Abstract
Background
Maternal allergy is believed to be a risk factor for peanut allergy (PNA) in children. However, there is no direct evidence of maternal transmission of PNA susceptibility, and it is unknown whether maternal peanut (PN) exposure affects the development of PNA in offspring.
Objective
To investigate the influence of maternal PNA on offspring reactions to the first PN exposure, and whether maternal low dose PN exposure during pregnancy and lactation influences these reactions and PN sensitization in a murine model.
Methods
Five-week-old offspring of PNA C3H/HeJ mothers (PNA-M) were challenged intragastrically (i.g.) with PN (first exposure), and reactions were determined. In a subset of the experiment, PNA-M were fed low dose of PN (PNA-M/PN) or not fed PN (PNA-M/none) during pregnancy and lactation. Their 5-week-old offspring were challenged intragastrically with PN, and reactions were determined. In another subset of the experiment, offspring of PNA-M /PN or PNA-M/none were sensitized with PN i.g. for 6 weeks and serum PN-specific antibodies were determined.
Results
PNA-M offspring exhibited anaphylactic reactions at first exposure to PN which were associated with PN-specific IgG1 levels, and prevented by a platelet activation factor antagonist. In a subset experiment, PNA-M/PN offspring showed significantly reduced first exposure PN reactions, increased IgG2a, and reduced mitogen-stimulated splenocyte cytokine production compared to PNA-M/none offspring. In additional experiment, PNA-M/PN offspring showed reduction of PN-specific IgE to active PN sensitization.
Conclusion
We show for the first time maternal transmission of susceptibility to first exposure PN reactions and active PN sensitization. Low dose PN exposure during pregnancy and lactation reduced this risk.
Clinical Implications
Maternal peanut allergy is a risk factor for offspring peanut anaphylaxis in a mouse model. Low dose peanut exposure during pregnancy and lactation reduced first PN exposure reactions, and inhibited active peanut sensitization after weaning.
Capsule Summary
Low dose peanut exposure of peanut allergic mice during pregnancy and lactation reduced susceptibility of offspring to peanut allergy. Strict avoidance of PN and other food allergens during pregnancy and lactation may be counterproductive.
Keywords: Murine model, maternal peanut allergy, IgG1 and IgG2a, PAF, maternal PN exposure
INTRODUCTION
Peanut allergy (PNA), affecting ~1% of children,(1;2) accounts for approximately 80% of fatal and near-fatal anaphylactic reactions,(3) and the prevalence is increasing.(4) Approximately 80% of anaphylactic reactions occur on first known ingestion.(5) Maternal atopy is believed to be a risk factor of developing childhood PNA.(5;6) However, the mechanisms underlying first exposure PNA reactions are largely unknown. For many years, the American Academy of Pediatricians (AAP) and the United Kingdom government recommended maternal dietary avoidance during pregnancy and lactation to reduce PNA. (7) However, there is no conclusive data that maternal PN restriction is protective, (2;7;8) and the AAP guidelines were recently revised.(9) It has been suggested that introduction of small amounts of PN early in life may prevent sensitization.(2;10) Further work is important to define the effects of early PN exposure on development of PNA, in high risk offspring.
Murine models of PNA which mimic human PNA are useful tools for initial investigation of interventions for PNA.(11–14) Several animal models have been used to determine the risk factor of maternal transmission of sensitivity to asthma and allergy.(6) Hamada et al(15) showed that offspring of mother mice with ovalbumin (OVA) induced `chronic asthma' were more susceptible to developing OVA-induced asthma. Herz et al.(16) demonstrated that prenatal maternal antigen exposure induced mitogen-stimulated Th2-type immune responses in offspring. Interestingly, Melkild et al(17) showed that immunization of naïve mice with OVA and adjuvant intraperitoneally during pregnancy and lactation significantly protected their adult offspring from OVA sensitization.(17) An additional study assessed the impact of airborne antigen exposure of lactating mice on the development of allergic asthma in their progeny. When the offspring reached adulthood, they were sensitized and challenged with OVA. As compared to mice breastfed by unexposed mothers, those breastfed by OVA-exposed mothers showed decreased allergic airway response. (18) These previous studies suggested that allergen exposure in normal mothers during pregnancy and /or lactation may protect offspring from allergic asthma. Nowadays, there is no direct evidence of maternal transmission of risk of PNA development, and no study investigating whether maternal PN exposure or restriction in PNA-M affects this risk.
In the present study, we characterized the susceptibility of PNA-M offspring to PNA. Offspring of PNA-M developed anaphylaxis following the first oral challenge dose of PN. These reactions were partially mediated by PAF and might be associated with maternal transmission of PN-specific IgG1, and were significantly reduced by maternal low dose PN consumption during pregnancy and lactation (PNA-M/PN). Protection was associated with a higher PN-specific IGg2a to IgG1 ratio. PN stimulation of splenocytes from these mice did not induce cytokine secretion, suggesting an absence of T cell transmission. However, concanavalin A (Con A) induced cytokine production was also inhibited in PNA-M/PN offspring. PNA-M /PN offspring also exhibited reduced IgE production in response to active PN sensitization. These findings show that low dose PN exposure during pregnancy and lactation reduced offspring risk of first exposure PN reactions, and reduced active PN-IgE sensitization.
