Abstract
Rapid infant weight gain is associated with increased abdominal adiposity, but there is no published report of the relationship of early infant growth to differences in specific adipose tissue depots in the abdomen, including visceral adipose tissue (VAT). In this study, we tested the associations of birth weight, infant weight gain, and other early life traits with VAT, abdominal subcutaneous adipose tissue (ASAT), and other body composition measures using magnetic resonance imaging (MRI) and dual-energy X-ray absorptiometry in middle adulthood (mean age = 46.5 years). The sample included 233 appropriate for gestational age singleton white children (114 males) enrolled in the Fels Longitudinal Study. Multivariate-adjusted general linear models were used to test the association of infant weight gain (from 0 to 2 years), maternal BMI, gestational age, parity, maternal age, and other covariates with adulthood body composition. Compared to infants with slow weight gain, rapid weight gain was associated with elevated risk of obesity (adjusted odds ratio = 4.1, 95% confidence interval = 1.4, 11.1), higher total body fat (+7 kg, P = 0.0002), percent body fat (+5%, P = 0.0006), logVAT mass (+0.43 kg, P = 0.02), logASAT mass (+0.47 kg, P = 0.001), and percent abdominal fat (+5%, P = 0.03). There was no evidence that the increased abdominal adipose tissue was due to a preferential deposition of VAT. In conclusion, rapid infant weight gain is associated with increases in both VAT and ASAT, as well as total adiposity and the risk of obesity in middle adulthood.
INTRODUCTION
Lifecourse epidemiology is a relatively new subspecialty of chronic disease epidemiology that elucidates critical developmental periods and the timing and duration of environmental exposures over the lifecourse as risk factors for cardiovascular disease, obesity, diabetes, cancer, and other common chronic diseases (1,2). The prenatal–neonatal transition is considered one such critical period that impacts these complex multifactorial diseases (2,3). In particular, individuals with a faster rate of weight gain in the first months of life tend to have a higher risk of later obesity (reviewed by refs. 4–6) and abnormal β-cell function and insulin sensitivity (7–10) than individuals with slower or gradual infant weight gain.
One proximate mechanism explaining the association of infant weight gain to the risk of diabetes is that growth-restricted fetuses who experience rapid “catch-up” growth during infancy preferentially deposit adipose tissue in the abdominal region in comparison to slower growing infants (11,12). Body fat centralization involves a heterogeneous set of tissue types, including both abdominal visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (ASAT), which have distinguishable effects on the metabolic syndrome and diabetes risk (13–16), circulating adipokine levels (15,17,18), and possibly mortality (19). However, the link between rapid infant weight gain and the deposition of adipose tissue in specific compartments within the abdomen, including VAT, has not been tested. Further, it is not clear whether or not infant weight gain contributes significantly to later body composition in normal birth weight infants growing up in well-nourished environments (as well as growth-restricted infants) (12). The aim of this study is to test the associations of birth weight, infant weight gain, and other early life traits with total, abdominal, and VAT mass in middle adulthood in a well-characterized cohort of apparently healthy individuals.
METHODS AND PROCEDURES
Sample
The data set comprised a subset of 233 appropriate for gestational age white singleton children (114 males, 119 females) enrolled in the Fels Longitudinal Study, who also participated as adults in a magnetic resonance imaging (MRI) study conducted in 2004–2007. The Fels Longitudinal Study began in 1929 as a study of individual variation in the growth and development of children (20) and has continued as a study of early life antecedents of cardiovascular and diabetes risk factors in adulthood. Of the 630 infants in the Fels Longitudinal Study having measured weights at birth and age 2, and who were followed longitudinally thereafter, 316 were eligible for the MRI study (i.e., were ≥18 years of age, were scheduled for a visit between 2003 and 2007 (when the MRI study was being conducted), were not pregnant or nursing, did not exceed the weight/size limits of the MRI or dual-energy X-ray absorptiometry, had no contraindications to MRI, and did not report claustrophobia), and 74% agreed to participate. These 233 subjects did not differ from those not in the MRI study in birth weight (P = 0.56), infant weight gain (P = 0.67), or birth year (P = 0.68). Those in the MRI study had a lower BMI throughout childhood and at age 18 (BMI: 21.9 kg/m2 vs. 22.5 kg/m2, P = 0.04), which may reflect the exclusion due to weight and size limits of body composition assessment. All protocols and informed consent materials were approved by the Wright State University Institutional Review Board.
Measurements
Birth year varied from 1930 to 1988, and therefore we included birth year as a covariate in all models. Gestational age at birth was calculated from maternal report of last menstrual period at the time of the infant’s birth. Maternal age and parity were obtained via maternal interview. Infants were weighed in the hospital at birth and then at age 2, at the study center.
