Abstract
Background
Maternal obesity represents a risk factor for pregnancy-related complications. Glucocorticoids are known to promote obesity in adults.
Methods
We evaluated maternal and fetal metabolic changes during and after three weekly courses of betamethasone (BM) administered to pregnant baboons (Papio spp.) at doses equivalent to those given to pregnant women.
Results
BM administration during the second half of pregnancy increased maternal weight, but neither maternal food intake nor fetal weight, as assessed at the end of gestation. BM increased maternal serum glucose concentration, IGF-I:IGFBP-3 ratio, and serum leptin during treatment (normalized by 17, 35, and 45 days post-treatment respectively for each parameter). Maternal and fetal serum leptin concentrations did not differ between groups at the end of gestation.
Conclusion
Prolonged maternal hyperleptinemia caused by BM administration in the second half of gestation did not change fetal metabolic parameters measured and placental leptin distribution at the end of gestation.
Keywords: glucocorticoid, insulin-like growth factor, IGF binding protein, leptin, baboon, pregnancy
Introduction
Leptin, insulin-like growth factors (IGF-I and IGF-II), and glucocorticoids are important regulators of appetite, metabolism, growth, development, and energy balance in humans.1,2 Several rodent and human studies confirm that IGFs and leptin act as important placental and fetal growth factors.3-5 Glucocorticoids exert essential actions on critical metabolic processes and elevated circulating glucorticoid levels due to stress, pathological conditions, or exogenous administration alter homeostasis and induce obesity, hypertension, and diabetes.6
Each year, 7-10% of pregnant women living in Europe and North America receive synthetic glucocorticoids to promote lung maturation if the fetus is at risk of pre-term delivery.7 Widespread maternal and fetal metabolic responses to antenatal corticosteroid therapy have been described,8,9 but the mechanisms underlying these effects remain unclear. Information on the effect of administration of clinically relevant doses of glucocorticoids to women during pregnancy on leptin and the IGF axis is limited.10,11 Human studies on the effects of glucocorticoids administered during pregnancy have focused on fetal cord blood leptin levels, which are increased in premature babies whose mothers receive glucocorticoids.11 Glucocorticoid regulation of maternal and fetal hormonal parameters and metabolism is well described in ruminants and rodents.12,13 In the rat, for example, dexamethasone administration causes both maternal and fetal hyperleptinemia.14 However, since placental function and structure in ruminants and rodents differ significantly from humans, especially with respect to leptin and IGF metabolism,15-17 studies of leptin and IGF physiology in a non-human primate model of pregnancy are needed.
We evaluated the effect of maternal glucocorticoid (betamethasone) administration during the second half of gestation on maternal and fetal metabolic status, as evaluated by maternal weight gain and appetite, fetal and placental weight, maternal and fetal biochemistry (glucose, cholesterol), and endocrinology (circulating IGF-I and -II, IGFBP-3, GH, leptin) during (maternal) and after (fetal and maternal) treatment of the mother. We based our betamethasone administration on National Institute of Child Health and Human Development recommendations for prevention of neonatal complications associated with prematurity,8,18 the timing of treatment corresponds to human second trimester pregnancy at 24, 26 and 27 weeks of gestation.
Given the observed in vitro and in vivo effects of glucocorticoids on leptin and IGF physiology in humans, rats, and ruminants, we hypothesized that exposure to glucocorticoids at doses equivalent to those administrated clinically to pregnant women would increase maternal and fetal serum levels of leptin and IGFs during treatment in this important experimental model.
Material and Methods
Experimental animals
Pregnant baboons (Papio cynocephalus, n = 31) 10-15 years of age from the colony maintained at the Southwest National Primate Research Center (SNPRC), Southwest Foundation for Biomedical Research (SFBR, San Antonio, TX, USA) were studied. Baboons were housed in outdoor metal and concrete gang cages, each containing 10-16 females and 1 breeding male. Animals were fed Purina Monkey Diet 5038 (Purina, St. Louis, MO) and water ad libitum. All animal procedures were performed in accordance with accepted standards of humane animal care, approved by the SFBR Institutional Animal Care and Use Committee (IACUC), and conducted in AAALAC, Inc. approved facilities. Animals were observed three times per week for evaluation of perineal skin swelling. Gestational age was calculated, using estimated day of conception (the time of last maximal perineal skin swelling minus 2 days).
Study design and maternal blood sampling
Normal baboon gestation lasts 180 days. At 90 days of gestation (dG) (0.5 gestation, 0.5 G), pregnant baboons maintained in group caging were weighed, underwent a detailed ultrasound examination, and placed in individual indoor cages. Baboons were observed twice each day, and their health status was continuously monitored and recorded. All animals received 25 standard biscuits twice daily. The number of biscuits eaten by each individual was recorded at the end of the day. Baboons were randomized to receive saline (control group, control; n = 14) or betamethasone phosphate (Celestan Solubile, Essex Pharma, Munich, Germany) (BM, betamethasone group; n = 17) in doses of 175 μg·kg-1·day-1 – a weight-adjusted dose equivalent to 12 mg administered to a 70 kg woman. Treatments were administered intramuscularly once daily at 0800 hours at 111, 112, 118, 119, 125, and 126 dG (equivalent of 24, 26 and 27 weeks of human pregnancy). Femoral vein blood samples were obtained from non-pregnant and pregnant baboons, following tranquilization with ketamine hydrochloride (Ketaset, Fort Dodge, IA), 10 mg/kg administered intramuscularly. Fasting blood samples were drawn from the femoral vein under sterile conditions directly into a 4-mL Vacutainer tube without additives (Becton Dickinson, Franklin Lakes, NJ, USA). Serum samples and animal weights were obtained at 0800 hours the day after completing each course of betamethasone or saline injections (on 113, 120, and 127 dG) and at 144 ± 2 (mean ± range), 162 ± 2 (mean ± range) and 175 ± 5 dG (mean ± range). The final sample was taken at the time of cesarean section.
Cesarean section, fetal blood sampling, fetal and maternal morphometry
Pregnant baboons were weighed 30 minutes before Cesarean section. Cesarean sections were performed at 174.3 ± 0.62 dG (mean ± SE) in control animals and at 174.1 ± 0.67 dG in BM-treated baboons.