METHODS
Animals and reagents
Six-week-old female and male C3H/HeJ mice purchased from the Jackson Laboratory (Bar Harbor, ME) were maintained on PN-free chow under specific pathogen-free conditions according to standard guidelines for the care and use of animals.(19) Freshly ground whole roasted PN prepared as previously described (20) was used as an antigen. Endotoxin levels in ground PN were tested using the Pyrogent Plus assay kit (Lonza, MA) as previous described(21), and were lower than the detectable level. Crude PN extract (CPE) was prepared as described previously.(22) Cholera toxin (CT) was purchased from List Biological Laboratories Inc (Campbell, CA). Concanavalin A (ConA), avidin-peroxidase, cyproheptadine, CV-3988 and dinitrophenyl-phosphate (DNP) were purchased from Sigma Aldrich (St. Louis, MO). Antibodies for ELISA were purchased from Accurate Scientific (Westbury, NY) or Pharmingen (San Diego, CA). ABTS (2,2'–azino-di(3-ethylbenzthiazoline-6-sulfonate) was purchased from KPL (Gaithersburg, MD).
Experimental protocol
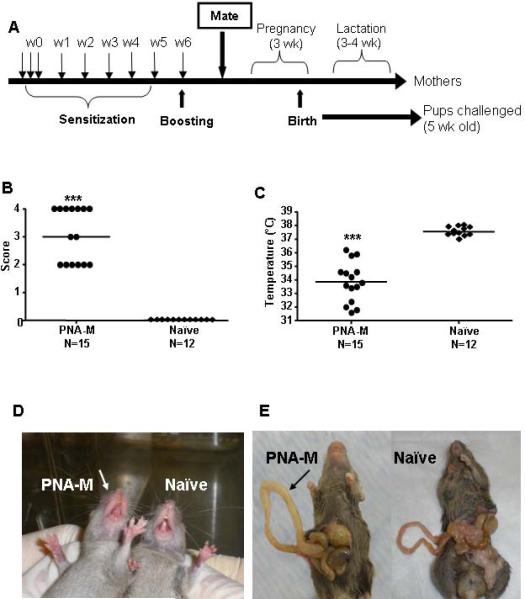
To determine the influence of maternal PNA on first exposure PN reactions in offspring, female mice were sensitized i.g. with ground PN (10 mg/mouse) and CT (20μg/mouse) weekly for five weeks and boosted with 50 mg/mouse on week 6 as previously described (Fig. 1, A)(23) but without week 8 boosting to avoid the stressful anaphylactic reactions of mothers. Subsequently, they were mated with naïve males. During pregnancy and lactation these mice were on the normal mouse chow, which does not contain PN. Naïve mice were employed as controls. Offspring were weaned at approximately 3 and a half to 4 weeks. Five week-old offspring in each group were challenged i.g. with 200 mg of PN/mouse. (We noted in the first set of experiments, that the majority of offspring of PNA-M developed severe reactions (near fatal), which makes blood collection rather difficult. We therefore did not give week 6 boosting to PNA-M for the following sets experiments). To determine the role of mediators such as histamine and PAF in the induction of PN anaphylaxis, in a second set of experiment, mice received PN plus CT sensitization weekly for 5 weeks. Subsequently, they were mated with naïve males as in Fig 1A. Five week old PNA-M offspring received 50 μg cyproheptadine i.p (histamine antagonist) or 75μg CV-3988 i.v. (PAF antagonist)(24) 30 minutes before PN challenge. None treated PNA-M and naïve mice were used as controls.
FIG 1.
A, Experimental protocol. B, Symptoms of anaphylaxis. C, Temperatures. Bars indicate the median of 15 offspring from PNA-M and 12 offspring in the naïve group. ***P<0.001 vs naïve. D, Offspring from PNA-M experiencing redness and swelling around the mouth in comparison with a naïve mouse. E, Signs of intestinal anaphylaxis; note edema in the small intestine of an offspring of PNA-M in comparison with a naïve mouse intestine.
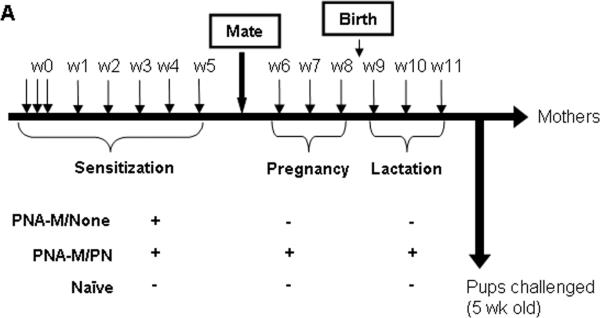
To determine the effect of PN exposure on first exposure PN-induced anaphylaxis, in a third set of experiments female C3He/J mice were sensitized with PN+CT i.g. (10 mg PN/mouse; 20μg CT/mouse) for 5 weeks at weekly intervals (Fig. 4, A). After sensitization, they were mated with naïve males. During pregnancy and lactation these PNA-M were divided into 2 groups. One group did not receive PN/CT during pregnancy and lactation (PNA-M/None), and the other PNA-M group received the weekly immunization regimen throughout pregnancy and lactation (PNA-M/PN). Feeding PNA-M with a low dose of PN and CT primes/maintains PN immune responses but does not elicit clinical reactions. Five-week-old offspring from each group were then challenged with 200 mg PN/mouse i.g.
