induce alakaloids in Escholzia californica, suggesting they might have biological activity. We synthesized the tryptamine conjugate of JA and tested its ability to induce agravitropism in Arabidopsis. Figure 1A shows that a response is seen, albeit weaker than for JA-Trp. Plants also contain a variety of JA-related compounds, including dihydroJA (DHJA) and hydroxylated derivatives. DHJA-Trp, along with JA-Trp, has been found in Asparagus officianalis,17 so the activity of this conjugate was tested next. Figure 1B shows that DHJA-Trp is actually more effective than JA-Trp as an IAA antagonist in root growth assays. As species vary in the abundance of individual JA-related compounds,18 they may also differ in which of these act as Trp conjugates to regulate auxin action.
Figure 1.
(A) Effect of JA-Trp and JA-tryptamine on Arabidopsis root gravitropic response. Root deviation from the gravity vector was measured as described previously.13 Values are the means with SE (n = 24 to 30 seedlings). (B) Suppression of auxin-inhibited root growth by JA-Trp and dihydroJA-Trp (DHJA-Trp). Seedling root length was measure after 7 d growth on MS agar plates with 2 µM IAA plus the indicated amount of each conjugate. Values are the means (n = 21 roots) with SEs.
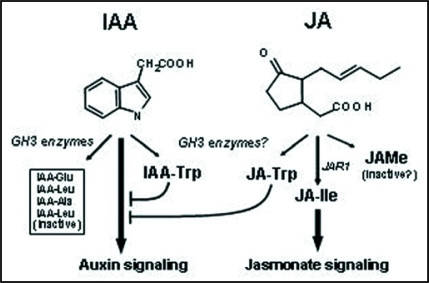
Plants have seemingly myriad ways to regulate auxin, including the control of IAA synthesis, degradation, availability and transport. Regulation of receptor activity and post-signaling control of auxin-related gene expression are also crucial to optimize auxin responses. So why were Trp conjugates recruited to add to this complex regulatory network? As mentioned earlier, conjugation is an important mechanism to control auxin availability. Synthesis of IAA-Trp would add a potent new dimension to this mechanism by not only removing IAA from the active auxin pool, but also by converting the signal to its own antagonist. JA-Trp, on the other hand, by negatively regulating an entirely different signaling pathway potentially contributes to the coordination of these two hormones, which is already known to occur at other levels.7,19–22 These relationships are summarized in the model shown in Figure 2.
Figure 2.
The role of amino acid conjugates in JA and IAA activity. Free IAA acts directly as a signal for the auxin receptor TIR1, whereas JA must be conjugated to Ile in order to function with the presumed jasmonate receptor COI1. JAR1 is the enzyme primarily responsible for JA-Ile synthesis in Arabidopsis, while related GH3 enzymes may synthesize Trp conjugates. Both JA-Trp and IAA-Trp inhibit IAA signaled responses by an unknown mechanism. The other amino acid conjugates of IAA shown are known to be inactive auxins.
Unlike JA-Ile, we do not yet know which enzymes synthesize JA-Trp and IAA-Trp. Likely candidates are the JAR1-related GH3 family of adenylating enzymes, several of which are known to conjugate IAA.23 The mechanism of Trp conjugate action is also unknown. The action of JA-Trp and IAA-Trp in auxin-inhibited root growth is additive, not synergistic, so they may involve the same biochemical machinery. Mutants with altered sensitivity to JA-Trp are being studied to search for an answer. In summary, conjugation can no longer be viewed as simply a means to dispose of excess hormones. To conjugate, or not to conjugate, is a decision of signal importance to plants.