All baboons were given ketamine (10 mg/kg intramuscularly) as described above and ampicillin (0.25 mg/kg) was administered as an intravenous infusion (lactated Ringer's solution, 250 mL/procedure). After intubation, isoflurane (2%, 2 L/min) was used to maintain a surgical plane of anesthesia throughout surgery and fetal blood sampling. Umbilical vein blood sampling was performed without exteriorizing the fetus from the uterine cavity. While still under anesthesia, the fetus was delivered and euthanized by an intracardiac injection of 7.8 mg pentobarbital sodium (Euthasol; Delmarva Laboratories, Inc. Midlothian, VA, USA). The placenta was removed from the uterus and immediately submitted, together with fetus, for complete pathologic evaluation and sampling. The uterus and abdominal wall were closed in layers.19
Post-operative analgesia was provided with buprenorphine hydrochloride, 0.015 mg·kg-1·day-1 for 3 post-operative days (IM, twice daily), (Buprenex Injectable, Reckitt Benckiser Health Care Ltd., Hull, UK). Ampicillin (25 mg/kg IM, twice daily) was administered for 7 days. Post-surgical management has been previously described in detail.19
Two of 14 control baboons and three of 17 BM-treated baboons delivered spontaneously 2 to 24 hours before scheduled cesarean section. One spontaneously delivered fetus from the BM-treated group was stillborn due to trauma during delivery. Maternal and fetal data from this pair were not included in the data analysis.
Glucose and cholesterol
Glucose and cholesterol were measured in fasting serum using a Beckman Synchron CX5CE (Beckman Coulter, Inc., Fullerton, CA).20
Leptin measurement in serum, amniotic fluid, and umbilical cord blood
Leptin radioimmunoassay (LINCO Research, Inc., St. Charles, MO) was performed according to the manufacturer's instructions. The intra-assay coefficient of variation (CV) was 3.0% at a leptin concentration of 4.9 ng/mL.
IGF-II measurement in serum, amniotic fluid, and umbilical cord blood
Total IGF-II concentrations in fetal and maternal serum, and amniotic fluid in samples taken at cesarean section were quantified by enzyme-linked immunosorbent assay (Diagnostic Systems Laboratories, Inc, Webster, TX, USA). Samples were measured in duplicate. Intra-assay CV was 7%, with an inter-assay CV of 8% at IGF-II concentration of 300 ng/mL.
IGF-I, IGFBP-3, growth hormone (GH), and cortisol measurements
IGF-I, IGFBP-3, GH, and cortisol were measured using an Immulite 1000 analyzer using test kits from Diagnostic Products Corporation (Los Angeles, CA). Pooled maternal serum was first tested to validate the performance of this system for baboon samples. Assay precision was determined by testing the pooled samples using five replicates in each of two assays. These assays were repeated at two dilutions to assess linearity of the results. All test samples were run at dilutions estimated to achieve values in the middle of the assay's calibration range. Intra-/inter-assay CV for cortisol, GH, IGF-I, and IGFBP-3 were 5.6/8.4, 3.4/7.0, 2.9/5.9, and 2.4/3.5, respectively. The correlation coefficients (r values) for dilutions for cortisol, GH, IGF-I, and IGFBP-3 were 0.90, 0.98, 0.98, and 0.93, respectively, for recovery with known standards.
Immunohistochemistry for leptin and long isoform of leptin receptor (Ob-R)
Tissues were immersion-fixed for 24 h in 4% buffered paraformaldehyde, embedded in paraffin, sectioned at 5 μm, deparaffinized in xylene, rehydrated in descending grades of alcohol (100%, 95%, 70%, and 50%) to water, immersed in citrate buffer (0.01 M citrate buffer, pH 6.0) and heated to boiling for 15 min. After cooling for 15 min, the sections were rinsed in potassium phosphate-buffered saline (KPBS containing 0.04 M K2HPO4, 0.01 M KH2PO4, 0.154 M NaCl, pH 7.4; 7 rinses, 6 min each), washed for 10 min in a solution of 1.5% H2O2/methanol, and then washed for 5 min in KPBS. Sections were placed in diluted (10%) normal serum for 20 min, then covered overnight at 4°C with primary antibody to leptin or to Ob-R at final dilutions of 1:50 for both antibodies (cat no. SC-842 and SC-1832, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). After overnight incubation, sections were rinsed in KPBS and incubated for 1 h at 22°C with secondary antibody (1/1000 dilution): biotinylated anti-rabbit IgG for leptin and anti-goat IgG for leptin receptor, then rinsed 40 min in KPBS, incubated in A(avidin)B(biotin) 1:333 for 1 h at 25°C, then rinsed 15 min in KPBS and 15 min in 0.175 M sodium acetate, incubated in nickel sulfate diaminobenzidine, and rinsed in sodium acetate and KPBS for 30 min. Sections were then handled as described by the manufacturer. Serial dehydration in 50%, 70%, 95%, and 100% ethanol was followed by Histoclear (3 times, 2 to 5 min each). Sections were then mounted in Histomount (National Diagnostics, Atlanta, GA, USA). Image analyses were performed as previously described.21
Statistical analysis
Variables with skewed distributions (leptin and IGF-II) were normalized using a natural logarithm transformation. For subjects with missing data, but without systematic differences on the fully observed variables, these data was assumed to be completely random. The missing values were estimated using the information of repeated measures; total number of points missing for leptin was four. For those cases with complete data, a linear regression for the value was performed based on all other repeated measurements. Using the estimated equation, we calculated the predicted data (between one and four data points for each set of repeated measurements).22
Comparison of treatment effect between groups used a generalized linear model for repeated measurements (GLMR), considering the weight and basal conditions as covariates.23 We applied Friedman analysis to calculate changes in the variables over time, including the initial measurement without any adjustment. Figures show the P value for each basal analysis. Results from group comparisons and Friedman analysis are listed in the text or legends. Data are presented throughout as mean ± SE (with control values first, followed by betamethasone when comparisons are made), except as noted.