FIG 4.
Experimental protocol. A, Intragastrically sensitized PN-allergic female mice were mated after a 5 weeks PN sensitization. During pregnancy and lactation mice were divided into 2 groups: (PNA-M/None; n=5) and (PNA-M/PN; n=5). A naïve group (n=5) was employed as a control. Five-week-old offspring from each group were then challenged with 200 mg PN/mouse.
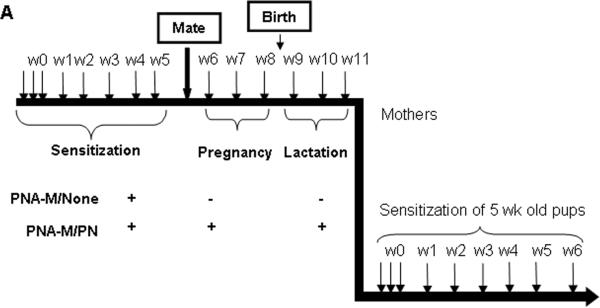
To determine the effect of PN exposure on active PN-sensitization of offspring, in the last set of experiments, 5-week old PNA-M/None and PNA-M/PN offspring were sensitized with PN+CT i.g. (10 mg PN/mouse; 20 μg CT/mouse) for 6 weeks at weekly intervals as depicted in Fig. 6, A. This protocol induces substantial PN-IgE by wk 6.(25) Offspring were bled at weeks 0, 3 and 6 and PN-specific IgE, IgG1 and IgG2a were measured by ELISA, as described below (23).
FIG 6.
Experimental protocol. A, Intragastrically sensitized PN-allergic female mice were mated after a 5 weeks PN sensitization. During pregnancy and lactation mice were divided into 2 groups regarding their PN feeding (10mg PN+CT/mouse) during pregnancy and lactation: (PNA-M/None; n=5) and (PNA-M/PN; n=5). Five-week-old offspring from each group were then sensitized weekly during 6 weeks (10mg PN+CT/mouse).
Assessment of systemic anaphylactic signs and measurement of rectal temperature
Anaphylactic signs were evaluated 30 to 40 minutes after the commencement of the challenge by 2 investigators using the following scoring: 0, no signs; 1, scratching and rubbing around the mouth and head; 2, puffiness and redness around the eyes and mouth, diarrhea, pilar erecti, reduced activity, and/or decreased activity with increased respiratory rate; 3, wheezing, labored respiration and cyanosis around the mouth and tail; 4, no activity after prodding or tremor and convulsions: and 5, death (20;21).
Rectal temperatures were measured 30 minutes after the PN challenge by using a thermal probe (Harvard Apparatus, Newark, NJ).(20; 21)
Measurement of plasma histamine and Mouse Mast Cell Protease -1 (MMCP-1) levels
Plasma was obtained 30 minutes after the second challenge, histamine (Immunotech, Marseille, France) and MMCP-1 (Moredun Scientific, Midlothian, Scotland) levels were analyzed by using an enzyme immunoassay kit as described by the manufacturer.
Measurement of serum antibodies
Retro-orbital venous blood was collected from mothers or offspring as indicated. Sera were collected and stored at −80° C until analyzed. PN-specific IgE, IgG1 and IgG2a levels were determined using monoclonal antibody as previously described.(13; 26; 27).
Cell culture and cytokine measurements
Following final challenge, splenocytes, prepared as previous described, (28) were resuspended in RPMI 1640 supplemented with fetal bovine serum and cultured in 24-well plates (4 × 106/well/mL) in the presence or absence of CPE (200 μg/mL) or ConA (2.5 μg/mL). 72 h later, supernatants were collected and cytokine levels were determined by ELISA according to manufacturer's instructions (R&D Systems and BD-Pharmingen).
Passive cutaneous anaphylaxis (PCA) test
Abdomens of 8 to 12 month-old BALB/c male naïve mice were shaved 1 day before intradermal (i.d.) injection of 50 μL of heat-inactivated (56°C for 3 hours) and unheated pooled sera (1:5 dilution) from 6–10 offspring of PNA-M. Control mice received an equal amount of diluted naïve serum. Twenty-four hours later, mice were injected i.v. with 100 μL of 0.5% Evan's Blue dye, immediately followed by an i.d. injection of 50 μL of CPE. Thirty minutes after the last injection, the mice were killed, the skin of the abdomen was inverted, and reactions were examined for visible blue color. A reaction was scored as positive if the bluing of the skin at the injection sites was greater than 3 mm in diameter in any direction. (20)
Statistical analysis
We performed an ANOVA followed by a Bonferroni's t test for all pairwise comparisons if the data were approximately normal. Differences between groups were analyzed by ANOVA on ranks followed by all pairwise comparisons if the data were not normally distributed. P values < 0.05, based on two-tailed tests, is considered statistical significant. All statistical analyses were performed with Sigma Stat 3.5 (Systat Software Inc, Chicago, IL).
