Abstract
VERNALIZATION INSENSITIVE 3 (VIN3) encodes a PHD domain chromatin remodelling protein that is induced in response to cold and is required for the establishment of the vernalization response in Arabidopsis thaliana.1 Vernalization is the acquisition of the competence to flower after exposure to prolonged low temperatures, which in Arabidopsis is associated with the epigenetic repression of the floral repressor FLOWERING LOCUS C (FLC).2,3 During vernalization VIN3 binds to the chromatin of the FLC locus,1 and interacts with conserved components of Polycomb-group Repressive Complex 2 (PRC2).4,5 This complex catalyses the tri-methylation of histone H3 lysine 27 (H3K27me3),4,6,7 a repressive chromatin mark that increases at the FLC locus as a result of vernalization.4,7–10 In our recent paper11 we found that VIN3 is also induced by hypoxic conditions, and as is the case with low temperatures, induction occurs in a quantitative manner. Our experiments indicated that VIN3 is required for the survival of Arabidopsis seedlings exposed to low oxygen conditions. We suggested that the function of VIN3 during low oxygen conditions is likely to involve the mediation of chromatin modifications at certain loci that help the survival of Arabidopsis in response to prolonged hypoxia. Here we discuss the implications of our observations and hypotheses in terms of epigenetic mechanisms controlling gene regulation in response to hypoxia.
Key words: arabidopsis, VIN3, FLC, hypoxia, vernalization, chromatin remodelling, survival
The Regulation of VIN3 Expression
The regulation of VIN3 expression in response to low temperatures or hypoxia appears to occur via different mechanisms; de novo protein synthesis is required for VIN3 expression in response to hypoxia but not low temperatures.11 This response is similar to the regulation of ADH1 under low oxygen conditions where AtMYB2 must be translated before ADH1 is induced.12 Promoter motifs that have been defined experimentally are essential for the hypoxic induction of ADH1 by AtMYB212 and, like the ADH1 locus, the VIN3 promoter contains a MYB/GT motif to which AtMYB2, or another MYB protein may bind (Fig. 1).
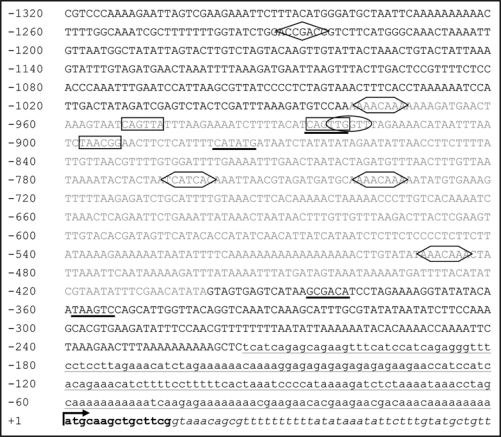
Figure 1.
The presence of DNA regulatory elements and a Mutator element in the VIN3 promoter. The promoter region of the VIN3 gene is in upper case. The VIN3 cDNA sequence is in lower case. The 5′UTR is underlined. The start of translation of the VIN3 gene is represented by the upward arrow. Exon 1 of the VIN3 gene is in bold. Intron 1 of the VIN3 gene is italicised. Numbers refer to nucleotides relative to the start of translation (+1). The PLACE database31,32 predicted the following binding sites and DNA regulatory motifs upstream of the start of the VIN3 cDNA: MYB transcription factor binding sites (rectangle); MYC-recognition motif (bold underlined); low temperature response element (diamond); Anaerobic response elements (hexagon); and a GT-motif essential for low oxygen induction of ADH1 (oval).12 The majority of the DNA regulatory motifs lie within a Mutator (Mu) transposable element. The sequence of the Mu element, which is named AT5TE83600 and is a member of the Mu element family AT9TSD1,33 is in grey.
The MYB/GT motif lies within a Mutator (Mu) transposable element that exists in the proximal promoter region of the VIN3 gene (Fig. 1). This Mu element also contains other putative DNA regulatory elements. Most transposable elements are clustered in heterochromatic regions of the genome and are transcriptionally silenced via DNA methylation and the deposition of di-methylated lysine 9 on histone H3 (H3K9me2) by an RNAi-mediated mechanism.13–15 Whole genome profiling of DNA methylation and H3K9me2 indicated that the Mu element within the VIN3 promoter is not associated with DNA methylation and has little if any H3K9me2.16–18 This contrasts with other Mu elements that reside in euchromatic DNA, for example a Mu element within intron 1 of the FLC gene in the Ler ecotype is decorated with repressive chromatin modifications directed by siRNAs that originate from dispersed copies of the Mu element; the repressive chromatin modifications contribute to the low level of FLC expression in this ecotype.19 It will be of interest to determine if the putative DNA regulatory elements located in the Mu element and the element itself play a role in the regulation of VIN3 expression.