Infant weight standard deviation scores (SDSs) were calculated at birth and at age 2 using sex- and age-specific sample mean weights and standard deviations in the 233 infants. Infant weight gain was calculated as the difference between weight SDS at birth and age 2 and was used primarily as a continuous variable. In addition, we categorized infants as having “rapid,” “slow,” and “gradual” infant growth according to Ong et al. (21) definition of “clinically significant” weight increment between birth and age 2 greater than +0.67 SDS (rapid), less than −0.67 SDS (slow), or between −0.67 and +0.67 SDS (gradual).
Maternal BMI was collected within 5 years of the infant’s birth using the nonpregnancy weight closest to the birth date, and was available for a subset of 160 of the subjects (the remainder of the women had measured BMI outside of that range, or the mother of the infant did not agree to being measured). The time difference between the infant’s birth date and the date of the maternal BMI measurement was not associated with maternal BMI (r = 0.04, P = 0.73), with birth weight (r = 0.04, P = 0.67), or with infant weight gain (r = −0.03, P = 0.78). Maternal BMI was standardized to the sample mean and standard deviation (maternal BMI SDS). There were no differences between subjects with, as opposed to without, maternal BMI data in the main variables analyzed in this study: birth weight SDS, infant weight gain SDS, adulthood BMI, VAT mass, ASAT mass, or waist. Subjects with data on maternal BMI were older at the MRI visit (mean age 49.9 years vs. 39.1 years, P < 0.0001) and had a lower rate of current cigarette smoking (17% vs. 32%, P = 0.01).
At the adulthood (MRI) visit, participants wore light clothing (shorts, sleeveless shirts) during all measurements. Weight was measured to the nearest 0.01 kg using a digital scale and stature to the nearest 0.01 cm using a digital stadiometer. Waist circumference (waist) was measured to the nearest centimeter immediately superior to the left iliac crest following protocols of the National Health and Nutrition Examination Survey (22). Total body fat (TBF) mass, fat-free mass (FFM), and percent body fat (%BF) were measured using the Hologic QDR 4500 DXA system (Hologic, Bedford, MA; software version 9.8D). Abdominal MRI was conducted at the Good Samaritan Hospital Greater Dayton MRI Consortium in Dayton, Ohio, using a protocol described previously (14). Images were obtained every 1 cm from the 9th thoracic vertebra (T9) to the first sacral vertebra (S1). Segmentation of the axial images into VAT and ASAT areas (cm2) was performed by two trained observers using image analysis software (Slice-O-matic, version 4.2; TomoVision, Montreal, Quebec, Canada). VAT and ASAT areas were summed across all 24 images to obtain VAT and ASAT volume, and then these were multiplied by 0.916 g/cm3, the approximate density of adipose tissue to obtain total VAT mass (kg) and total ASAT mass (kg). Interobserver variation was typical of MRI studies (coefficient of variation% = 7.75% for VAT mass and 2.2% for ASAT mass). Derived variables were created to characterize the extent of central and VAT deposition in the body (abdominal fat = VAT + ASAT; %abdominal fat = abdominal fat/TBF × 100; %VAT/TBF, and %VAT/ASAT).
Responses on current cigarette smoking (yes/no), educational attainment (4-year university degree/less than a 4-year university degree), and engagement in sports using the sport activity index score (≥3 = high; <3 = low sports activity) of the Baecke Habitual Physical Activity Questionnaire (23) were obtained via interview at the time of the MRI.
Statistical analysis
Distributional characteristics of the variables were examined and those with significant skewness (VAT and ASAT) were log transformed prior to analysis. χ2-tests for frequencies and independent sample t-tests for continuously distributed variables, were used to assess significant differences between males and females; Bonferroni corrections were made to reduce the risk of inflated type 1 error. Minimally adjusted (sex, age, birth weight SDS, and adult stature) general linear models were first used to examine differences in the least squares means for body composition variables by infant weight gain group (rapid, gradual, and slow). We then used multivariate-adjusted general linear models to test the association of birth weight SDS and change in infant weight SDS with all adulthood body composition variables. In each of these models, gestational age at birth, birth order, maternal age, birth year, age at adulthood MRI, and adulthood stature were included as continuous covariates, whereas sex (male vs. female), infant feeding mode (ever breastfed vs. never breastfed), adulthood educational attainment, adulthood cigarette smoking status, and adulthood sports activity level were included as categorical covariates. Sex was examined as an interaction term to detect differential relationship in males and females; none was significant. These models were also applied to the subset of 160 participants with maternal BMI SDS data, first with, and then without, the addition of maternal BMI SDS as an independent variable.
Because only birth weight SDS, infant weight gain SDS, maternal BMI SDS, sex, maternal age, age at MRI, sport activity, and smoking status reached statistical significance (P < 0.05) in any model, only the estimates from these terms are presented in the tables, although for consistency, all covariates were included in all models. SAS (version 9.1; SAS Institute, Cary, NC) was used for all analyses.