RESULTS
PNA-M offspring developed anaphylactic reactions following first oral PN challenge
All offspring of PNA-M developed first exposure PN anaphylactic reactions, with a median anaphylactic score of 3 (Fig 1 B). Systemic anaphylactic symptoms including puffiness around the eyes and swelling and redness around the mouth (Fig 1 D; score 2, 40%), labored respiration (score 3, 13%) and near fatal reactions (no activity after prodding or tremor and convulsions; score 4, 47%). No offspring of naïve mothers (naive-M) reacted to PN challenge. Core body temperatures of PNA-M offspring were significantly lower than naïve mice (33.8°C vs 37.55°C; P<0.001, Fig 1, C). PNA-M offspring also developed intestinal anaphylactic reactions as evidenced by intestinal edema (Fig 1, E).
First exposure PN anaphylactic reactions in PNA-M offspring were partially PAF mediated
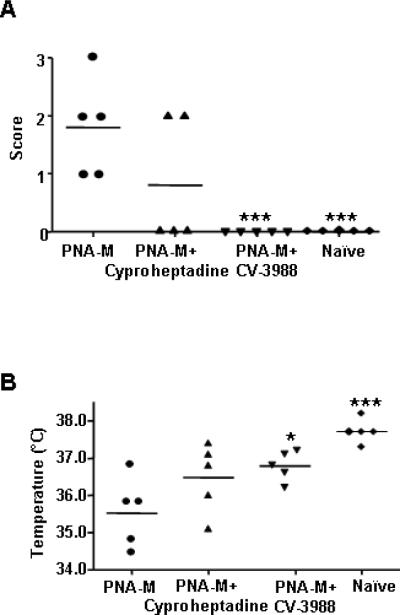
PAF plays a critical role in human and murine anaphylaxis.(29; 30) To investigate the mediators involved in initial anaphylactic reactions. we determined whether PAF and/or histamine were essential, as reported previously.(24) Treatment of PNA-M offspring with the antihistamine cyproheptadine 30 min prior to PN challenge showed a trend of reduction of symptom scores, and hypothermia following challenge when compared with untreated offspring, but the reduction did not reach statistical significance (Fig 2, A and B). Administration of the PAF antagonist CV-3988 30 min prior to PN challenge completely abrogated anaphylactic reactions (Fig 2, A, p<0.001). Core body temperatures were also significantly higher than in non-treated offspring (Fig 2 B. 35.52°C vs 36.78°C; P<0.05). These results demonstrate PAF might be a critical mediator in the development of anaphylaxis in the PNA-M offspring. Furthermore, plasma histamine levels and MMCP-1 levels were determined. Both histamine and MMCP-1 levels were moderately higher in PNA-M offspring than in naïve (4588 nM vs 2213 nM for histamine, P<0.05; 10.77 ng/mL vs 7.22 ng/mL for MMCP-1, P<0.01).
FIG 2.
Mechanisms of PN-induced systemic anaphylaxis in offspring of PNA-M. A, 30 minutes before challenge mice were pretreated with either i.p injection of cyproheptadine or i.v. injection of CV-3988. Symptoms of anaphylaxis were scored as described in the methods. B, Temperatures were measured 30 minutes after challenge with a rectal probe digital thermometer. Bars indicate the median of 5 mice per group. *P<0.05, ***P<0.001 vs PNA-M.
First exposure PN anaphylaxis in PNA-M offspring was associated with maternal transmission of PN specific IgG1
To determine whether the initial anaphylactic reactions in PNA-M offspring were associated with maternal transmission of PN-specific antibodies, serum PN-specific IgE, IgG1 and IgG2a levels in PNA-M offspring one day prior to PN-oral challenge were determined. PN-specific IgE was undetectable. High levels of PN-specific IgG1 and IgG2a were found, and IgG1 levels were higher than IgG2a levels (Fig 3 A and B. IgG1 vs IgG2a=1.64 ± 0.10). As expected no PN-specific IgG1 or IgG2a was detected in naive-M offspring sera. A previous study demonstrated that PN IgG1 can induce PN- anaphylaxis in the absence of IgE.(31) To confirm the role of PN IgG1 in this model, we performed PCA testing. PCA reactions in naïve mice receiving sera from PNA-M offspring following CPE administration (Fig 3, C) were not affected by heat-inactivation of immune sera (Fig 3, D). No PCA reactions were observed in mice receiving naïve sera (Fig 3, E). These results demonstrated that PN-specific IgG1 might be responsible for first exposure PN-induced anaphylaxis.
FIG 3.
