Abstract
The loss of firm texture is one of the most characteristic physiological processes that occur during the ripening of fleshy fruits. It is generally accepted that the disassembly of primary cell wall and middle lamella is the main factor involved in fruit softening. In this process, polygalacturonase (PG) has been implicated in the degradation of the polyuronide network in several fruits. However, the minor effect of PG downregulation on tomato softening, reported during the nineties, minimized the role of this enzyme in softening. Further works in other fruits are challenging this general assumption, as is occurring in strawberry. The strawberry (Fragaria × ananassa) fruit undergoes an extensive and fast softening that limit its shelf life and postharvest. Traditionally, it has also been considered that PG plays a minor role on this process, due to the low PG activity found in ripened strawberry fruits. Transgenic strawberry plants expressing an antisense sequence of the ripening-specific PG gene FaPG1 have been generated to get an insight into the role of this gene in softening. Half of the transgenic lines analyzed yielded fruits significantly firmer than control, without being affected other fruit parameters such as weight, color or soluble solids. The increase on firmness was maintained after several days of posharvest. In these firmer lines, FaPG1 was silenced to 95%, but total PG activity was only minor reduced. At the cell wall level, transgenic fruits contained a higher amount of covalently bound pectins whereas the soluble fraction was diminished. A microarray analysis of genes expressed in ripened receptacle did not show any significant change between control and transgenic fruits. Thus, contrary to the most accepted view, it is concluded that PG plays a key role on pectin metabolism and softening of strawberry fruit.
Key words: Fragaria x ananassa, cell wall, softening, fruit ripening, fruit texture, pectin metabolism
Texture is one of the most important quality attributes of fruits. During its ripening, fleshy fruits undergo a softening process which determines its shelf life, frequency of harvest, postharvest deterioration, transportation and storage. Several factors contribute to the overall fruit texture but it is generally accepted that the disassembly of cell walls and the dissolution of the middle lamella, both processes mediated by hydrolytic enzymes, are the main factors that cause fruit softening.1,2 In the case of strawberry fruit, main cell wall changes during softening are the increased solubilization of polyuronides and the slight depolymerization of hemicellulosic polymers.3,4 Among the cell wall hydrolytic enzymes involved in fruit softening, polygalacturonase (PG) is the best characterized. Several commodities such as tomato, avocado or peach show a peak of PG activity during ripening, and it has been related to the degradation of cell wall polyuronides.5 In tomato, which has been largely adopted as the model species to study fruit softening, the downregulation of a PG gene suppressed pectin depolymerization but not pectin solubilization.
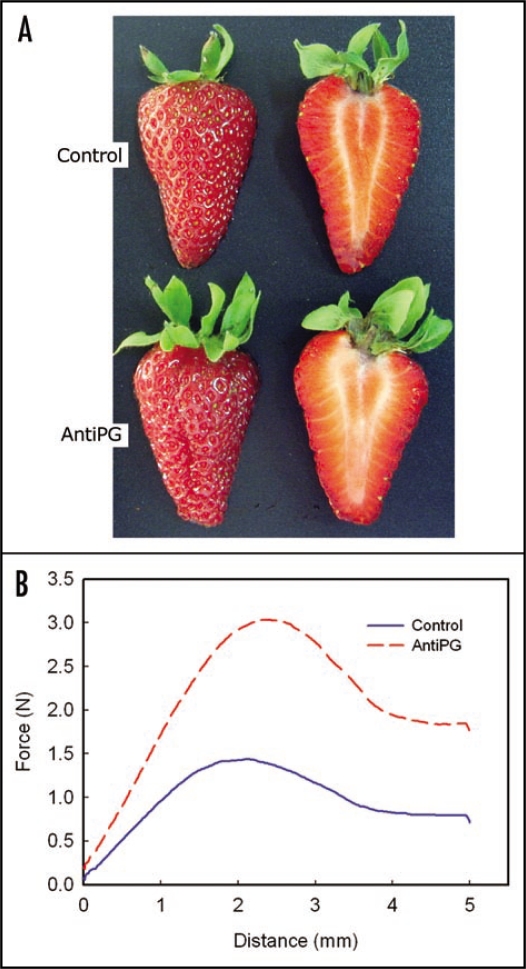