Addendum to: Staswick P. The tryptophan conjugates of jasmonic and indole-3-acetic acids are endogenous auxin inhibitors. Plant Physiol. 2009 doi: 10.1104/pp.109.138529. In press.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/9180
References
- 1.Thiman K. Hormone action in the whole life of plants. Amherst: University of Massachusetts Press; 1977. [Google Scholar]
- 2.Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol Biol. 2002;50:309–332. doi: 10.1023/a:1016024017872. [DOI] [PubMed] [Google Scholar]
- 3.Rampey A, LeClere S, Kowalczyk M, Ljung K, Sandberg G, Bartel B. A family of auxin-conjugate hydrolases that contributes to free indole-3-acetic acid levels during Arabidopsis germination. Plant Physiol. 2004;135:1–11. doi: 10.1104/pp.104.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramell R, Miersch O, Hause B, Ortel B, Parthier B, Wasternack C. Amino acid conjugates of jasmonic acid induce jasmonate-responsive gene expression in barley (Hordeum vulgare L.) leaves. FEBS Lett. 1997;414:197–202. doi: 10.1016/s0014-5793(97)01005-3. [DOI] [PubMed] [Google Scholar]
- 6.Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16:2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- 9.Staswick PE. JAZing up jasmonate signaling. Trends Plant Sci. 2008;13:66–71. doi: 10.1016/j.tplants.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA. 2008;105:7100–7105. doi: 10.1073/pnas.0802332105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heil M, Ton J. Long-distance signaling in plant defence. Trends Plant Sci. 2008;13:265–272. doi: 10.1016/j.tplants.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Tamogami S, Rakwal R, Agrawal GK. Interplant communication: airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochem Biophys Res Commun. 2008;376:723–727. doi: 10.1016/j.bbrc.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 13.Staswick P. The tryptophan conjugates of jasmonic and indole-3-acetic acids are endogenous auxin inhibitors. Plant Physiol. 2009 doi: 10.1104/pp.109.138529. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brückner C, Kramell R, Schneider G, Schmidt J, Preiss A, Sembdner G. Schreiber K, N-[(-)-Jasmonoyl]-s-tryptophan and a related tryptophan conjugate from Vicia faba. Phytochem. 1988;27:275–276. [Google Scholar]
- 15.Brückner C, Kramell R, Schneider G, Schmidt J, Knofel HD, Sembdner G, Schreiber K. N-(Jasmonoyl)tyrosine: A conjugate of jasmonic acid from Vicia faba. Phytochemistry. 1986;25:2236–2237. [Google Scholar]
- 16.Kramell R, Schmidt J, Herrmann G, Schleimann W. N-(Jasmonoyl)tyrosine-derived compounds from flowers of broad beans (Vicia faba) J Nat Prod. 2005;68:1345–1349. doi: 10.1021/np0501482. [DOI] [PubMed] [Google Scholar]
- 17.Gapper NE, Norris GE, Clarke SF, Lill RE, Jameson PE. Novel jasmonate amino acid conjugates in Asparagus officinalis during harvest-induced and natural foliar senescence. Physiol Plant. 2002;114:116–124. doi: 10.1034/j.1399-3054.2002.1140116.x. [DOI] [PubMed] [Google Scholar]
- 18.Miersch O, Neumerkel J, Dippe M, Stenzel I, Wasternack C. Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol. 2008;177:114–127. doi: 10.1111/j.1469-8137.2007.02252.x. [DOI] [PubMed] [Google Scholar]
- 19.Schwechheimer C, Serino G, Deng XW. Multiple ubiquitin ligase-mediated processes require COP9 signalosome and AXR1 function. Plant Cell. 2002;14:2553–2563. doi: 10.1105/tpc.003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray WM, Muskett PR, Chuang HW, Parker JE. Arabidopsis SGT1b is required for SCF(TIR1)-mediated auxin response. Plant Cell. 2003;15:1310–1319. doi: 10.1105/tpc.010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagpal P, Ellis CM, Weber H, Ploense SE, Barkawi LS, Guilfoyle TJ, et al. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development. 2005;132:4107–4118. doi: 10.1242/dev.01955. [DOI] [PubMed] [Google Scholar]
- 22.Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell. 2007;19:2225–2245. doi: 10.1105/tpc.106.048017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;17:616–627. doi: 10.1105/tpc.104.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]