Results
Maternal weight and food consumption before, during, and after betamethasone treatment
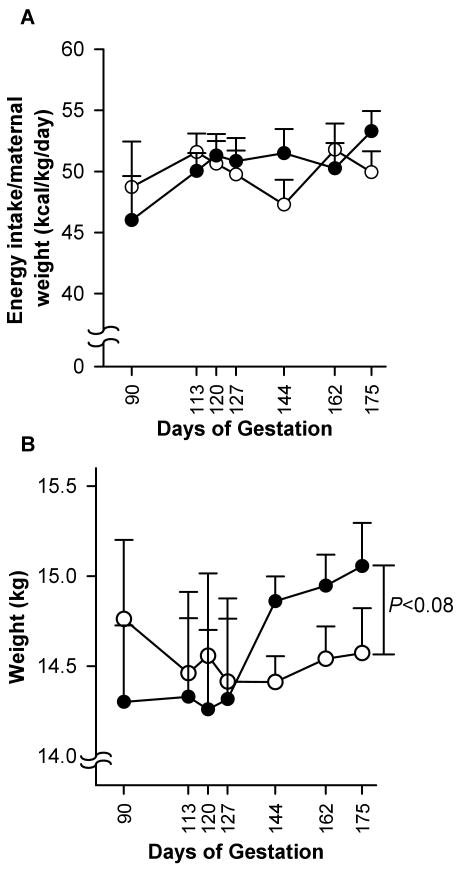
Maternal energy intake during the whole observation period did not differ between groups (49.9 ± 1.04 vs. 50.5 ± 0.96 kcal·kg-1·day-1; Fig. 1A). During gestation, energy intake increased by 15.8% in the BM-treated group (46.0 ± 3.6 kcal·kg-1·day-1 at 0.5 G vs. 53.29 ± 1.6 kcal·kg-1·day-1 at 0.95 G), while in the control group this increase was 2.5% (48.7 ± 1.7 kcal·kg-1·day-1 at 0.5 G vs. 49.9 ± 1.7 kcal·kg-1·day-1 at 0.95 G). This difference (15.8% vs. 2.5%) was not significant. The weight of the BM-treated mothers was higher at the end of gestation (0.95 G) compared to mid-gestation (0.5 G) (15 ± 0.2 kg vs. 14.3 ± 0.4 kg; P = 0.005). The weight of the control animals did not increase (14.8 ± 0.4 kg vs. 14.6 ± 0.3 kg, respectively) (Fig. 1B). At term, the difference between the groups approached significance (P < 0.08).
Fig. 1.
Maternal energy intake (A) and weight (B). Data are presented for the control group (○, n = 14) and betamethasone-treated group (●, n = 16) (mean ± SE) during and after treatment. The Friedman coefficient for the betamethasone group was P < 0.0005 and for control P < 0.054.
Fetal and placental morphometry at time of cesarean section
Placental weight (control 191.4 ± 8.8 g vs. BM-treated 188.6 ± 8.3 g) and fetal weight (control 860.0 ±34.8 g vs. BM-treated 839.5 ± 30.7 g) at 0.95 G were similar between groups; maternal weight correlated positively with fetal weight at cesarean section in the BM-treated group (r = 0.47, P = 0.001), but not in the control group.
Maternal and fetal glucose and cholesterol during and after treatment
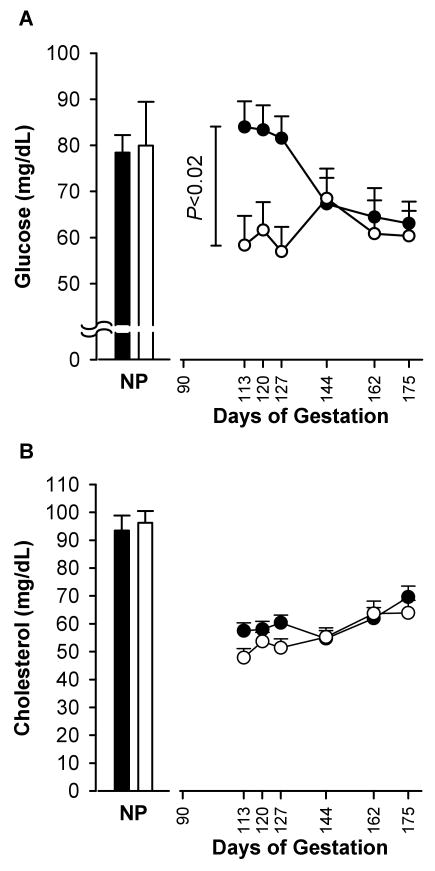
Maternal blood glucose concentration was higher in the BM-treated group than in the control group following each treatment (Fig. 2A). This difference disappeared 17 days after completion of treatment. During treatment, maternal serum cholesterol level rose 14.2% higher in the BM-treated group than the control group (P < 0.08). At the end of the study, cholesterol concentrations were similar in the two groups. Cholesterol levels rose at the end of pregnancy compared to the second trimester in a similar manner in both control and BM-treated groups; for combined control and BM-treated groups, maternal serum cholesterol was 52.7 ± 1.9 mg/dL at 0.6 G vs. 66.8 ± 2.6 mg/dl at 0.95 G (P < 0.001 vs. baseline; Fig. 2B). At 0.95 G, glucose and cholesterol levels in umbilical venous blood of fetuses from the control mothers did not differ from the BM-treated mothers (38.1 ± 4.0 vs. 31.9 ± 5.3 mg/dL for glucose; and 58.1 ± 3.6 vs. 61.7 ± 3.7 mg/dL for cholesterol, respectively).
Fig. 2.
Maternal glucose (A) and cholesterol (B) concentrations. Data are presented for the control group (○, n = 14) and betamethasone-treated group (●, n = 16) (mean ± SE) during and after administration. NP: non-pregnant animals. Friedman coefficient for both groups, P < 0.0001. For treatment differences, P < 0.38 after adjustment by weight from 0.5 G to 0.95 G.
Circulating leptin profiles
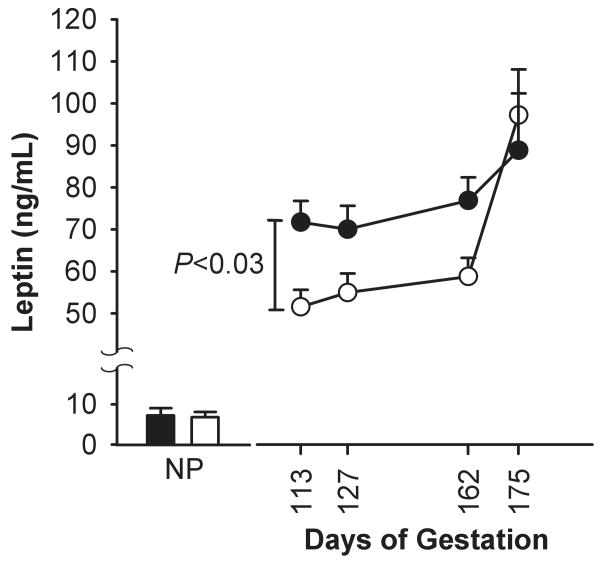
Maternal leptin concentration did not differ prior to pregnancy in females who entered the control and BM- treated groups (4.6 ± 3.2 ng/mL and 4.9 ± 4.1 ng/mL, respectively). Maternal leptin level was higher in the BM-treated group 35 days after treatment than control (74.9 ± 5 ng/mL vs. 58.7 ± 4 ng/mL respectively; P < 0.05). Leptin levels rose in control and BM-treated mothers during gestation and was higher at the end of gestation compared to 0.6 G (P < 0.05), but did not differ between groups (Fig. 3). The control group displayed a higher slope of leptin concentration rise compared with the BM group. The simple regression analysis between 0.9 and 0.95G showed a leptin increase of 1.6 ng·mL-1·day-1 for the control group (P < 0.003) vs. 0.36 ng·mL-1·day-1 for the BM group (P = 0.05), these slopes were different between the two groups (P < 0.002). Maternal leptin concentration at the end of gestation (0.95 G) tended to correlate positively with maternal weight in both groups (r = 0.38, P < 0.07). Fetal umbilical venous leptin concentration was similar in both groups: 1.6 ± 0.4 ng/mL in the control (n = 12) and 1.6 ± 1.0 ng/mL (n = 10) in the BM-treated group. Fetal leptin concentration correlated positively with maternal leptin in BM-treated group (r = 0.69, P < 0.02) but not in the control group. Maternal:fetal serum leptin ratio did not differ between BM-treated and control groups at term (53.3 ± 4.3 vs. 66.7 ± 4, P = 0.13). At 0.95 G, maternal GH levels correlated positively with maternal leptin levels in the control group (r=0.78, P < 0.02), but not in the BM-treated group.
