Abstract
Cotyledons of tomato seedlings that germinated in a 20 µM AlK(SO4)2 solution remained chlorotic while those germinated in an aluminum free medium were normal (green) in color. Previously, we have reported the effect of aluminum toxicity on root proteome in tomato seedlings (Zhou et al.1). Two dimensional DIGE protein analysis demonstrated that Al stress affected three major processes in the chlorotic cotyledons: antioxidant and detoxification metabolism (induced), glyoxylate and glycolytic processes (enhanced), and the photosynthetic and carbon fixation machinery (suppressed).
Key words: aluminum, cotyledons, proteome, tomato
Different biochemical processes occur depending on the developmental stages of cotyledons. During early seed germination, before the greening of the cotyledons, glyoxysomes enzymes are very active. Fatty acids are converted to glucose via the gluconeogenesis pathway.2,3 In greening cotyledons, chloroplast proteins for photosynthesis and leaf peroxisomal enzymes in the glycolate pathway for photorespiration are metabolized.2–4 Enzymes involved in regulatory mechanisms such as protein kinases, protein phosphatases, and mitochondrial enzymes are highly expressed.3,5,6
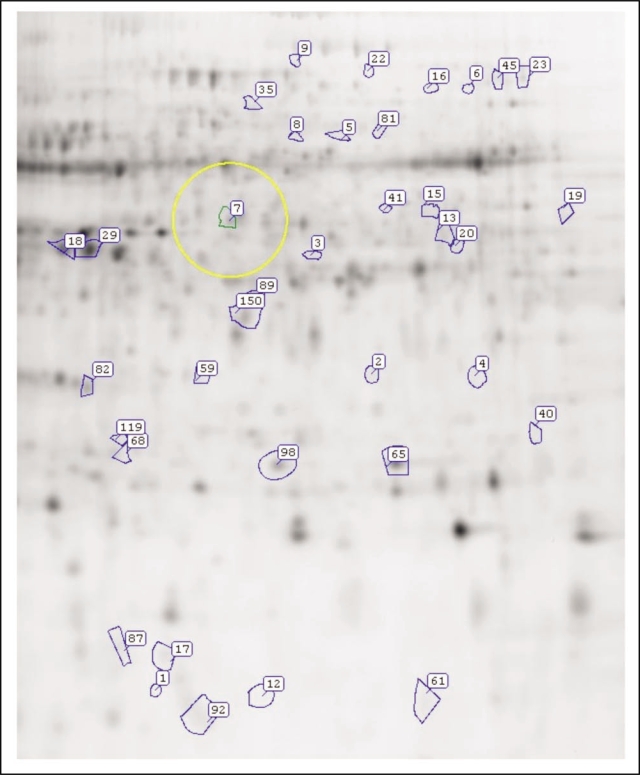
The chlorotic cotyledons are similar to other chlorotic counterparts in that both contains lower levels of chlorophyll, thus the photosynthetic activities are not as active. In order to understand the impact of Al on tomato cotyledon development, a comparative proteome analysis was performed using 2D-DIGE following the as previously described procedure.1 Some proteins accumulated differentially in Al-treated (chlorotic) and untreated cotyledons (Fig. 1). Mass spectrometry of tryptic digestion fragments of the proteins followed by database search has identified some of the differentially expressed proteins (Table 1). The proteome changes affected by Al stress are summarized in the followings.
Figure 1.
Image of protein spots generated by Samspot analysis of Al treated and untreated tomato cotyledons proteomes separated on 2D-DIGE.
Table 1.