Low oxygen conditions produced by waterlogging, where the roots of seedlings are completely submerged in water, induced the expression of VIN3 in the aerial tissue of these seedlings.11 This suggests that there must be a systemic signal that originates in the roots and which is transmitted to the aerial tissue. This signal may be important for both local and systemic induction of VIN3. Root-to-shoot communication appears to play a role in the response to low oxygen environments.20 In contrast, early experiments suggest that there is no transmissible signal involved in the vernalization response.21 The transmissible signal involved in regulating VIN3 expression during hypoxia should be identified so that it can be determined whether this signal is also involved in the induction of VIN3 expression during cold exposure.
The Function of VIN3 during Low Oxygen Conditions
Our data indicate that VIN3 is required for the survival of Arabidopsis following a prolonged period of hypoxia.11 At the end of 120 hours under low oxygen conditions all plants (both the wild-type and the vin3 mutant) remained green although some leaf tissue appeared to be wilting. The treated seedlings only became chlorotic and died during the 10-day recovery period after the low oxygen treatment; the vin3 mutant had a lower survival rate than wild-type plants.11 Plants need to survive not only the low oxygen stress but also the consequences of a return to normal atmospheric oxygen.22 During low oxygen conditions, some of the genes that are induced have a role in the protection of cell metabolism from subsequent exposure to oxygen. One protective system involves Superoxide Dismutase (SOD) which converts superoxide radicals, generated when atmospheric oxygen is decreased for prolonged periods, to hydrogen peroxide that is further reduced to water.22 In addition, numerous changes to the transcriptional and metabolomic profiles occur when Arabidopsis seedlings are re-exposed to oxygen.23,24 We found no difference in gene expression between wild-type and vin3–4 mutant plants after 24 hours under low oxygen.11 We hypothesise that VIN3 is needed to establish a gene expression state in response to hypoxia; this state is then maintained during the recovery phase when seedlings are returned to atmospheric oxygen (Fig. 2). For example, VIN3 may initiate the formation of repressive chromatin at a gene and prevent it from being (re)-expressed during the return to atmospheric oxygen. This proposed role for VIN3 in low oxygen is comparable to its function in the vernalization response—VIN3 initiates the vernalization-induced repression of FLC during cold exposure, which continues after low temperature treatment.1
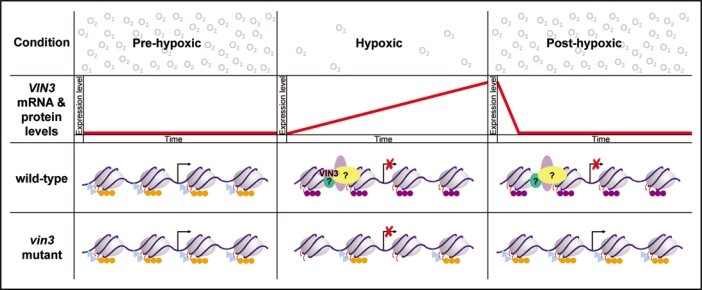
Figure 2.
One model for VIN3 action during low oxygen conditions; a comparison of wild-type and vin3 mutant plants. The proposed action of VIN3 during hypoxia is modelled on the action of VIN3 in the vernalization response where VIN3 is required for the repression of FLC expression during vernalization.1 VIN3 gene products (second row; red line) are not expressed under normal atmospheric oxygen levels (pre-hypoxic). Under these conditions VIN3 target genes are expressed in both wild-type plants (third row) and vin3 mutant plants (fourth row). The chromatin marks associated with actively transcribed genes are indicated: acetylated histone H3 and H4 (H3Ac and H4Ac; light blue triangles) and H3K4me3 (orange circles). Under hypoxic conditions the expression of some genes decreases in a VIN3 independent manner consistent with our gene expression microarray that did not identify genes that were differentially expressed in wild-type and vin3-4 mutant plants after 24 hours of hypoxia.11 In wild-type plants, where VIN3 expression is induced in a quantitative manner, VIN3 (pink) interacts with other chromatin remodelling proteins (green, purple and yellow) to set up a repressive chromatin state at VIN3 target genes. For example: the removal of H3Ac, H4Ac and H3K4me3 and the addition of repressive chromatin modifications, such as H3K27me3 (purple circles). The addition of H3K27me3 ensures that VIN3 target genes remain repressed on return to normal atmospheric conditions (post-hypoxic). Even though VIN3 is no longer present, the recruited chromatin remodelling complex maintains VIN3 target genes in a repressed state to promote the survival of Arabidopsis seedlings following prolonged hypoxia. In contrast to wild-type plants, the absence of VIN3 in a vin3 mutant would prevent the recruitment and formation of a chromatin remodelling complex at VIN3 target genes and thus there would be no addition of repressive chromatin modifications* to maintain VIN3 target genes in a repressed state post-hypoxia. *there maybe some removal of active modifications, as this could be performed by another chromatin remodelling complex.