Fig. 3.
Maternal serum leptin concentration. Data are presented for the control group (○; n = 8) and the betamethasone group (●; n = 10) at 13, 127, 162, and 175 days of gestation (mean ± SE). NP: non-pregnant animals.
Placental histology
The distribution of immunoreactivity of leptin receptor long isoform (cytotrophoblast shell in the basal plate and in the syncytiotrophoblast of stem villi) and leptin (syncytiotrophoblast and cytotrophoblast of peripheral villi) in the placenta was the same in BM-treated and control placentas (Fig. 4).
Fig. 4.
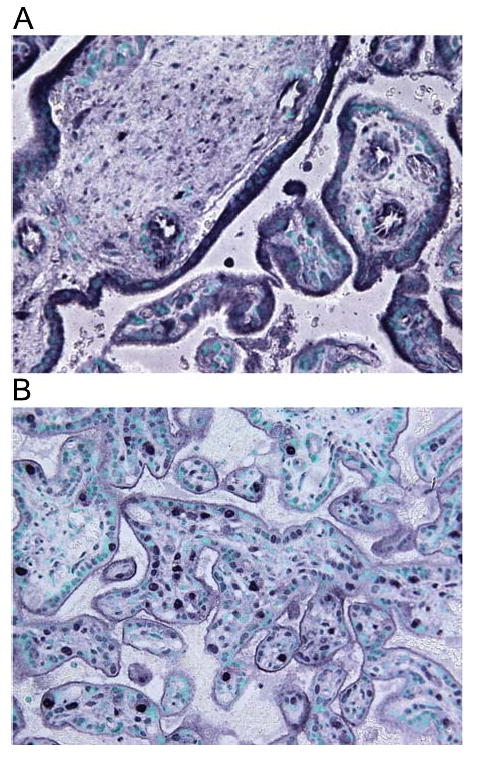
Representative microphotograph of the distribution of immunoreactivity of long isoform of leptin receptor: cytotrophoblast shell in the basal plate and in the syncytiotrophoblast of stem villi (A) and leptin: syncytiotrophoblast and cytotrophoblast of peripheral villi in the placenta (B). Magnification 40×.
Maternal and fetal IGF-II levels during and after betamethasone treatment
Total IGF-II (free and bound to IGFBPs) in maternal serum was unchanged by betamethasone treatment (Fig. 5). Maternal IGF-II level was lower at 0.95 G compared to 0.6 G in both controls and BM-treated groups (P < 0.05). At 0.95 G, fetal total IGF-II level was 2044 ± 1498 ng/mL vs. 659 ± 50 ng/mL in plasma and 860 ± 62 ng/mL vs. 1136 ± 218 ng/mL in amniotic fluid from control and BM-treated groups, respectively. These values did not differ. There were no significant correlations among total IGF-II concentrations in fetal and maternal circulations, amniotic fluid, fetal weight, or placental weights in either group.
Fig. 5.
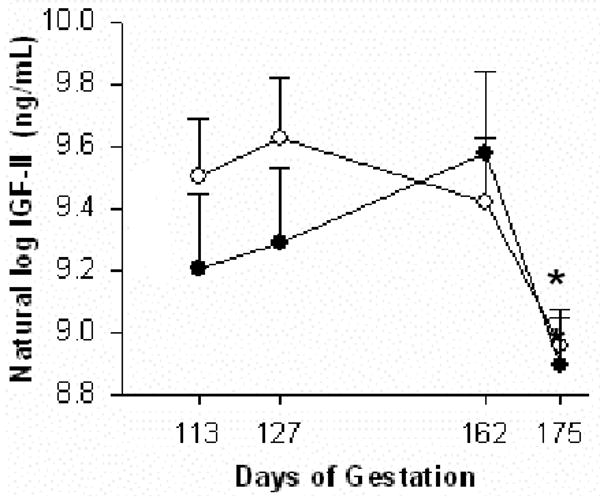
Maternal serum total IGF-II levels during baboon pregnancy in control (○, n = 7) and betamethasone-treated (●, n = 7) groups (mean ± SE). Maternal IGF-II level was lower at the end of third trimester (175 days of gestation) compared to second trimester (113 days of gestation) (*P < 0.05) in both groups.