RESULTS
Descriptive statistics are presented in Table 1. The infants in this analysis displayed birth weights in the normal range (average birth weight percentile was 49.5% compared to the National Center for Health Statistics, Centers for Disease Control and Prevention 2000 weight-for-age charts) (24). For the subset with maternal BMI, maternal BMI was also in the normal range (mean BMI 22 kg/m2). At the time of their MRI, the subjects were middle aged (mean age 44 years) and in the overweight range for BMI, on average. Expected sex differences in infant body size and adulthood body composition were observed (P < 0.0001).
Table 1.
Characteristics of the study sample during infancy and adulthood (mean (s.d.), range)
| Males (N = 114) | Females (N = 119) | |
|---|---|---|
| Infancy characteristics | ||
| Birth weight (kg) | 3.53 ± 0.49 (2.1, 4.7) | 3.22 ± 0.49 (1.9, 4.9)a |
| Weight at age 2 years (kg) | 12.53 ± 1.23 (9.5, 16.2) | 11.82 ± 1.25 (9.0, 15.3)a |
| Birth weight SDS | <0.000000 ± 1.0 (−2.6, 2.4) | <0.000000 ± 1.0 (−3.5, 3.5) |
| Infant weight gain SDS (0–2 years) | <0.000000 ± 1.1 (−3.2, 3.3) | <0.000000 ± 1.1 (−2.5, 4.3) |
| Gestational age at birth (weeks) | 39.8 ± 1.2 (36, 44) | 39.7 ± 1.5 (35, 43) |
| Birth order (1 = first born) | 2.3 ± 1.3 (1, 8) | 2.3 ± 1.2 (1, 6) |
| Maternal age (years) | 29.2 ± 5.6 (17.5, 43.6) | 27.8 ± 5.5 (17.8, 43.2) |
| Maternal BMIb (kg/m2, median, range) | 23.1 (18.3, 39.4) | 21.7 (17.9, 36.5) |
| Maternal BMI SDS | 0.14 ± 1.0 (−1.3, 4.1) | −0.14 ± 1.0 (−1.4, 3.7) |
| Birth year (median, range) | 1958 (1930–1986) | 1957 (1930–1988) |
| Ever breastfed (%yes) | 45.6 | 49.6 |
| Adulthood characteristics | ||
| Age at MRI (years) | 46.0 ± 15.3 (18.0, 75.6) | 47.0 ± 14.1 (18.4, 74.3) |
| Current smoker (%) | 19.3 | 23.5 |
| High sport activity (%)c | 25.4 | 11.8a |
| Education (% completed university degree) | 18.4 | 21.9 |
| Adulthood body composition | ||
| Stature (cm) | 179.7 ± 6.8 (160.4, 197.7) | 165.3 ± 6.4 (148.4, 181.3)a |
| BMI (kg/m2) | 27.7 ± 5.0 (18.3, 46.0) | 27.1 ± 6.1 (16.4, 47.7)a |
| Abdominal circumference (waist, cm) | 100.8 ± 14.2 (72.1, 141.3) | 92.7 ± 14.7 (67.3, 135.7)a |
| Total body fat (TBF, kg) | 20.2 ± 8.0 (5.9, 41.5) | 26.1 ± 9.8 (8.1, 48.5)a |
| Fat-free mass (FFM, kg) | 67.0 ± 8.0 (47.7, 88.3) | 47.6 ± 7.0 (36.3, 71.4)a |
| Percent body fat (%BF) | 22.8 ± 6.3 (9.3, 39.4) | 34.7 ± 6.8 (16.2, 49.4)a |
| Visceral adipose tissue (VAT) mass (kg) | 4.05 ± 2.69 (0.27, 12.1) | 1.96 ± 1.52 (0.23, 7.4)a |
| Abdominal subcutaneous adipose tissue (ASAT) mass (kg) | 4.96 ± 3.00 (0.77, 16.5) | 5.96 ± 3.81 (0.99, 18.3)a |
| %VAT/ASAT (VAT/ASAT × 100) | 42.4 ± 10.3 (16.0, 66.8) | 23.8 ± 7.5 (9.6, 45.7)a |
| %VAT/TBF (VAT/TBF × 100) | 21.4 ± 12.2 (3.8, 67.4) | 7.3 ± 4.2 (1.37, 26.1)a |
| %Abdominal fat ((VAT + ASAT)/TBF × 100) | 37.9 ± 10.8 (13.9, 63.9) | 27.2 ± 7.8 (12.98, 46.56)a |
MRI, magnetic resonance imaging; SDS, standard deviation score.
Significantly different from males (P < 0.0001).
Maternal BMI was available for 81 males and 79 females, and was measured within 5 years before or after the birth of the infant.
High sport activity = score of ≥3 (out of a maximum of 5) on the Sport Index from the Baecke Physical Activity Questionnaire.