Given the known role of VIN3 in the chromatin dynamics during the vernalization response it is highly likely that VIN3 activity in hypoxic conditions is also epigenetic in nature. It has been suggested that VIN3 might play a role in histone deacetylation of the FLC locus during vernalization.1 VIN3 contains a PHD domain;1 these domains have been reported to bind methylated lysine residues on histone tails with a high affinity for tri-methylated lysine 4 of histone H3 (H3K4me3).25,26 Recognition of H3K4me3 by a PHD domain is thought to be an initial event that leads to changes in chromatin modifications such as histone deacetylation in the vicinity of H3K4me3. The Drosophila PCL protein of the Pcl-PRC2 complex contains a PHD domain which physically interacts with the histone deacetylase RPD3.27,28 However, whether the PHD domain of VIN3 binds H3K4me3 has not been reported and no histone deacetylase has been associated with the PRC2 complex in Arabidopsis.
Post-translational modifications of histones can change on target genes in response to abiotic stresses (reviewed in ref. 29). Thus an epigenetic aspect in the regulation of hypoxic stress is highly likely. Apart from prolonged cold treatment, members of the PRC2 complex have not been reported to regulate abiotic stress responses. VIN3 may be acting in a PRC2 complex during hypoxia, or perhaps in another chromatin remodelling complex. During vernalization VIN3 interacts not only with the conserved members of the PRC2 complex but also with VIN3-like proteins.4 This interaction occurs via their VID domains9,10 and is an important step in the initiation of the vernalization response; without VIN3, VIL1/VRN5 does not associate with FLC chromatin and the epigenetic repression of FLC does not occur unless plants are exposed to an even longer period of cold.4,30 One step to finding out the function of VIN3 is to determine if other members of the VIN3 gene family or components of the PRC2 complex are required together with VIN3 for survival during prolonged low oxygen conditions.
Concluding Remarks
Our observation that VIN3 is critical for the survival of Arabidopsis plants exposed to prolonged periods of hypoxia indicates that the VIN3 family of proteins may play a more diverse role in plant responses to environmental challenges, than previously recognised. The employment of dynamic epigenetic gene regulation systems for a diverse range of processes reflects the need of sessile organisms to manage the effects of the environment to which they are exposed. Future work will identify regulators of VIN3 expression and VIN3 binding partners and improve the knowledge of how VIN3 mediates the vernalization and low oxygen responses. Characterisation of VIN3 function could therefore change the understanding of how epigenetic mechanisms affect the response of plants to stress and open new perspectives for applied research.
Addendum to: Bond DM, Wilson IW, Dennis ES, Pogson BJ, Finnegan EJ. VERNALIZATION INSENSITIVE 3 (VIN3) is required for the response of Arabidopsis thaliana seedlings exposed to low oxygen conditions. Plant J. 2009 doi: 10.1111/j.1365-313X. In press.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/9178
References
- 1.Sung S, Amasino RM. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature. 2004;427:159–164. doi: 10.1038/nature02195. [DOI] [PubMed] [Google Scholar]
- 2.Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES. The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell. 1999;11:445–458. doi: 10.1105/tpc.11.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Lucia F, Crevillen P, Jones AME, Greb T, Dean C. A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci USA. 2008;105:16831–16836. doi: 10.1073/pnas.0808687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood CC, Robertson M, Tanner G, Peacock WJ, Dennis ES, Helliwell CA. The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc Natl Acad Sci USA. 2006;103:14631–14636. doi: 10.1073/pnas.0606385103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang D, Wang Y, Wang Y, He Y. Repression of FLOWERING LOCUS C and FLOWERING LOCUS T by the Arabidopsis polycomb repressive complex 2 components. PLoS ONE. 2008;3:3404. doi: 10.1371/journal.pone.0003404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schubert D, Primavesi L, Bishopp A, Roberts G, Doonan J, Jenuwein T, Goodrich J. Silencing by plant polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J. 2006;25:4638–4649. doi: 10.1038/sj.emboj.7601311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finnegan EJ, Dennis ES. Vernalization-induced trimethylation of histone H3 lysine 27 at FLC is not maintained in mitotically quiescent cells. Curr Biol. 2007;17:1978–1983. doi: 10.1016/j.cub.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Greb T, Mylne JS, Crevillen P, Geraldo N, An H, Gendall AR, Dean C. The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr Biol. 2007;17:73–78. doi: 10.1016/j.cub.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 10.Sung S, Schmitz RJ, Amasino RM. A PHD finger protein involved in both the vernalization and photoperiod pathways in Arabidopsis. Genes Dev. 2006;20:3244–3248. doi: 10.1101/gad.1493306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bond DM, Wilson IW, Dennis ES, Pogson BJ, Finnegan EJ. VERNALIZATION INSENSITIVE 3 (VIN3) is required for the response of Arabidopsis thaliana seedlings exposed to low oxygen conditions. Plant J. 2009 doi: 10.1111/j.1365-313X.2009.03891.x. In Press. [DOI] [PubMed] [Google Scholar]