IGF-I, IGFBP-3, GH, and cortisol measurements in maternal serum during and after treatment and in fetal serum at 0.95 G
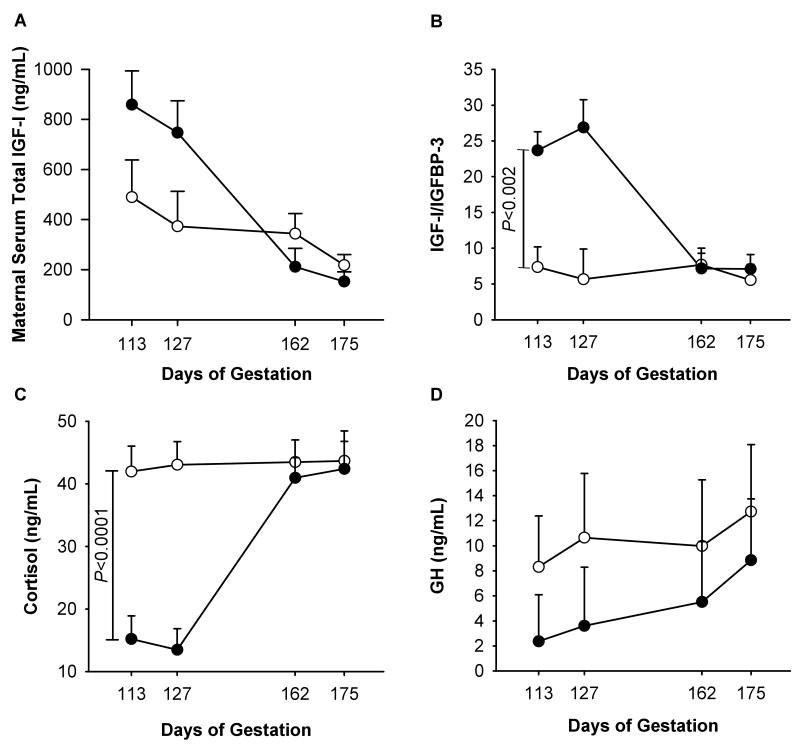
Maternal serum total IGF-I concentrations did not differ between control and BM-treated groups (356 ± 91 ng/mL vs. 493 ± 83 ng/mL, as means for the whole period of treatment, P < 0.3). Maternal total IGF-I decreased during gestation from 489 ± 147 ng/mL at 0.6 G to 218 ± 42 ng/mL at 0.95 G in control and 858 ± 135 ng/mL to 152 ± 38 ng/mL over the same period in the BM-treated group (P < 0.05) (Fig. 6A). This decrease was more pronounced in the BM-treated group than the control group: 82% vs. 55% (P < 0.03). During treatment at 0.6 G and 0.7 G, the IGF-I:IGFBP-3 ratio was significantly higher in the BM-treated than in the control group: 16.21 ± 2.3 vs. 6.57 ± 2.53 at 0.6G (P < 0.05) and 16.21 ± 2.3 vs. 6.57 ± 2.53 at 0.7 G (P < 0.05) (Fig. 6B). Circulating maternal GH levels were unchanged by BM treatment (Fig. 6C). During gestation, serum GH level rose by 53% in the control group and by 271% in the BM-treated group (P < 0.04 for both groups vs. baseline).
Fig. 6.
Maternal serum total IGF-I level (A), IGF-I:IGFBP-3 ratio (B), cortisol level (C), and growth hormone (GH) level (D). Data are presented for the control group (○, n = 5) and the betamethasone group (●, n = 6) at 113, 127, 162, and 175 days of gestation (dG) (mean ± SE).
Discussion
Effect of betamethasone treatment on maternal and fetal morphometry
Since Cushing's classic clinical description of elevated glucocorticoid secretion over 100 years ago, glucocorticoids have been known to promote obesity and redistribute body fat in adults.24 In agreement with clinical data, we observed that maternal weight increased following betamethasone treatment in pregnant baboons in the absence of increased food consumption, which may indicate changes in metabolic rate during betamethasone administration in pregnancy. Absence of increased food consumption during glucocorticoid treatment has also been observed in pregnant rats.25 A similar phenomenon of presence of weight gain with rising total leptin level was described in humans with glucocorticoid excess—who gain weight and have good appetite26 despite rising total leptin levels.27 Glucocorticoids affect energy storage28 via decreased thermogenesis,29 increased lipogenesis (with increased insulin levels)30,31 and altered central neural regulation of energy homeostasis.32
Betamethasone did not change fetal weight at term in this study. The published results on the effect of multiple courses of prenatal glucocorticoids on fetal growth in different species have been inconclusive. Some studies have demonstrated that glucocorticoids inhibit fetal growth when administered during later stages of gestation in sheep, rats, nonhuman primates, and humans33 while, similar to our observations, other studies report no adverse effects of antenatal glucocorticoid on fetal weight in human pregnancy.34 Our findings agree with several human reports showing that prenatal glucocorticoids do not lower birth weight.35-37 Discrepancies between studies in both humans and non-human primates (regarding fetal weight changes after glucocorticoid administration) may reflect usage of dexamethasone, and not betamethasone. Additionally, the trajectory of fetal development differs among primates and non-primate species.38 We have discussed the influence of betamethasone treatment on placental structure in different animal models and humans in a recent publication.39 Our experimental design, which utilized bethamethasone, closely resembles the clinical situation, where bethamethasone has been recommended over dexamethasone administration.18.
Effect of betamethasone treatment on maternal and fetal glucose levels
Glucocorticoids cause a transient increase in hepatic glucose release in adults and a transient surge in fetal insulin synthesis in human pregnancy.40 Similarly, we observed an initial rise in maternal glucose concentration during treatment. However, circulating glucose levels in mother and fetus were similar in the control and betamethasone groups after completion of the treatment course and at the end of gestation.
Effect of betamethasone treatment on maternal and fetal leptin concentrations
In our present study, maternal leptin concentration increased at 0.95 G compared to 0.6 G in both control and betamethasone groups. This observation supports other recent reports that leptin concentration increases in the maternal circulation as gestation advances in baboons,41 Japanese monkeys,42 humans,43 rodents,14 and sheep.44
Glucocorticoids stimulate leptin gene expression in sheep fetal perirenal adipose tissue45 and leptin release in human subjects.46 We observed maternal leptin concentrations in the BM-treated group to be significantly higher than in the control group, even 35 days after completing the treatment course. This long-term effect is surprising because bethamethasone is cleared from the maternal circulation by 7 days after injection.47 In non-pregnant humans and sheep treated with cortisol or dexamethasone, the rise in plasma leptin was short-lived (3-4 days)46,48 and the maternal endocrine system appeared to recover rapidly, with cortisol levels returning to normal within 1 to 2 days after treatment.49 The differences between reported short-term effects of betamethasone treatment on leptin serum concentration in the non-pregnant state and the long-term effects observed in the present study are likely due to specific, pregnancy-associated mechanisms involving leptin secretion and utilization in pregnancy (e.g., the gene promoters regulating leptin synthesis differ between placental and adipose tissues27,50). Glucocorticoids induce a state of “leptin resistant” obesity.51 When administered in pregnancy glucocorticoids are acting in a milieu that is already leptin resistant, which is not so in the normal non-pregnant state.52 Recently published human data36 support our observation of a prolonged effect of betamethasone administration on maternal leptin concentration. Marinoni et al.37 found that leptin levels were elevated at least 7 days after completing two treatment courses. One could speculate—since leptin directly stimulates Kiss-1 peptide,53 a potent vasoconstrictor54—that prolonged exposure to higher leptin levels may induce maladaptation to pregnancy-related cardiovascular changes, especially during the second half of gestation. Of interest, pre-eclampsia and maternal obesity are other two conditions associated with hyperleptinemia55.
The control group displayed a higher slope of leptin concentration rise compared with BM-treated group. The reason for these different slopes could be related to delivery. C. sections were performed around the time of delivery in the baboon (175 ± 11 dG). Maternal leptin concentration at the end of the study did not differ between two groups. A potential explanation may be that there is a maximal pre-delivery leptin concentration peak56, much of which has been already driven by exogenous glucocorticoids and the controls catch up to this level
The correlation between maternal leptin and GH concentrations contradicts experimental data published for GH deficiency in human adults,57 but agrees with experimental data from rats58 and peripubertal children.59 The observed differences may relate to altered leptin receptor sensitivity during pregnancy and the placental contribution to leptin metabolism.