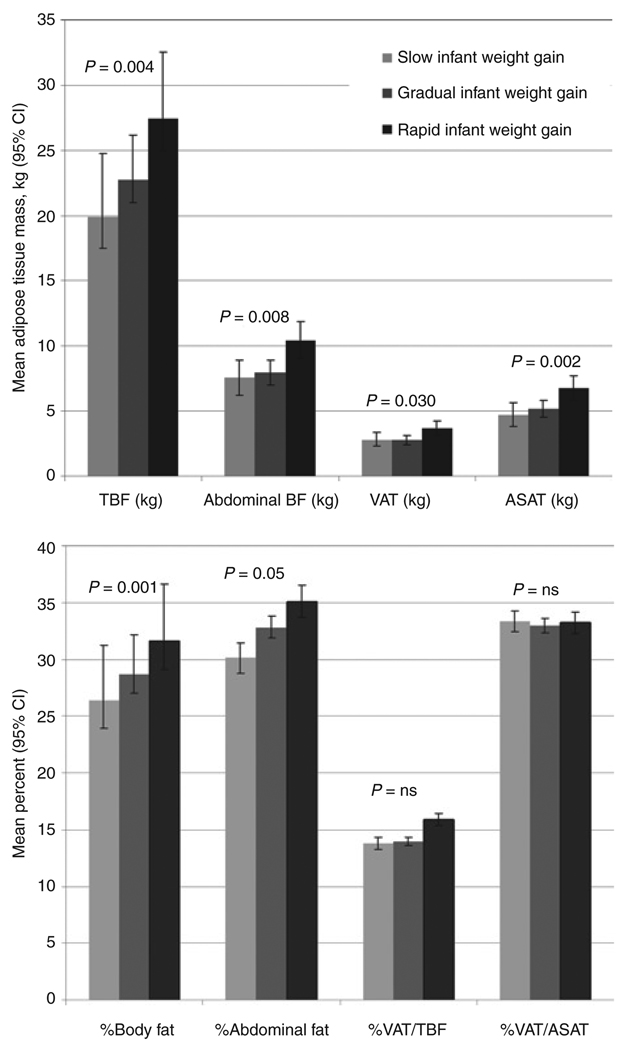
Our primary aim was to examine the relationship of infant weight gain to obesity and total and abdominal adipose tissue levels in adulthood. Compared to infants with slow infant weight gain, infants categorized as having rapid infant weight gain had a significantly increased risk of overweight (multivariate- adjusted odds ratio = 5.54, 95% confidence interval 1.88, 16.31) and obesity (multivariate-adjusted odds ratio = 4.08, 95% confidence interval 1.44, 11.6) (Table 2). Both total and central adiposity were higher among infants with rapid weight gain, who had ~7 kg higher TBF and 3 kg higher abdominal adipose tissue (comprised of 1 kg higher VAT mass and 2 kg higher ASAT mass) than infants who were categorized as having slow infant weight gain (Figure 1). Although infants with rapid weight gain had greater relative adiposity and central adiposity (i.e., higher %BF and %abdominal fat), there was no evidence that VAT in particular was elevated; neither VAT/TBF% nor VAT/ASAT% varied significantly across infant weight gain groups.
Table 2.
Rapid infant weight gain and the risk of overweight and obesity in adulthood, N = 233
| Overweight (BMI >25 kg/m2) | Obesity (BMI >30 kg/m2) | |||
|---|---|---|---|---|
| ORa(minimally adjusted) | ORb(fully adjusted) | ORa(minimally adjusted) | ORb(fully adjusted) | |
| Rapid infant weight gain (change in SDS greater than +0.67) |
2.27 (1.04, 4.94) | 5.54 (1.88, 16.31) | 2.41 (1.09, 5.37) | 4.08 (1.44, 11.6) |
| Gradual infant weight gain (change in SDS −0.67 to +0.67) |
1.35 (0.70, 2.58) | 2.37 (1.04, 5.42) | 0.82 (0.39, 1.76) | 1.07 (0.45, 2.58) |
| Slow infant weight gain (change in SDS less than −0.67) |
(ref) | (ref) | (ref) | (ref) |
OR, odds ratio; MRI, magnetic resonance imaging; SDS, standard deviation score.
Adjusted for sex, gestational age at birth, and age at MRI.
Adjusted for the above, and birth weight SDS, stature, birth year, mother’s age at birth, birth order, breastfeeding (ever/never), education (university degree yes/no), sport activity (high/low), and current cigarette smoking status (yes/no).
Figure 1.
Differences in adulthood total and abdominal adiposity, by infant weight gain from 0 to 2 years. Infant weight gain groups: slow = less than −0.67 weight standard deviation score (SDS) change; gradual = −0.67 to +0.67 weight SDS change; rapid = greater than +0.67 weight SDS change. Least squares means (95% confidence intervals for the mean) were from general linear models adjusted for sex, birth weight SDS, age at adulthood magnetic resonance imaging (MRI), and adulthood stature. TBF, total body fat from dual-energy X-ray absorptiometry; VAT, visceral adipose tissue mass from MRI; ASAT, abdominal subcutaneous adipose tissue mass from MRI; abdominal BF, total abdominal adipose tissue mass (VAT mass + ASAT mass); %BF, %body fat from dual-energy X-ray absorptiometry; %abdominal fat, abdominal fat/TBF × 100; %VAT/TBF, VAT/TBA × 100; %VAT/ASAT, VAT/ASAT × 100. P value is for the test of a linear trend across weight gain groups.