- 12.Hoeren FU, Dolferus R, Wu Y, Peacock WJ, Dennis ES. Evidence for a role for AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase gene (ADH1) by low oxygen. Genetics. 1998;149:479–490. doi: 10.1093/genetics/149.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan SWL, Henderson IR, Jacobsen SE. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat Rev Genet. 2005;6:351–360. doi: 10.1038/nrg1601. [DOI] [PubMed] [Google Scholar]
- 14.Jackson J, Johnson L, Jasencakova Z, Zhang X, PerezBurgos L, Singh P, et al. Dimethylation of histone H3 lysine 9 is a critical mark for DNA methylation and gene silencing in Arabidopsis thaliana. Chromosoma. 2004;112:308–315. doi: 10.1007/s00412-004-0275-7. [DOI] [PubMed] [Google Scholar]
- 15.Lippman Z, Martienssen R. The role of RNA interference in heterochromatic silencing. Nature. 2004;431:364–370. doi: 10.1038/nature02875. [DOI] [PubMed] [Google Scholar]
- 16.Bernatavichute YV, Zhang X, Cokus S, Pellegrini M, Jacobsen SE. Genome-wide association of histone H3 lysine nine methylation with CHG DNA methylation in Arabidopsis thaliana. PLoS ONE. 2008;3:3156. doi: 10.1371/journal.pone.0003156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild CD, et al. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452:215–219. doi: 10.1038/nature06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. http://epigenomics.mcdb.ucla.edu/
- 19.Liu J, He Y, Amasino R, Chen X. siRNAs targeting an intronic transposon in the regulation of natural flowering behavior in Arabidopsis. Genes Dev. 2004;18:2873–2878. doi: 10.1101/gad.1217304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey-Serres J, Chang R. Sensing and signalling in response to oxygen deprivation in plants and other organisms. Ann Bot. 2005;96:507–518. doi: 10.1093/aob/mci206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwabe WW. Factors controlling flowering in the Chrysanthemum: IV. The site of vernalization and the translocation of the stimulus. J Exp Bot. 1954;5:389–400. [Google Scholar]
- 22.Drew MC. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- 23.Branco-Price C, Kaiser KA, Jang CJH, Larive CK, Bailey-Serres J. Selective mRNA translation coordinates energetic and metabolic adjustments to cellular oxygen deprivation and reoxygenation in Arabidopsis thaliana. Plant J. 2008;56:743–755. doi: 10.1111/j.1365-313X.2008.03642.x. [DOI] [PubMed] [Google Scholar]
- 24.Branco-Price C, Kawaguchi R, Ferreira RB, Bailey-Serres J. Genome-wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Ann Bot. 2005;96:647–660. doi: 10.1093/aob/mci217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi X, Hong T, Walter KL, Ewalt M, Michishita E, Hung T, et al. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature. 2006;442:96–99. doi: 10.1038/nature04835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, et al. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- 27.Tie F, Prasad-Sinha J, Birve A, Rasmuson-Lestander A, Harte PJ. A 1-megadalton ESC/E (Z) complex from Drosophila that contains polycomb-like and RPD3. Mol Cell Biol. 2003;23:3352–3362. doi: 10.1128/MCB.23.9.3352-3362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tie F, Stratton CA, Kurzhals RL, Harte PJ. The N terminus of Drosophila ESC binds directly to histone H3 and is required for E(Z)-dependent trimethylation of H3 lysine 27. Mol Cell Biol. 2007;27:2014–2026. doi: 10.1128/MCB.01822-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinnusamy V, Gong Z, Zhu J-K. Abscisic acid-mediated epigenetic processes in plant development and stress responses. J Integr Plant Biol. 2008;50:1187–1195. doi: 10.1111/j.1744-7909.2008.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheldon CC, Finnegan EJ, Peacock WJ, Dennis ES. Mechanisms of gene repression by vernalization in Arabidopsis. Plant J. 2009 doi: 10.1111/j.1365-313X.2009.03883.x. In Press. [DOI] [PubMed] [Google Scholar]
- 31.Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database. Nucl Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prestridge DS. SIGNAL SCAN: a computer program that scans DNA sequences for eukaryotic transcriptional elements. Comput Appl Biosci. 1991;7:203–206. doi: 10.1093/bioinformatics/7.2.203. [DOI] [PubMed] [Google Scholar]
- 33. http://www.arabidopsis.org.