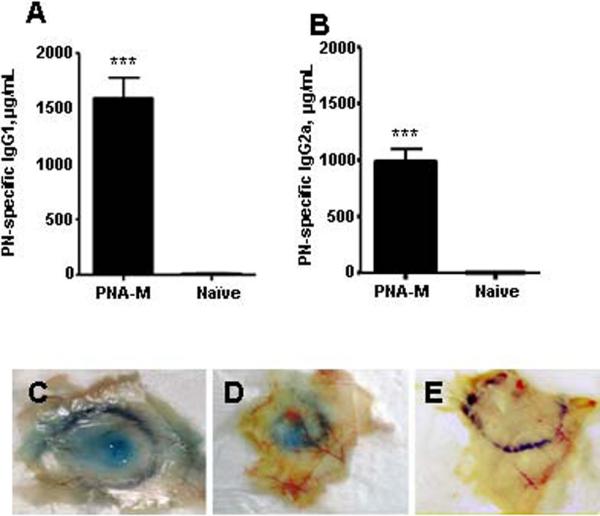
Humoral and cellular response in offspring of PNA-M and naïve mothers. A, Serum PN-specific IgG1 (μg/mL) and B, Serum PN-specific IgG2a. Data are expressed as means ± SEMs for each group (n=13 for PNA-M group and n=5 for naïve). ***P<0.001 vs naïve. PCA reactions after injection of C, unheated, D, heated and E, naïve sera.
Low-dose PN consumption by PNA-M during pregnancy and lactation provided partial protection against first exposure PN-anaphylaxis in their offspring
We next determined whether administering a low dose of PN during pregnancy and lactation affected PNA-M offspring first exposure PN anaphylaxis. We found that only 28% of PNA-M/PN offspring exhibited anaphylactic reactions following first PN challenge, as compared to 80% of PNA-M/None offspring (Table I). In addition, median symptom scores of PNA-M/PN offspring were significantly lower than those of PNA-M/None offspring (0 vs. 2; P<0.01). Mean core body temperatures were significantly higher in PNA-M/PN offspring than PNA-M/None offspring (36.8°C vs 35.8°C; P<0.05). These results show that feeding of low doses of PN to PNA-M during pregnancy and lactation provided significant protection against first exposure PN-anaphylaxis in offspring as compared to offspring of PNA-M not exposed to PN.
TABLE I.
First exposure PN anaphylaxis in offspring of PNA-M/None and PNA-M/PN
| Anaphylactic Score | Temperatures (°C) | ||
|---|---|---|---|
| Group | N/total (%) | Median (range) | Median (range) |
| PNA-M/None | 12/15 (80%) | 2 (0–3)### | 35.8 (32.2–37.5)### |
| PNA-M/PN | 7/25 (28%) | 0 (0–3)** | 36.8 (32.7–37.7)###* |
| Naϊve | 0/14 (0%) | 0 | 37.7 (37.0–38.2) |
Offspring from PNA-M/none and PNA-M/PN were i.g. challenged with 200 mg PN/mouse. Symptoms of anaphylaxis were scored. Temperatures were measured 30 minutes after the challenge with a rectal probe thermometer.
P<0.05
P<0.01 vs PNA-M/None
P<0.001 vs naïve.
Protection against first exposure PN anaphylaxis in PNA-M/PN offspring was associated with increased PN-specific IgG2a concentrations
We next investigated the influence of low-dose PN exposure during pregnancy and lactation on offspring humoral responses. PN-specific IgE was undetectable (data not shown), but PNA-M/PN offspring had significantly higher PN-specific IgG2a levels than that in PNA-M/None offspring (p<0.001; Fig 5, A). Although PN-specific IgG1 levels were also significantly higher in offspring from PNA-M/PN group, IgG2a levels were greater than IgG1 levels, and the ratio of PN-specific IgG2a to IgG1 was significantly higher in PNA-M/PN offspring than in PNA-M/None offspring (P<0.05; Fig. 5, B and C). The high levels of IgG2a in PNA-M/PN offspring may be due to increased maternal IgG2a levels in response to following low dose of PN feeding because PN-specific IgG2a levels were higher in PNA-M/PN mothers than in PNA-M/None mothers (data not shown).
FIG 5.
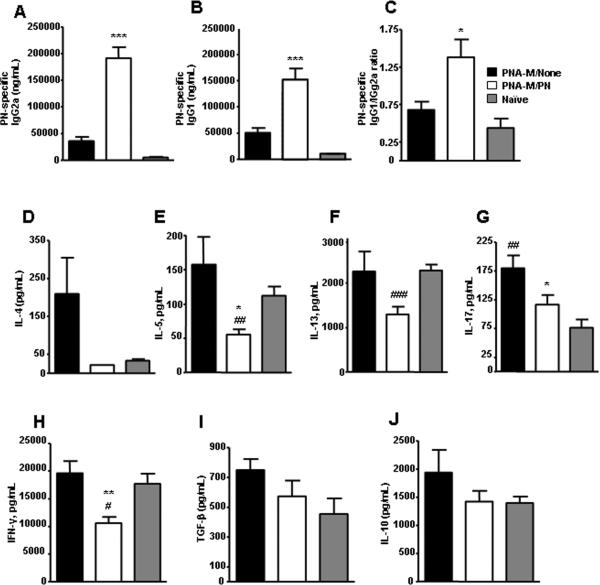
A, Serum PN-specific IgG2a (μg/mL) and B, serum PN-specific IgG1 (μg/mL). C, Ratio between PN-specific IgG1/IgG2a. D, IL-4, E, IL-5, F, IL-13, G, IL-17, H, IFN-γ, I, TGF-β and J, IL-10 levels in culture supernatants (pg/mL). Data in (A–J) are expressed as means ± SEMs of duplicates of ELISA for each group (n=7). *P<0.05, **P<0.01, ***P<0.001 vs PNA-M/None; #P<0.05, ##P<0.01, ###P<0.001 vs naïve.