Comparing the effects of infant weight gain to other early life factors (Table 3), participants with faster weight gain in the first 2 years of life had higher BMI, waist circumference, TBF mass, FFM, logVAT mass, logASAT mass, %BF, and %AF than infants with slower weight gain in the first 2 years. Again, faster infant weight gain was not associated with increased %VAT/TBF or %VAT/ASAT. Birth weight SDS was positively associated with adulthood BMI, waist, FFM, and logASAT, but not with logVAT mass, %abdominal fat, %VAT/TBF, or %VAT/ ASAT. As expected, low sport activity level was associated with greater total and central adiposity. Older maternal age appeared to be protective against centralization of body fat, as it was associated with lower logVAT, %abdominal fat, %VAT/TBF, and %VAT/ASAT. Cigarette smoking was associated with increased %VAT.
Table 3.
Birth weight and infancy weight gain (0–2 years) as predictors of total and regional adiposity in adulthood (β coefficient, standard error), N = 233
| Predictors | BMI (kg/m2) |
Waist (cm) |
TBF (kg) |
FFM (kg) |
%BF (%) |
logVAT (kg) |
logASAT (kg) |
%VAT/ ASAT |
%Abdominal fat |
%VAT/ TBF |
|---|---|---|---|---|---|---|---|---|---|---|
| Birth weight (SDS) | 1.48 (0.51)** |
3.13 (1.23)* |
1.20 (0.86) |
1.88 (0.64)** |
0.58 (0.62) |
0.11 (0.07) |
0.13 (0.06)* |
−0.33 (0.78) |
0.37 (0.90) |
−0.24 (0.85) |
| Infant weight gain (SDS change) |
1.43 (0.42)** |
3.70 (1.01)** |
2.51 (0.68)** |
1.81 (0.51)** |
1.59 (0.49)** |
0.14 (0.06)* |
0.17 (0.05)** |
−0.42 (0.64) |
1.42 (0.72)* |
0.07 (0.68) |
| Sex (male) | 2.22 (1.13)* |
7.99 (2.73)** |
−6.74 (1.86)** |
12.31 (1.39)*** |
−9.92 (1.33)*** |
0.68 (0.16)*** |
−0.17 (0.12) |
18.1 (1.73)*** |
12.15 (1.95)*** |
14.76 (1.8)*** |
| Age at MRI (years) | 0.02 (0.09) |
0.25 (0.21) |
0.04 (0.14) |
0.02 (0.10) |
0.06 (0.10) |
0.01 (0.01) |
0.00 (0.01) |
0.27 (0.13)* |
0.10 (0.15) |
0.19 (0.14) |
| Maternal age (years) | −0.08 (0.08) |
−0.28 (0.18) |
−0.10 (0.12) |
−0.11 (0.09) |
−0.06 (0.09) |
−0.03 (0.01)* |
−0.01 (0.01) |
−0.22 (0.11)* |
−0.43 (0.13)** |
−0.41 (0.12)** |
| Sport activity (low) | 2.60 (0.94)** |
8.04 (2.28)** |
6.18 (1.53)*** |
0.59 (1.15) |
5.02 (1.10)*** |
0.46 (0.13)** |
0.48 (0.10)*** |
−0.66 (1.44) |
5.34 (1.61)** |
1.75 (1.52) |
| Current smoking (no) | −1.08 (0.90) |
−3.38 (2.18) |
−1.11 (1.45) |
−1.68 (1.09) |
−0.48 (1.05) |
−0.16 (0.13) |
−0.09 (0.10) |
−1.82 (1.38) |
−2.71 (1.53) |
−3.19 (1.44)* |
| Model R2 | 0.16 | 0.32 | 0.29 | 0.76 | 0.58 | 0.37 | 0.23 | 0.63 | 0.40 | 0.52 |
In addition to the focal variables shown, the model also included gestational age, stature, birth year, parity, breastfed status (ever/never), and education (completed university/did not complete university) as covariates.
%Body fat = TBF/Weight × 100%. VAT/ASAT% = VAT/ASAT × 100%. %Abdominal = ((VAT + ASAT)/TBF) × 100%. %VAT/TBF = (VAT/TBF) × 100%.
ASAT, abdominal subcutaneous adipose tissue; %BF, percent body fat; FFM, fat-free mass; MRI, magnetic resonance imaging; SDS, standard deviation score; TBF, total body fat; VAT, visceral adipose tissue.
P values for the individual parameters estimates are as follows:
P < 0.05;
P < 0.01;
P < 0.0001.