Effect of betamethasone treatment on maternal and fetal IGF concentrations
Observations of glucocorticoid action on the circulating IGF axis vary. Glucocorticoid administration has been reported to reduce IGF-I release from bone60 or to have no effect.61 Glucocorticoids suppress IGF-I production during pregnancy in rats.62 In fetal sheep, cortisol suppresses IGF-II mRNA abundance in liver, skeletal muscle, and adrenal tissues, and exerts tissue-specific effects on IGF-I gene expression in placenta.63 Of interest, the recent studies have reported that fetal exposure to a maternal hyper-glucocorticoid environment disturbs glucose metabolism and the IGF axis in later life, demonstrating persistent effect of the glucocorticoid exposure. 64, 65 The mechanism of these changes remains to be elucidated.
Because the serum of pregnant baboons lacks the IGFBP protease activity detected in human pregnancy serum,66 it is unlikely that the increase in circulating IGF levels results from increased IGFBP protease activity in our study. Maternal IGF-II concentration at 0.95 G was lower than at the middle of gestation in our study. These data agree with observations from others, who found that plasma IGF-II levels decrease close to term.46
Conclusion
We described here for the first time the protracted effect of maternal betamethasone treatment on both IGF-I:IGFBP-3 ratio and leptin in the maternal circulation, with a more prolonged elevation of leptin, than of IGF-I, levels. The observed differences in IGF and leptin responses might be due to different mechanisms by which glucocorticoids influence the production of these major hormones. Our data show that the prolonged elevation of leptin level after betamethasone treatment is not associated with changes in IGF, glucose, cholesterol, or GH status. The clinical observations of Marinoni et al. 37 reinforce the direct application of our own findings to human pregnancy. The baboon is a promising model to study key questions related to the metabolic changes associated with prenatal glucocorticoid exposure. The molecular mechanisms and full consequences of the protracted leptin rise following betamethasone administration to the mother remain to be elucidated.
Acknowledgments
We are grateful to Ms. C. Jett for performing the leptin analyses. We acknowledge the excellent assistance of Ms. Cathy Snider and Mrs. Xiaojing Xu in the conduct of the biochemical estimates. The authors express their gratitude to the personnel of SNPRC and UTHSCSA for their assistance and expertise, especially Ms. Susan L. Jenkins of UTHSCSA for her help with part of statistical analysis and Mrs. Gloria Matthews of the Division of Pediatric Endocrinology at UTHSCSA for administrative support.
Grant support: This work was supported in part by NIH grants R01 HD21350 (Dr. Nathanielsz), P51 RR013986 (SNPRC base grant), and K08 DK02876 and the SPC Russell H. Nahvi Memorial Fund for Pediatric Research at UTHSCSA (Dr. Ferry).
Contributor Information
Natalia E. Schlabritz-Loutsevitch, Email: schlabritzlu@uthscsa.edu.
Juan C. Lopez-Alvarenga, Email: jalvaren@sfbrgenetics.org.
Anthony G. Comuzzie, Email: tony@sfbrgenetics.org.
Myrna M. Miller, Email: myrna.miller@ars.usda.gov.
Stephen P. Ford, Email: spford@uwyo.edu.
Cun Li, Email: lic@uthscsa.edu.
Gene B. Hubbard, Email: ghubbard@sfbr.org.
Robert J. Ferry, Jr., Email: bob@utmem.edu.
Peter W. Nathanielsz, Email: nathanielsz@uthscsa.edu.
References
- 1.Ferry RJ, Jr, Cohen P. The insulin-like growth factor axis in pediatrics. Clin Pediatr Endocrinol. 1999;8:1–10. [Google Scholar]
- 2.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 3.Christou H, Connors JM, Ziotopoulou M, et al. Cord blood leptin and insulin-like growth factor levels are independent predictors of fetal growth. J Clin Endocrinol Metab. 2001;86:935–938. doi: 10.1210/jcem.86.2.7217. [DOI] [PubMed] [Google Scholar]
- 4.Considine RV. Regulation of leptin production. Rev Endocrinol Metab Dis. 2001;2:357–363. doi: 10.1023/a:1011896331159. [DOI] [PubMed] [Google Scholar]
- 5.Ong K, Kratzsch J, Kiess W, et al. Size at birth and cord blood levels of insulin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-1 (IGFBP-1), IGFBP-3, and the soluble IGF-II/mannose-6-phosphate receptor in term human infants. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab. 2000;85:4266–4269. doi: 10.1210/jcem.85.11.6998. [DOI] [PubMed] [Google Scholar]
- 6.Adam TC, Epel ES. Stress, eating and the reward system. Proceedings from the 2006 Meeting of the Society for the Study of Ingestive Behavior; 2007. pp. 449–458. [DOI] [PubMed] [Google Scholar]
- 7.Dudley DJ, Waters TP, Nathanielsz PW. Current status of single-course antenatal steroid therapy. Clin Obstet Gynecol. 2003;46:132–149. doi: 10.1097/00003081-200303000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Lee BH, Stoll BJ, McDonald SA, Higgins RD, National Institute of Child Health and Human Development Neonatal Research Network Adverse neonatal outcomes associated with antenatal dexamethasone versus antenatal betamethasone. Pediatrics. 2006;117:1503–1510. doi: 10.1542/peds.2005-1749. [DOI] [PubMed] [Google Scholar]
- 9.Mariotti V, Marconi AM, Pardi G. Undesired effects of steroids during pregnancy. J Matern Fetal Neonatal Med. 2004;16:5–7. doi: 10.1080/14767050410001727099. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad I, Beharry KD, Valencia AM, et al. Influence of a single course of antenatal betamethasone on the maternal-fetal insulin-IGF-GH axis in singleton pregnancies. Growth Horm IGF Res. 2006;16:267–275. doi: 10.1016/j.ghir.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Shekhawat PS, Garland JS, Alex C, et al. Cord blood and postnatal serum leptin and its relationship to steroid use and growth in sick preterm infants. J Pediatr Endocrinol Metab. 2000;13:1571–1576. doi: 10.1515/jpem.2000.13.9.1571. [DOI] [PubMed] [Google Scholar]