Low-dose PN consumption by PNA-M during pregnancy and lactation inhibited cytokine production by splenocytes from offspring
We determined offspring cytokine profiles- including Th1 (INF-γ), Th2 (IL-4, IL-5 and IL-3), Th3 (TGF-β and IL-10) and Th 17 (IL-17) – of splenocytes taken immediately following initial challenge in response to CPE or Con A stimulation. Splenocytes from offspring in all groups did not respond to CPE stimulation (data not shown). However, there was an overall trend of higher levels of Th2, Th17, and Th3, but not Th1cytokine production to Con A stimulation by splenocytes of PNA-M offspring compared to naive-M offspring. Surprisingly, overall cytokine levels to Con A stimulation were reduced in PNA-M/PN offspring (Fig 5, D–J) and the reductions of IL-5, IL 17 and IFN-γ were statistically significant compared to offspring in the PNA/None group (Fig 5 E, G and H: P<0.05 to P<0.01). IL-5, IL-13, IL 17 and IFN-γ production in PNA-M/PN offspring was even significantly lower than that in naive-M offspring Fig. 5, E–H: P<0.05 to P<0.01).
Exposure of PNA-M to PN during pregnancy and lactation suppressed antibody production in response to active PN sensitization of their offspring
Since low dose PN exposure of PNA-M during pregnancy and lactation induced overall reduced cytokine levels in response to Con A, we hypothesized that this approach may also cause reduced antibody production in response to PN sensitization. One day prior to PN-sensitization, offspring from PNA-M/PN and PNA-None showed any detectable PN-specific IgE levels. PN-specific IgE levels were markedly elevated in PNA-M/None offspring as early as 3 wks following sensitization and remained higher at wk 6 (Fig 7A), however, they were undetectable in PNA-M/PN offspring at wk 3, and were significantly lower than that in PNA-M/None offspring at 6 wks following sensitization. IgG1 levels were not different, but IgG2a levels were significantly higher in PNA-M/PN offspring than in PNA-M/None one day prior to PN sensitization. Both IgG1 and IgG2a levels in PNA-M/PN offspring declined at week 3, and were significantly lower at wks 3 and 6 than that in PNA-M/None offspring following PN sensitization (Fig 7A, B).
FIG 7.
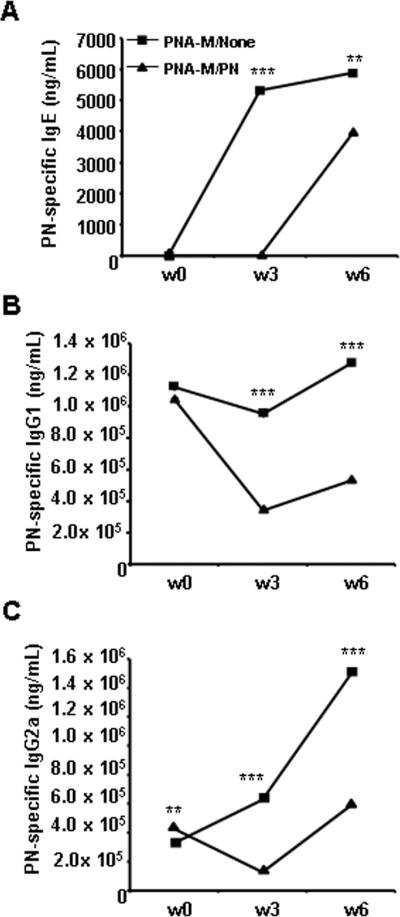
Humoral response of offspring of PNA-M/None and PNA-M/PN mothers. A, Serum PN-specific IgE (ng/mL). B, Serum PN-specific IgG1 (μg/mL) and C, Serum PN-specific IgG2a were evaluated by antigen-specific ELISA. Data are expressed as means ± SEMs of triplicates for each group (pooled samples; n=5 for PNA-M/None group and n=10 for PNA-M/PN). **P<0.01, ***P<0.001 vs PNA-M/PN.