Maternal BMI was correlated with birth weight (r = 0.29, P = 0.0002) and infant weight gain (r = −0.15, P = 0.05). Relationships between offspring body composition and all three exposures (maternal BMI, birth weight SDS, and infant weight gain SDS) were tested in a subset analysis (Table 4). Maternal BMI was positively associated with offspring BMI, Waist, %BF, and ASAT. However, in this subset, infant weight gain was a significant predictor only of FFM.
Table 4.
Maternal BMI as a predictor of total and regional adiposity in adulthood (β coefficient, standard error), N = 160
| Model 1a | Model 2b | ||||||
|---|---|---|---|---|---|---|---|
| Outcomes | Birth weight (SDS) |
Infant weight gain (SDS) |
R2 | Birth weight (SDS) |
Infant weight gain (SDS) |
Maternal BMI (SDS) |
R2 |
| BMI (kg/m2) | 1.32 (0.63)* | 0.77 (0.48) | 0.21 | 0.86 (0.62) | 0.73 (0.47) | 1.54 (0.43)*** | 0.28 |
| Waist (cm) | 2.82 (1.56)* | 2.23 (1.20) | 0.40 | 1.72 (1.54) | 2.14 (1.16)* | 3.61 (1.07)*** | 0.44 |
| TBF (kg) | 1.31 (1.07) | 1.46 (0.81) | 0.28 | 0.63 (1.06) | 1.36 (0.78) | 2.18 (0.71) | 0.33 |
| FFM (kg) | 1.94 (0.85)* | 1.35 (0.64)* | 0.77 | 1.60 (0.86)* | 1.30 (0.64)* | 1.08 (0.57) | 0.79 |
| %BF | 0.76 (0.80) | 0.88 (0.61) | 0.57 | 0.33 (0.80) | 0.82 (0.60) | 1.38 (0.54)** | 0.59 |
| logVAT (kg) | 0.17 (0.09) | 0.10 (0.07) | 0.42 | 0.16 (0.09) | 0.10 (0.07) | 0.11 (0.07) | 0.42 |
| logASAT (kg) | 0.11 (0.07) | 0.09 (0.06) | 0.23 | 0.06 (0.07) | 0.09 (0.05) | 0.16 (0.05)*** | 0.29 |
| %VAT/ASAT | 1.72 (1.02) | 0.32 (0.79) | 0.64 | 2.10 (1.0)* | 0.35 (0.79) | −1.24 (0.72) | 0.65 |
| %Abdominal fat | 1.00 (1.18) | 0.80 (0.89) | 0.44 | 0.83 (1.21) | 0.77 (0.90) | 0.55 (0.81) | 0.44 |
| %VAT/TBF | 0.01 (0.01) | 0.001 (0.001) | 0.52 | 0.01 (0.01) | <0.000 (0.009) | 0.003 (0.008) | 0.52 |
ASAT, abdominal subcutaneous adipose tissue; %BF, percent body fat; FFM, fat-free mass; SDS, standard deviation score; TBF, total body fat; VAT, visceral adipose tissue.
Model 1: did not include maternal BMI, and was adjusted for sex, gestational age at birth, age at MRI, birth year, mother’s age at birth, birth order, breastfeeding (ever/never), stature, education (university degree yes/no), sport activity (high/low), and current cigarette smoking status (yes/no).
Model 2: included maternal BMI and was adjusted for the same covariates as Model 1.
P values for the individual parameter estimates are as follows:
P < 0.05,
P < 0.01, and
P < 0.0001.
DISCUSSION
This study presents novel data that disaggregates the relationship of weight gain during infancy and adult abdominal adiposity into its visceral and subcutaneous components, and it is also among the few that have prospectively examined the association of early life factors to total body adiposity and FFM over a long follow-up period. We found that both total and abdominal adipose tissue levels were significantly greater among adults who had experienced rapid infant weight gain than those who had experienced slower infant weight gain, adjusting for important potential confounders (sex, stature), and covariate effects (birth weight, age, educational attainment, cigarette smoking, physical activity). These results confirm the findings in previous cohorts (reviewed by refs. 5,6) and particularly the results of recent studies in which weight gain in infancy was an independent predictor of measured trunk adipose tissue mass in early (25) and late (26) adolescence. This study extends these findings by showing that the increased abdominal adiposity of faster-growing infants consisted of increases in both VAT and ASAT in adulthood.