Proteins identified from tomato cotyledons of seeds germinating in Al-solution
| Spot No. | Fold (treated/ctr) | ANOVA (p value) | Annotation | SGN accession |
| 1 | 2.34 | 0.001374 | 12S seed storages protein (CRA1) | SGN-U314355 |
| 2 | 2.13 | 0.003651 | unidentified | |
| 3 | 2.0 | 0.006353 | lipase class 3 family | SGN-U312972 |
| 4 | 1.96 | 0.002351 | large subunit of RUBISCO | SGN-U346314 |
| 5 | 1.95 | 2.66E-05 | arginine-tRNA ligase | SGN-U316216 |
| 6 | 1.95 | 0.003343 | unidentified | |
| 7 | 1.78 | 0.009219 | Monodehydroascorbate reductase (NADH) | SGN-U315877 |
| 8 | 1.78 | 0.000343 | unidentified | |
| 9 | 1.75 | 4.67E-05 | unidentified | |
| 12 | 1.70 | 0.002093 | unidentified | |
| 13 | 1.68 | 0.004522 | unidentified | |
| 15 | 1.66 | 0.019437 | Glutamate dehydrogenase 1 | SGN-U312368 |
| 16 | 1.66 | 0.027183 | unidentified | |
| 17 | 1.62 | 2.01E-08 | Major latex protein-related, pathogenesis-related | SGN-U312368 |
| 18 | −1.61 | 0.009019 | RUBisCo activase | SGN-U312543 |
| 19 | 1.61 | 0.003876 | Cupin family protein | SGN-U312537 |
| 20 | 1.60 | 0.000376 | unidentified | |
| 22 | 1.59 | 0.037216 | unidentified | |
| 0.003147 | unidentified | |||
| 29 | −1.56 | 0.001267 | RUBisCo activase | SGN-U312543 |
| 35 | 1.52 | 0.001955 | unidentified | |
| 40 | 1.47 | 0.007025 | unidentified | |
| 41 | 1.47 | 0.009446 | unidentified | |
| 45 | 1.45 | 0.001134 | unidentified | |
| 59 | −1.40 | 5.91E-05 | 12 S seed storage protein | SGN-U314355 |
| 61 | 1.39 | 1.96E-05 | MD-2-related lipid recognition domain containing protein | SGN-U312452 |
| 65 | 1.37 | 0.000608 | triosephosphate isomerase, cytosolic | SGN-U312988 |
| 68 | 1.36 | 0.004225 | unidentified | |
| 81 | 1.32 | 0.001128 | unidentified | |
| 82 | −1.31 | 0.001408 | 33 kDa precursor protein of oxygen-evolving complex | SGN-U312530 |
| 87 | 1.30 | 0.002306 | unidentified | |
| 89 | −1.3 | 0.000765 | unidentified | |
| 92 | 1.29 | 0.000125 | superoxide dismutase | SGN-U314405 |
| 98 | 1.28 | 0.000246 | triosephosphate isomerase, cytosolic | SGN-U312988 |
Antioxidant and Detoxification Proteins were Strongly Induced in Al-Treated Chlorotic Cotyledons
Aluminum and other heavy metals activate generation of reactive oxygen species (ros).7,8 Excessive production of ros may lead to oxidative stress that result in elevated levels of oxidized proteins and oxidized lipids, loss of cell function, and ultimately apoptosis or necrosis. Antioxidant mechanisms are activated to protect against damages produced by ros.1,9 Mondehydroascorbate reductase (mdar) is an enzymatic component of the ascorbate-glutathione cycle. The tomato homologous protein of mdar was induced 1.78 fold in al treated tissues (spot 7). Superoxide dismutase (sod), an enzyme that repairs cells and reduces the damage done to them by superoxide, and the corresponding enzyme protein (spot 92) were induced 1.29 fold.
Two proteins associated with proline synthesis, important component of stress responses in plants, were induced. Arginine-tRNA ligase, an enzyme that participates in arginine and proline metabolism, (spot 5) was induced 1.96 fold. Glutamate dehydrogenase (spot 15) was increased 1.66 fold in Al treated tissues. Glutamate dehydrogenase was found to be activated by ROS in order to detoxify ammonia and to produce Glu for the synthesis of praline.10
The Glyoxylate and Glycolytic Processes were more Active in Al-Treated Seedlings
During the early stages of seed germination, before the onset of photosynthesis, plants rely on stored nutrients to synthesize the various cellular structural molecules essential for cell division, growth, and for the production of ATPs needed to drive these reactions. Conversion of high energy lipids into glucose via the glyoxylate cycle occurs in cotyledons during the early stages of seed germination. Triglyceride lipases promote fatty acid β-oxidation to produce free fatty acids which are further converted to glucose. In Al treated tomato tissues, the lipase class 3 family (spot 3) was induced 2.0 fold, indicating that treated seeds were using fat in order to sustain the growth of seedlings while the control plants had became autotrophic through photosynthesis in the green cotyledons. Triosephosphate isomerase (TPI) plays an important role in glycolysis and is essential for efficient energy production. Two isoforms of the cytosolic TPI (spot 65, 1.37 fold, spot 98, 1.28 fold) were identified, and both were induced in Al treated tomato tissues.