However, despite these biochemical changes, fruit softening was not significantly altered.6,7 Furthermore, the overexpression of a PG gene in the non-softening tomato mutant rin failed to induce softening, although polyuronide solubilization was restored to wild type level.8 These previous works led to the hypothesis that PG mediated pectin solubilization is neither necessary nor sufficient to induce fruit ripening.5 Contrary to tomato, in strawberry fruits PG activity is low, and several studies suggested that softening is not mediated by PG, which would be in accordance with the low pectin depolymerization observed during the ripening of this soft fruit.9 At the molecular level, two fruit-specific PG genes have been described in strawberry, FaPG1 and FaPG2. We have shown that the expression of both genes is induced during strawberry ripening and they are also repressed by auxin, as occurs with most ripeningrelated genes in strawberry.10 Previous studies on FaPG1 expression in fruits at different developmental stages indicated that this gene could be involved in the release of pectic oligosaccharins, small pectin fragments which could be elicitors of the ripening process.11 To ascertain the role of FaPG1 on strawberry softening, we have obtained transgenic plants expressing an antisense sequence of this gene under the control of the constitutive promoter CaMV35S.10 Eight out of the 16 independent transgenic lines analyzed showed fruit firmness significantly higher than wild type, without being modified other fruit characteristics such as weight, color or soluble solids (Fig. 1).
Figure 1.
(A) Control and transgenic strawberry fruit expressing the antisense FaPG1 gene (AntiPG) harvested at the full ripened stage. (B) Firmness of control and transgenic AntiPG fruits at the full ripened stage. Firmness was estimated by a puncture test using a TAXT-Plus texturometer, and curves correspond to mean values of 10 independent fruits.
Further characterization was performed in four selected lines. These lines showed a 95% silencing on FaPG1 expression in red ripened fruits. This silencing did not affect FaPG2 which was expressed at the same level than in wild type fruits. Despite the high FaPG1 silencing achieved, total PG activity was only moderately reduced. Cell walls from control and transgenic fruits have been isolated and sequentially extracted to isolate several pectin fractions based on their solubility on different solvent that are used to extract them from the wall, i.e., water-, chelator- and sodium carbonate-soluble polyuronides. These fractions correspond to pectins that are freely soluble in the apoplast, ionically or covalently bound to the wall, respectively.1 Transgenic fruits showed a higher amount of ionically and, especially, covalently linked pectins than controls, whereas the soluble fraction was diminished.10 Fraction of sodium carbonate-soluble pectins is mainly composed by polyuronides located within the cell wall, and presumably, pectin solubilization during softening occurs at the expense of this fraction.1 Size exclusion chromatography of covalently bound pectins extracted from green-unripe strawberry fruits shows two predominant peaks, one of large average size and the other one of medium size.12 At the ripened stage, both peaks remained although pectins were slightly displaced toward lower molecular sizes, and the first peak, comprising large molecular weight pectins, was notably reduced.12 This fact could indicate that depolymerization and solubilization of large pectins occurs simultaneously during strawberry ripening. The chromatographic profile of covalently bound pectins from antiPG ripened fruits showed enrichment on the amount of pectins within the first peak, and interestingly, both peaks were displaced toward larger polyuronide sizes (unpublished results). Histological observations of ripened transgenic fruits showed a higher extent of cell to cell contact areas when compared with control tissues.