- 12.Forhead AJ, Thomas L, Crabtree J, et al. Plasma leptin concentration in fetal sheep during late gestation: ontogeny and effect of glucocorticoids. Endocrinology. 2002;143:1166–1173. doi: 10.1210/endo.143.4.8762. [DOI] [PubMed] [Google Scholar]
- 13.Ousey JC, Rossdale PD, Dudan FE, Fowden AL. The effects of intrafetal ACTH administration on the outcome of pregnancy in the mare. Reprod Fertil Dev. 1998;10:359–367. doi: 10.1071/r98045. [DOI] [PubMed] [Google Scholar]
- 14.Smith JT, Waddell BJ. Leptin distribution and metabolism in the pregnant rat: transplacental leptin passage increases in late gestation but is reduced by excess glucocorticoids. Endocrinology. 2003;144:3024–3030. doi: 10.1210/en.2003-0145. [DOI] [PubMed] [Google Scholar]
- 15.Ehrhardt RA, Bell AW, Boisclair YR. Spatial and developmental regulation of leptin in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1628–R1635. doi: 10.1152/ajpregu.00750.2001. [DOI] [PubMed] [Google Scholar]
- 16.Malik NM, Carter ND, Wilson CA, et al. Leptin expression in the fetus and placenta during mouse pregnancy. Placenta. 2005;26:47–52. doi: 10.1016/j.placenta.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Thomas L, Wallace JM, Aitken RP, et al. Circulating leptin during ovine pregnancy in relation to maternal nutrition, body composition and pregnancy outcome. J Endocrinol. 2001;169:465–476. doi: 10.1677/joe.0.1690465. [DOI] [PubMed] [Google Scholar]
- 18.Lee BH, Stoll BJ, McDonald SA, et al. Neurodevelopmental outcomes of extremely low birth weight infants exposed prenatally to dexamethasone versus betamethasone. Pediatrics. 2008;121:289–296. doi: 10.1542/peds.2007-1103. [DOI] [PubMed] [Google Scholar]
- 19.Schlabritz-Loutsevitch NE, Hubbard GB, et al. Normal concentrations of essential and toxic elements in pregnant baboons and fetuses (Papio species) J Med Primatol. 2004;33:152–162. doi: 10.1111/j.1600-0684.2004.00066.x. [DOI] [PubMed] [Google Scholar]
- 20.Schlabritz-Loutsevitch NE, Hubbard GB, Jenkins SL, et al. Ontogeny of hematological cell and biochemical profiles in maternal and fetal baboons (Papio species) J Med Primatol. 2005;34:193–200. doi: 10.1111/j.1600-0684.2005.00109.x. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Levitz M, Hubbard GB, et al. The IGF axis in baboon pregnancy: placental and systemic responses to feeding 70% global ad libitum diet. Placenta. 2007;28:1200–1210. doi: 10.1016/j.placenta.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allison P. Missing Data. Sage University Paper Series on Quantitative Applications in the Social Sciences 07-36. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 23.Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester, England: John Wiley & Son Ltd.; 1999. pp. 199–259. [Google Scholar]
- 24.Ishida-Takahashi R, Uotani S, Abe T, et al. Rapid inhibition of leptin signaling by glucocorticoids in vitro and in vivo. J Biol Chem. 2004;279:19658–19664. doi: 10.1074/jbc.M310864200. [DOI] [PubMed] [Google Scholar]
- 25.Sugden MC, Langdown ML, Munns MJ, Holness MJ. Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression and materno-fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early-growth-retarded adult offspring. Eur J Endocrinol. 2001;145:529–539. doi: 10.1530/eje.0.1450529. [DOI] [PubMed] [Google Scholar]
- 26.Tataranni PA, Larson DE, Snitker S, et al. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 27.Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 28.Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol. 2008;197:189–204. doi: 10.1677/JOE-08-0054. [DOI] [PubMed] [Google Scholar]
- 29.Tokuyama K, Himms-Hagen J. Adrenalectomy prevents obesity in glutamate-treated mice. Am J Physiol. 1989;257(2 Pt 1):E139–E144. doi: 10.1152/ajpendo.1989.257.2.E139. [DOI] [PubMed] [Google Scholar]
- 30.Dallman MF, la Fleur SE, Pecoraro NC, et al. Minireview: glucocorticoids--food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- 31.Björntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 32.Nishida Y, Yoshioka M, St-Amand J. Regulation of hypothalamic gene expression by glucocorticoid: implications for energy homeostasis. Physiol Genomics. 2006;25:96–104. doi: 10.1152/physiolgenomics.00232.2005. [DOI] [PubMed] [Google Scholar]
- 33.Reinisch JM, Simon NG, Karow WG, Gandelman R. Prenatal exposure to prednisone in humans and animals retards intrauterine growth. Science. 1978;202:436–438. doi: 10.1126/science.705336. [DOI] [PubMed] [Google Scholar]
- 34.Hasbargen U, Reber D, Versmold H, Schulze A. Growth and development of children to 4 years of age after repeated antenatal steroid administration. Eur J Pediatr. 2001;160:552–555. doi: 10.1007/s004310100804. [DOI] [PubMed] [Google Scholar]
- 35.Crowther CA, Haslam RR, Hiller JE, et al. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet. 2006;367:1913–1919. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- 36.Crowther CA, Doyle LW, Haslam RR, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. New Engl J Med. 2007;357:1179–1189. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- 37.Marinoni E, Letizia C, Ciardo F, et al. Effects of prenatal betamethasone administration on leptin and adiponectin concentrations in maternal and fetal circulation. Am J Obstetr Gynecol. 2008;199:141.e1–141.e6. doi: 10.1016/j.ajog.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 38.Benirschke, Kaufmann . Pathology of the human placenta. Springer Verlag; 2000. [Google Scholar]
- 39.Schlabritz-Loutsevitch N, Ballesteros B, Dudley C, et al. Moderate maternal nutrient restriction, but not glucocorticoid administration, leads to placental morphological changes in the baboon (Papio sp.) Placenta. 2007;28:783–793. doi: 10.1016/j.placenta.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogueh O, Miell JP, Jones JC, et al. Antenatal dexamethasone and the growth hormone-insulin-like growth factor axis. Hum Reprod. 2000;15:1403–1406. doi: 10.1093/humrep/15.6.1403. [DOI] [PubMed] [Google Scholar]
- 41.Henson MC, Castracane VD. Leptin: roles and regulation in primate pregnancy. Semin Reprod Med. 2002;20:113–122. doi: 10.1055/s-2002-32502. [DOI] [PubMed] [Google Scholar]