DISCUSSION
The majority of PNA reactions occur at first known PN ingestion.(5) Although little is known about risk factors, it has been suggested that food allergy, including PNA, is associated with maternal allergy. (6) However, there is no direct evidence showing that maternal PNA increases development of PNA in offspring. Our study is the first attempt to determine the effect of maternal PNA on first exposure PN reactions of offspring in a mouse model. We found that 5-week-old C3H/HeJ mice of PNA-M developed anaphylactic reactions following the first PN challenge. A PAF antagonist, but not a histamine antagonist, completely blocked these reactions. An association between PAF and severe human anaphylactic reactions, including fatal anaphylaxis in PNA patients has been reported.(32) In this study, we also found that histamine and MMCP-1 levels increased during the anaphylactic reactions. A previous study reported that PN can contribute to anaphylactic shock by activating complement, C3a. (33) We speculate that although PAF appeared to be critical in these model, other mechanisms may also be involved. Further studies are required to clarify the mediators and cell types involved in anaphylaxis in this model. Finkelman has suggested two independent mechanisms involved in anaphylaxis, the classical pathway that is mediated by IgE antibody and alternative pathway that is mediated by IgG (IgG1) antibody. Because IgG but not IgE can cross the placenta, and offspring of PNA-M were fed PN free chow, were not actively sensitized with PN, and exhibited no detectable serum PN-IgE, but a high PN-specific IgG1, we hypothesized that the first exposure PN reactions in this model were mediated not by in utero PN sensitization but by transfer of maternal PN-specific IgG1. Although further work is needed to confirm this hypothesis, our finding that PCA reactions induced by heat-inactivated sera from PNA-M offspring at least suggest the role of IgG1 mediated reactions in this model. This finding is consistent with previous findings in which IgG1 mediated murine anaphylactic reaction in the absence of IgE.(29; 31) However, whether this mechanism also accounts for first-exposure reactions to PN in children is unknown and remains to be investigated. Most human anaphylaxis is IgE dependent, but, as noted by Finkelman, there is evidence that human anaphylaxis can also be IgG mediated.(29) Bock et al.(34) examined the natural history of adverse reactions to foods in 480 children followed from birth to their third birthday. During the first year of life, 80% of the initial complaints of food reactions occurred and almost all reproducible reactions appeared to be non IgE mediated. Niggemann et al(35) reported a subgroup of children with positive double blind, placebo controlled food challenge responses with no proven IgE sensitization. Whether non-IgE mediated food allergic reactions are IgG mediated remains to be defined.
Maternal avoidance of PN during pregnancy and lactation was recommended for many years in the US and UK. Recently, this recommendation has been abandoned because of lack of conclusive evidence of benefit,(9) and evidence that this approach may increase the risk of PNA development.(2) Hourihane et al (27) reported peanut allergy outcomes in a cohort of children born after the UK government's advice to mothers of high-risk infants to follow maternal avoidance during pregnancy and lactation, and to avoid introduction of peanut to their children until age 3 years. The rate of peanut allergy in this cohort was 1.8%, the highest recorded. Several studies indicated that early introduction of peanuts to infants may be beneficial.(2; 10) However whether maternal exposure to controlled low dose of PN prevents high-risk children from developing PNA is unknown. Our study is the first attempt to investigate the possible beneficial effect of maternal PN exposure during pregnancy and lactation in preventing/reducing PNA in high risk offspring in a mouse model in which it is known that IgG1 can precipitate a reaction. We found that weekly exposure of PNA-M to a low dose of PN (beneath the threshold of triggering clinical reactions) during pregnancy and lactation (PNA-M/PN) reduced first PN exposure reaction. This protection was associated with dominant IgG2a antibodies. As in previously reported studies in murine models,(17;25) we employed adjuvant, cholera toxin, to enhance the response to PN antigen exposure. We found that giving PN plus adjuvant during pregnancy and lactation was more effective in preventing PNA development in offspring than PN alone (López-Expósito et al and Song et al. unpublished data). As primary intervention for high risk offspring, maternal low dose exposure during pregnancy and lactation with PN plus a more Th1 driven adjuvant such as CpG ODN should be addressed in the future. Furthermore, one may question whether pregnant women with PNA should consume any PN because of concern of severe reactions. However, Clark et al(36) recently showed that peanut oral immunotherapy (OIT) can induce clinical tolerance to PN protein in humans. In this study, a marked increase in PN dose threshold was observed; from 5–50 mg (1/40–1/4 of a whole peanut) at pre OIT to at least 2.38 g protein (10 whole peanuts) post OIT. If this approach can be reproduced in large controlled study, it is possible that PNA women who are able to develop PN tolerance following OIT(36) or other interventions,(21;37;38) should be able to continue PN at a maintenance dose during pregnancy and lactation.
We found no evidence of maternal PN-specific T cell transmission, because there was no detectable PN-specific cytokine production by splenocytes from either PNA-M/PN or PNA/M-None offspring. Importantly, T cell suppression was observed in offspring of PNA-M/PN as evidenced by overall inhibition of mitogen-stimulated cytokine production. These findings prompted us to test possible inhibition of IgE synthesis of these offspring following active PN sensitization. We found a marked elevation of PN-specific IgE in offspring of PNA-M/None offspring as early as 3 wks following PN-sensitization, which was more than 10 fold high than naïve mice offspring in respond to PN-sensitization (data not shown). However, there was an overall suppression of PN-specific IgE as well as IgG1 and IgG2a synthesis in PNA-M/PN offspring at 3 weeks following active PN sensitization. However, PN-specific IgE, IgG1 and IgG2a in PNA-M-PN offspring developed following PN-sensitization at wk 6, although the levels were significantly lower than those of PNA-M/None offspring. These data demonstrated a short window of unresponsiveness (tolerance) to PN-sensitization. The mechanisms underlying reduced responsiveness (tolerance) in PNA-M/PN offspring following peanut sensitization was not investigated in this study. There are several potential explanations. First it may be mediated by T regulatory cells or low dose antigen transmitted by breast milk as a previous study showed breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma (18); Second, it may be due to CD80-CTLA-4 interactions, because a previous study suggested that CD80, but not CD 86 interaction with CTLA-4 is crucial for the induction of low dose tolerance to PN. (39) It may also be due to T cell anergy, as it has been shown that immature dendritic cells (DCs), which encounter antigen in the absence of inflammation, cause T cells anergy, while mature DCs, which encounter antigen in the presence of inflammation induce development of effector T cells. (40) Further characterizing of the mechanisms underlying the induction of offspring tolerance to PN sensitization by exposure PNA-M to low dose PN during pregnancy and lactation is required.