Altogether, these results indicate that FaPG1 silencing reduced pectin solubilization and depolymerization, and this was related to higher tissue integrity and, finally, fruit firmness. The suppression of a fruit specific pectate lyase gene in strawberry also induced cell wall changes similar to those described for antiPG fruits and increased fruit firmness, although the improvement on firmness achieved was lower than the observed with the FaPG1 silencing.12,13 As above mentioned, it was thought that FaPG1 could be involved in the release of oligosaccharins inducer of the ripening process. In that case, significant changes on gene expression profiles at ripening in transgenic fruits underexpressing this gene would be expected. However, transcriptomic analysis of gene expression in ripened receptacle tissues from control and transgenic antiPG fruits did not show any significant change in the 1250 receptacle expressed sequence tags analyzed.10 The effect of FaPG1 product on strawberry softening may be therefore structural rather than regulatory, because it does not seem to affect the expression of other genes during ripening. We hypothesized that FaPG1 product acts on specific homogalacturonan domains, releasing more freely bound pectins, which in turn has a significant effect on the weakening of the primary wall of cortical cells.
Cell wall changes in PG silenced tomato fruits were broadly similar to those described for antiPG strawberry fruits, but the enhancement on texture in tomato was very modest.14 Which could be the physiological factors that explain this difference?. First, strawberry and tomato have different developmental origins. Strawberry is an aggregate fruit originating from the development of the flower base, receptacle, whereas ovaries develop into one-seeded fruits, achenes, attached to the outer surface of the receptacle.9 By contrast, tomato is a berry which develops directly from a single ovary, and the fruit contains a liquid placenta at the ripe stage. These developmental differences yield structural differences. The molecular pathways that underline cell wall disassembly during ripening could also be different as well as its effect on softening. Another explanation to the different behavior between transgenic tomato and strawberries could be related to the contrasting level of PG activity in both fruits. It has been suggested that PG activity in tomato is present in at least 5-fold excess.6 Thus, the low PG mRNA level still present in transgenic tomato fruits underexpressing a PG gene could be enough to induce softening. By contrast, in strawberry fruit, where PG activity is 50 fold lower than in ripe tomato, the transgenic manipulation could have decreased the FaPG1 mRNA amount below a threshold level that could lead to a modification of texture. Along this line, it would be interesting to test this strategy in other fruits such as melon or apple, which contain low levels of PG activity but express PG ripening-specific genes,15,16 as occurs in strawberry. Finally, fruit texture is a complex trait influenced not only by the mechanical properties of the cell walls but by many other factors, such as turgor pressure, cell shape and the strength and extension of adhesion areas between neighboring cells.17 Recently, it has been suggested that turgor and transpirational water loss are more determinant than cell wall metabolism in the softening of tomato.18 In this model, the fruit cuticle has a key role in texture.18 Strawberry cuticle, by contrast, is a much softer structure than its corresponding in tomato, and it is likely that in this soft fruit the physical properties of the cell wall were the most relevant textural factor.
In summary, the downregulation of polygalacturonase10 and pectate lyase12,13 genes by antisense transformation indicates that pectin metabolism plays a key role in the textural changes that take place during strawberry ripening. Pectins linked to the cell wall by covalent interactions seem to be the target of these enzymes. In the case of antiPG plants, it has been shown that pectin degradation mediated by FaPG1 product does not significantly modify the expression of other ripening-related genes. This biotechnological approach could be therefore a useful strategy to improve the texture of this soft fruit.