There was little evidence, however, that the greater central adiposity of faster-growing infants was associated with preferential deposition of adipose tissue in the visceral compartment of the abdomen, as once VAT was adjusted for TBF (%VAT/TBF) or ASAT (%VAT/ASAT), no effect of infant weight gain remained. We had expected to find higher relative visceral adiposity in rapid growing infants given their greater tendency to develop glucose intolerance and insulin resistance, (10,27–29) which are more strongly linked to VAT than ASAT (14,16–18). It is important to keep in mind that our study was conducted in appropriate for gestational age infants living in the United States, because the strongest associations between infant weight gain and obesity-related disease outcomes (as opposed to obesity itself) tend to be among small for gestational age infants (27,30, and reviewed by ref. 12) and those born in developing nations, where maternal size and nutritional status are often constraining factors in prenatal growth (e.g., ref. 29). It is possible, then, that preferential VAT deposition may depend on the existence of prenatal growth restriction, followed by catch-up growth. An alternative explanation is that given the small absolute size of the VAT compartment relative to the whole (on average, %VAT/TBF was 14%) and relative to the measurement error inherent in VAT estimation from magnetic resonance images (typically ~5–10%), our sample size was not sufficient to detect a true effect of infant weight gain on %VAT. Nonetheless, these data show a strong effect of infant weight gain on adulthood total and central adiposity among normal birth weight individuals; the adverse effects of rapid infant weight gain on total and abdominal adiposity are not restricted to populations with prevalent fetal growth restriction.
Maternal BMI is an important predictor of offspring obesity, as has been consistently reported (reviewed by ref. 31), and was independently associated with BMI, waist, %BF, and ASAT in a subset of our subjects with maternal BMI data. However, because birth weight and infant weight gain had nonsignificant effects on adiposity in this subset, we could not discern the relationships among these early life exposures and the nature of their joint contribution to later adiposity.
Birth weight was not independently associated with VAT mass, %VAT/TBF, or %VAT/ASAT, in this study. This is in agreement with the findings by McNeely et al. (32) who found no association of birth weight with VAT in a sample of 91 Japanese-American adults, as well with the results of Choi et al. (33) who reported a low, nonsignificant association of birth weight with VAT area in 22 Korean adults (r = −0.22). The smaller sample sizes of these studies may have underpowered their ability to detect relatively low correlations, but we confirm in a larger cohort that birth weight is not strongly associated with VAT after accounting for the stronger effects of infant weight gain.
Greater maternal age was associated with lower waist, VAT mass, and VAT% in the offspring. Evidence in rodents (e.g., ref. 34) suggests that the hormonal environment during pregnancy varies with the age of the mother, with lower maternal serum estradiol levels in middle-aged compared to young adult pregnant dams. Offspring of middle-aged mice were found to have lower adulthood body weight than the offspring of young adult dams, which had higher circulating estradiol levels during pregnancy (34). Research on the relationship of maternal age, steroid hormone levels in pregnancy, and offspring body composition in humans is lacking.
Strengths of the study are the duration of the follow-up period and the use of MRI to disentangle abdominal adipose tissue into its VAT and ASAT compartments. Limitations of the study include limited availability of maternal BMI, which also was not exclusively measured prior to pregnancy. Long-term follow-up studies, such as this, carry the risk of survivor bias. However, our results are unlikely to be biased by differential survival among exposure status groups because the mean birth weight and the mean infant weight gain were not different among those who were in the MRI study compared to those who were not in the MRI study. Most individuals in this cohort were born prior to the initiation of the pediatric obesity epidemic in the United States; this is a weakness in terms of representing environmental conditions affecting children born today, yet is an inevitable aspect of long-term longitudinal studies. Our lack of detailed infant feeding data on this historical cohort reduced the precision with which we could detect protective effects, if any, of this important nutritional exposure. Finally, the ethnically homogenous (white) study population limits our ability to extend these findings to other ethnic/racial groups.
In conclusion, this is the first report to our knowledge of a significant positive association between infant weight gain and measured visceral and abdominal subcutaneous adiposity, as well as total adiposity, in middle adulthood. Further work is needed to identify maternal and nutritional factors involved in early rapid weight gain and to refine clinical guidelines on optimal weight gain in healthy infants.