The Photosynthetic Machinery was Affected by Aluminum Toxicity
Spot 82, identified to be 33 kDa precursor protein of oxygenevolving complex, was suppressed (−1.31 fold). Two isoforms of RUBisCo. activase in tomato were suppressed, (spot 29, −1.56 fold, and spot 18, −1.61 fold). The 33 kDa protein is one of the three subunits of the oxygen evolving complex (OEC) responsible for oxidation of water and releasing of molecular oxygen in the PSII complex. Rubisco activase serves as a chaperone for the promotion and maintenance of Rubisco's catalytic activities. The lower amount of such proteins could be related to chlorotic cotyledons. Low chlorophyll contents in chlorotic cotyledons of Al-treated seedlings could result in deficiency in absorbing light energy, consequently, fewer electrons were passed from PS I onto PSII. The Al-induced reduction in the photosynthetic machinery could delay the development of seedlings into mature plants.
The large subunit of RUBISCO (spot 4) was induced 1.96 fold. Several proteins associated with disease and stress resistance were induced. These included major latex protein-related, pathogenesis-related (spot 17, 1.62 fold), cupin family of proteins (spot 19, 1.61 fold) and MD-2-related lipid recognition domain containing protein (spot 61, 1.39 fold).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/9182
References
- 1.Zhou S, Sauvé R, Thannhauser TW. Proteome changes induced by aluminum stress in tomato roots. J Exp Bot. 2009;60:1849–1857. doi: 10.1093/jxb/erp065. [DOI] [PubMed] [Google Scholar]
- 2.Fukao Y, Hayashi M, Hara-Nishimura I, Nishimura M. Novel glyoxysomal protein kinase, GPK1, identified by proteomic analysis of glyoxysomes in etiolated cotyledons of Arabidopsis thaliana. Plant and Cell Physiol. 2003;44:1002–1012. doi: 10.1093/pcp/pcg145. [DOI] [PubMed] [Google Scholar]
- 3.Kagawa T, McGregor DI, Beevers H. Development of enzymes in the cotyledons of watermelon seedlings. Plant Physiol. 1973;51:66–71. doi: 10.1104/pp.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauncz Y, Gepstein S, Horwitz BA. Photocontrol of the accumulation of plastid polypeptides during greening of tomato cotyledons. Potentiation by a pulse of red light. Plant Physiol. 1992;100:1934–1939. doi: 10.1104/pp.100.4.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamura T, Minamikawa T, Koshiba T. Acid phosphatase in cotyledons of germinating Vigna mungo seeds. Plant and Cell Physiol. 1982;23:1155–1159. [Google Scholar]
- 6.Kollöffel C, Van Dijke HD. Mitochondrial arginase activity from cotyledons of developling and greening seeds of Vicia faba L. Plant Physiol. 1975;55:507–510. doi: 10.1104/pp.55.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002;128:63–72. [PMC free article] [PubMed] [Google Scholar]
- 8.Tani A, Inoue C, Tanaka Y, Yamamoto Y, Kondo H, Hiradate S, et al. The crucial role of mitochondrial regulation in adaptive aluminium resistance in Rhodotorula glutinis. Microbiology. 2008;154:3437–3446. doi: 10.1099/mic.0.2007/016048-0. [DOI] [PubMed] [Google Scholar]
- 9.Horemans N, Raeymaekers T, Van Beek K, Nowocin A, Blust R, Broos K, et al. Dehydroascorbate uptake is impaired in the early response of Arabidopsis plant cell cultures to cadmium. J Exp Bot. 2007;58:4307–4317. doi: 10.1093/jxb/erm291. [DOI] [PubMed] [Google Scholar]
- 10.Skopelitis DS, Paranychianakis NV, Paschalidis KA, Pliakonis ED, Delis ID, Yakoumakis DI, et al. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell. 2006;18:2767–2781. doi: 10.1105/tpc.105.038323. [DOI] [PMC free article] [PubMed] [Google Scholar]