Acknowledgements
This work was supported by the Ministerio de Educación y Ciencia of Spain and Feder EU Funds (grant references AGL2005-08128, AGL2008-02356 and MEC-BIO2007-67509-C02-02) and by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria of Spain (grant no. RTA04-079).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/9167
References
- 1.Brummell DA. Cell wall disassembly in ripening fruits. Funct Plant Bio. 2006;33:103–119. doi: 10.1071/FP05234. [DOI] [PubMed] [Google Scholar]
- 2.Vicente AR, Saladié M, Rose JKC, Labavitch JM. The linkage between cell wall metabolism and fruit softening: looking to the future. J Sci Food Agric. 2007;87:1435–1448. [Google Scholar]
- 3.Huber DJ. Strawberry fruit softening—the potential roles of polyuronides and hemicelluloses. J Food Sci. 1984;49:1310–1315. [Google Scholar]
- 4.Rosli HG, Civello PM, Martínez GA. Changes in cell wall composition of three Fragaria × ananassa cultivars with different softening rate during ripening. Plant Physiol Biochem. 2004;42:823–831. doi: 10.1016/j.plaphy.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Hadfield KA, Bennett AB. Polygalacturonases: many genes in search of a function. Plant Physiol. 1998;117:337–343. doi: 10.1104/pp.117.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CJS, Watson CF, Morris PC, Bird CR, Seymour GB, Gray JE, et al. Inheritance and effect on ripening of antisense polygalacturonase genes in transgenic tomatoes. Plant Mol Biol. 1990;14:369–379. doi: 10.1007/BF00028773. [DOI] [PubMed] [Google Scholar]
- 7.Kramer M, Sanders R, Bolkan H, Waters C, Sheehy RE, Hiatt WR. Postharvest evaluation of transgenic tomatoes with reduced levels of polygalacturonase: processing, firmness and disease resistance. Postharvest Biol Technol. 1992;1:241–255. [Google Scholar]
- 8.Giovannoni JJ, DellaPenna D, Bennett AB, Fischer RL. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell. 1989;1:53–63. doi: 10.1105/tpc.1.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins-Veazie P. Growth and ripening of strawberry fruit. Hortic Rev. 1995;17:267–297. [Google Scholar]
- 10.Quesada MA, Blanco-Portales R, Posé S, García-Gago JA, Jiménez-Bermúdez S, Muñoz-Serrano A, et al. Antisense downregulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol. 2009;150:1022–1032. doi: 10.1104/pp.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redondo-Nevado J, Moyano E, Medina-Escobar N, Caballero JL, Muñoz-Blanco J. A fruit-specific and developmentally regulated endopolygalacturonase gene from strawberry (Fragaria x ananassa cv. Chandler) J Exp Bot. 2001;52:1941–1945. doi: 10.1093/jexbot/52.362.1941. [DOI] [PubMed] [Google Scholar]
- 12.Santiago-Doménech N, Jiménez-Bermúdez S, Matas AJ, Rose JKC, Muñoz-Blanco J, Mercado JA, et al. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. J Exp Bot. 2008;59:2769–2779. doi: 10.1093/jxb/ern142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiménez-Bermúdez S, Redondo-Nevado J, Muñoz-Blanco J, Caballero JL, López-Aranda JM, Valpuesta V, et al. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol. 2002;128:751–759. doi: 10.1104/pp.010671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrington CM, Greve LC, Labavitch JM. Cell wall metabolism in ripening fruit VI. Effect of the antisense polygalacturonase gene on cell wall changes accompanying ripening in transgenic tomatoes. Plant Physiol. 1993;103:429–434. doi: 10.1104/pp.103.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadfield KA, Rose JKC, Yaver DS, Berka RM, Bennett AB. Polygalacturonase gene expression in ripe melon fruit supports a role for polygalacturonase in ripening-associated pectin disassembly. Plant Physiol. 1998;117:363–373. doi: 10.1104/pp.117.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q, Szakacs-Dobozi M, Hemmat M, Hrazdina G. Endopolygalacturonase in apples (Malus domestica) and its expression during fruit ripening. Plant Physiol. 1993;102:219–225. doi: 10.1104/pp.102.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harker FR, Redgwell RJ, Hallett IC, Murray SH. Texture of fresh fruit. Hortic Rev. 1997;20:121–224. [Google Scholar]
- 18.Saladié M, Matas AJ, Isaacson T, Jenks MA, Goodwin SM, Niklas KJ, et al. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol. 2007;144:1012–1028. doi: 10.1104/pp.107.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]