- 42.Wang C, Medan MS, Shimizu K, et al. Secretion of leptin throughout pregnancy and early postpartum period in Japanese monkeys: placenta as another potential source of leptin. Endocrine. 2005;27:75–81. doi: 10.1385/ENDO:27:1:075. [DOI] [PubMed] [Google Scholar]
- 43.Schubring C, Englaro P, Siebler T, et al. Longitudinal analysis of maternal serum leptin levels during pregnancy, at birth and up to six weeks after birth: relation to body mass index, skinfolds, sex steroids and umbilical cord blood leptin levels. Horm Res. 1998;50:276–283. doi: 10.1159/000023290. [DOI] [PubMed] [Google Scholar]
- 44.Ehrhardt RA, Slepetis RM, Bell AW, Boisclair YR. Maternal leptin is elevated during pregnancy in sheep. Domest Anim Endocrinol. 2001;21:85–96. doi: 10.1016/s0739-7240(01)00108-4. [DOI] [PubMed] [Google Scholar]
- 45.O'Connor DM, Blache D, Hoggard N, et al. Developmental control of plasma leptin and adipose leptin messenger ribonucleic acid in the ovine fetus during late gestation: role of glucocorticoids and thyroid hormones. Endocrinology. 2007;148:3750–3757. doi: 10.1210/en.2007-0310. [DOI] [PubMed] [Google Scholar]
- 46.Newcomer JW, Selke G, Melson AK, et al. Dose-dependent cortisol-induced increases in plasma leptin concentration in healthy humans. Arch Gen Psychiatry. 1998;55:995–1000. doi: 10.1001/archpsyc.55.11.995. [DOI] [PubMed] [Google Scholar]
- 47.Anderson AB, Gennser G, Jeremy JY, Ohrlander S, Sayers L, Turnbull AC. Placental transfer and metabolism of betamethasone in human pregnancy. Obstet Gynecol. 1977;49:471–474. [PubMed] [Google Scholar]
- 48.Ng PC, Lam CW, Lee CH, et al. Changes in serum leptin concentration after corticosteroid treatment in preterm infants. Acta Paediatr. 2002;91:684–690. doi: 10.1080/080352502760069124. [DOI] [PubMed] [Google Scholar]
- 49.Coe CL, Lubach GR. Developmental consequences of antenatal dexamethasone treatment in nonhuman primates. Neurosci Biobehav Rev. 2005;29:227–235. doi: 10.1016/j.neubiorev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Bi C, Gavrilova O, Gong DW, Mason M, Reitman M. Identification of a placental enhancer for the human leptin gene. J Biol Chem. 1997;272:30583–30588. doi: 10.1074/jbc.272.48.30583. [DOI] [PubMed] [Google Scholar]
- 51.Unger RH. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab. 2003;14:398–403. doi: 10.1016/j.tem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Zavalza-Gómez AB, Anaya-Prado R, Rincón-Sánchez AR, et al. Adipokines and insulin resistance during pregnancy. Diabetes Res Clin Pract. 2008;80:8–15. doi: 10.1016/j.diabres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 53.Brown RE, Imran SA, Ur E, et al. KiSS-1 mRNA in adipose tissue is regulated by sex hormones and food intake. Mol Cell Endocrinol. 2008;281:64–72. doi: 10.1016/j.mce.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Mead EJ, Maguire JJ, Kuc RE, et al. Kisspeptins: a multifunctional peptide system with a role in reproduction, cancer and the cardiovascular system. Br J Pharmacol. 2007;151:1143–53. doi: 10.1038/sj.bjp.0707295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rasmussen KM, Kjolhede CL. Maternal obesity: a problem for both mother and child. Obesity (Silver Spring) 2008;16:929–931. doi: 10.1038/oby.2008.36. [DOI] [PubMed] [Google Scholar]
- 56.Nuamah MA, Yura S, Sagawa N, et al. Significant increase in maternal plasma leptin concentration in induced delivery: a possible contribution of pro-inflammatory cytokines to placental leptin secretion. Endocr J. 2004;51:177–87. doi: 10.1507/endocrj.51.177. [DOI] [PubMed] [Google Scholar]
- 57.Engström BE, Burman P, Holdstock C, Karlsson FA. Effects of growth hormone (GH) on ghrelin, leptin, and adiponectin in GH-deficient patients. J Clin Endocrinol Metab. 2003;88:5193–5198. doi: 10.1210/jc.2003-030713. [DOI] [PubMed] [Google Scholar]
- 58.Carro E, Senaris R, Considine RV, Casanueva FF, Dieguez C. Regulation of in vivo growth hormone secretion by leptin. Endocrinology. 1997;139:2203–2206. doi: 10.1210/endo.138.5.5238. [DOI] [PubMed] [Google Scholar]
- 59.Ghizzoni L, Mastorakos G, Street ME, et al. Leptin, cortisol, and GH secretion interactions in short normal prepubertal children. J Clin Endocrinol Metab. 2001;86:3729–3734. doi: 10.1210/jcem.86.8.7758. [DOI] [PubMed] [Google Scholar]
- 60.Delany AM, Durant D, Canalis E. Glucocorticoid suppression of IGF-I transcription in osteoblasts. Mol Endocrinol. 2001;15:1781–1789. doi: 10.1210/mend.15.10.0704. [DOI] [PubMed] [Google Scholar]
- 61.Ward WE, Atkinson SA, Donovan SM, Paes B. Bone metabolism and circulating IGF-I and IGFBPs in dexamethasone-treated preterm infants. Early Hum Dev. 1999;56:127–141. doi: 10.1016/s0378-3782(99)00039-0. [DOI] [PubMed] [Google Scholar]
- 62.Luo JM, Murphy LJ. Dexamethasone inhibits growth hormone induction of insulin-like growth factor-I (IGF-I) messenger ribonucleic acid (mRNA) in hypophysectomized rats and reduces IGF-I mRNA abundance in the intact rat. Endocrinology. 1989;125:165–171. doi: 10.1210/endo-125-1-165. [DOI] [PubMed] [Google Scholar]
- 63.Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta. 2003;24:803–812. doi: 10.1016/s0143-4004(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 64.Gatford KL, Owens JA, Li S, et al. Repeated betamethasone treatment of pregnant sheep programs persistent reductions in circulating IGF-I and IGF-binding proteins in progeny. Am J Physiol Endocrinol Metab. 2008;295:E170–E178. doi: 10.1152/ajpendo.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Massmann A, Pulgar V, et al. Effects of Diet-Induced Obesity on Insulin Sensitivity in Adult sheep exposed anternatally to Glucocorticoids. Reprod Sci. 2008;15(Suppl Abstr763):276A. [Google Scholar]
- 66.Giudice LC, Dsupin BA, de las Fuentes L, et al. Insulin-like growth factor binding proteins in sera of pregnant nonhuman primates. Endocrinology. 1993;132:1514–1526. doi: 10.1210/endo.132.4.7681762. [DOI] [PubMed] [Google Scholar]