ACKNOWLEDGMENTS
We thank the participants in the Fels Longitudinal Study for their many years of dedicated support and involvement. We also thank Traci Rackett, Jean Payne, Gwen Hall, Carol Cottom, Frances Tyleshevski, and the entire staff of the Lifespan Health Research Center for their efforts in data collection, management, and analysis. Special appreciation goes to William Couch for developing and overseeing the image analysis protocols. Dr Ross was a consultant on the project and assisted us greatly in the development of the magnetic resonance imaging protocol. Mindy Shelley at Good Samaritan Hospital in Dayton, OH was invaluable for her knowledge and management of the magnetic resonance image acquisition and data transfer. Funding for the study was provided by the National Institutes of Health Grants R01-HD12252 (R.M.S.), R01-HD53685 (E.W.D.), R01-DK64870 (S.A.C.), and R01-DK64391 (B.T.). Two anonymous reviewers made helpful comments to improve the manuscript.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 2.Kuh D, Ben-Shlomo Y. A Lifecourse Approach to Chronic Disease Epidemiology. Oxford, UK: Oxford University Press; 2004. [PubMed] [Google Scholar]
- 3.Cameron N, Demerath EW. Critical periods in human growth and their relationship to diseases of aging. Am J Phys Anthropol. 2002 Suppl 35:159–184. doi: 10.1002/ajpa.10183. [DOI] [PubMed] [Google Scholar]
- 4.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 5.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 6.Stettler N. Nature and strength of epidemiological evidence for origins of childhood and adulthood obesity in the first year of life. Int J Obes (Lond) 2007;31:1035–1043. doi: 10.1038/sj.ijo.0803659. [DOI] [PubMed] [Google Scholar]
- 7.Crowther NJ, Cameron N, Trusler J, Gray IP. Association between poor glucose tolerance and rapid post natal weight gain in seven-year-old children. Diabetologia. 1988;41:1163–1167. doi: 10.1007/s001250051046. [DOI] [PubMed] [Google Scholar]
- 8.Crowther NJ, Trusler J, Cameron N, Toman M, Gray IP. Relation between weight gain and beta-cell secretory activity and non-esterified fatty acid production in 7-year-old African children: results from the Birth to Ten study. Diabetologia. 2000;43:978–985. doi: 10.1007/s001250051479. [DOI] [PubMed] [Google Scholar]
- 9.Ong KK, Dunger DB. Birth weight, infant growth and insulin resistance. Eur J Endocrinol. 2004;151 Suppl 3:U131–U139. doi: 10.1530/eje.0.151u131. [DOI] [PubMed] [Google Scholar]
- 10.Soto N, Bazaes RA, Peña V, et al. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. J Clin Endocrinol Metab. 2003;88:3645–3650. doi: 10.1210/jc.2002-030031. [DOI] [PubMed] [Google Scholar]
- 11.Law CM, Barker DJ, Osmond C, Fall CH, Simmonds SJ. Early growth and abdominal fatness in adult life. J Epidemiol Community Health. 1992;46:184–186. doi: 10.1136/jech.46.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells JC. The programming effects of early growth. Early Hum Dev. 2007;83:743–748. doi: 10.1016/j.earlhumdev.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 14.Demerath EW, Reed D, Rogers N, et al. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. Am J Clin Nutr. 2008;88:1263–1271. doi: 10.3945/ajcn.2008.26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 16.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 17.Motoshima H, Wu X, Sinha MK, et al. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002;87:5662–5667. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 18.Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 19.Kuk JL, Katzmarzyk PT, Nichaman MZ, et al. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006;14:336–341. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 20.Roche A. Growth, Maturation and Body Composition: The Fels Longitudinal Study 1929–1991. Cambridge, UK: Cambridge University Press; 1992. [Google Scholar]
- 21.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services PHS. US Government Printing Office Stock Number 017-022-01335-5. Washington, DC: GPO, Public Health Service; 1996. NHANES III Anthropometric Procedures Video. [Google Scholar]
- 23.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 24.Kuczmarski R, Ogden C, Guo S, et al. CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 25.Chomtho S, Wells JC, Williams JE, et al. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;87:1776–1784. doi: 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 26.Ekelund U, Ong K, Linné Y, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83:324–330. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 27.Ibáñez L, Ong K, Dunger DB, de Zegher F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J Clin Endocrinol Metab. 2006;91:2153–2158. doi: 10.1210/jc.2005-2778. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, et al. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia. 2006;49:1974–1984. doi: 10.1007/s00125-006-0311-7. [DOI] [PubMed] [Google Scholar]
- 29.Yajnik CS. The lifecycle effects of nutrition and body size on adult adiposity, diabetes and cardiovascular disease. Obes Rev. 2002;3:217–224. doi: 10.1046/j.1467-789x.2002.00072.x. [DOI] [PubMed] [Google Scholar]
- 30.Mericq V, Ong KK, Bazaes R, et al. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia. 2005;48:2609–2614. doi: 10.1007/s00125-005-0036-z. [DOI] [PubMed] [Google Scholar]
- 31.Hawkins SS, Law C. A review of risk factors for overweight in preschool children: a policy perspective. Int J Pediatr Obes. 2006;1:195–209. doi: 10.1080/17477160600943351. [DOI] [PubMed] [Google Scholar]
- 32.McNeely MJ, Fujimoto WY, Leonetti DL, Tsai EC, Boyko EJ. The association between birth weight and visceral fat in middle-age adults. Obesity (Silver Spring) 2007;15:816–819. doi: 10.1038/oby.2007.596. [DOI] [PubMed] [Google Scholar]
- 33.Choi CS, Kim C, Lee WJ, et al. Association between birth weight and insulin sensitivity in healthy young men in Korea: role of visceral adiposity. Diabetes Res Clin Pract. 2000;49:53–59. doi: 10.1016/s0168-8227(00)00131-5. [DOI] [PubMed] [Google Scholar]
- 34.Wang MH, vom Saal FS. Maternal age and traits in offspring. Nature. 2000;407:469–470. doi: 10.1038/35035156. [DOI] [PubMed] [Google Scholar]