Taken together, this study demonstrated that maternal PNA is a risk factor for developing PNA. Low dose PN-exposure during pregnancy and lactation reduced the risk of first exposure PN anaphylaxis and specific IgE production to active PN sensitization in offspring. Although murine models are not identical to human disease, our work may provide a rationale for a new concept regarding prevention of PNA in offspring of PN allergic women. More studies are required to further understand the underlying mechanisms.
Acknowledgements
This work was supported by the Food Allergy Initiative, and by National Institutes of Health grant # AT001495-01A1 awarded to Dr X-M Li, and The Spanish Ministry of Science and Innovation (MICINN)/Fulbright Postdoctoral Grants to Dr. I. López-Expósito. We thank Dr. Sampson for his continued support for this study, Mr. B Schofield for manuscript preparation, and Dr. TF Zhang for initial work related to this study. I. López-Expósito acknowledges to the American Academy of Allergy, Asthma and Immunology's for the Strategic Training in Allergy Research (ST*AR) Award which allowed the presentation of part of these results as an abstract during the 2009 AAAAI annual conference.
Abbreviations used
- ConA
Concanavalin A
- CPE
Crude Peanut Extract
- CT
Cholera Toxin
- DNP
Dinitrophenyl Phosphate
- MMCP-1
Mouse Mast Cell Protease-1
- PAF
Platelet Activation Factor
- PCA
Passive Cutaneous Anaphylaxis
- PN
Peanut
- PNA
Peanut Allergy
- PNA-M
Peanut-allergic mothers
- PNA-M/PN
Peanut allergic mothers/ fed peanut with CT during pregnancy and lactation
- PNA-M/None
Peanut allergic mothers / not fed PN with CT during pregnancy and lactation
- OVA
Ovalbumin
- Wk(s)
week (weeks)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007;120(3):491–503. doi: 10.1016/j.jaci.2007.07.015. [DOI] [PubMed] [Google Scholar]
- (2).Burks AW. Peanut allergy. Lancet. 2008;371(9623):1538–46. doi: 10.1016/S0140-6736(08)60659-5. [DOI] [PubMed] [Google Scholar]
- (3).Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327:380–4. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- (4).Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112(6):1203–7. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- (5).Hourihane JO, Dean TP, Warner JO. Peanut allergy in relation to heredity, maternal diet, and other atopic diseases: results of a questionnaire survey, skin prick testing, and food challenges. BMJ. 1996;313(7056):518–21. doi: 10.1136/bmj.313.7056.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lim RH, Kobzik L. Maternal transmission of asthma risk. Am J Reprod Immunol. 2009;61(1):1–10. doi: 10.1111/j.1600-0897.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- (7).Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2007. J Allergy Clin Immunol. 2008;121(6):1351–8. doi: 10.1016/j.jaci.2008.01.032. [DOI] [PubMed] [Google Scholar]
- (8).Hourihane JO, Aiken R, Briggs R, Gudgeon LA, Grimshaw KE, DunnGalvin A, et al. The impact of government advice to pregnant mothers regarding peanut avoidance on the prevalence of peanut allergy in United Kingdom children at school entry. J Allergy Clin Immunol. 2007;119(5):1197–202. doi: 10.1016/j.jaci.2006.12.670. [DOI] [PubMed] [Google Scholar]
- (9).Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121(1):183–91. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- (10).Wennergren G. What if it is the other way around? Early introduction of peanut and fish seems to be better than avoidance. Acta Paediatr. 2009 doi: 10.1111/j.1651-2227.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- (11).Helm RM. Food allergy animal models: an overview. Ann N Y Acad Sci. 2002;964:139–50. doi: 10.1111/j.1749-6632.2002.tb04139.x. 139–50. [DOI] [PubMed] [Google Scholar]
- (12).Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002;169(6):3284–92. doi: 10.4049/jimmunol.169.6.3284. [DOI] [PubMed] [Google Scholar]
- (13).Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172(11):6978–87. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- (14).Li XM. Beyond allergen avoidance: update on developing therapies for peanut allergy. Curr Opin Allergy Clin Immunol. 2005;5(3):287–92. doi: 10.1097/01.all.0000168796.20324.bd. [DOI] [PubMed] [Google Scholar]
- (15).Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, et al. Allergen-independent maternal transmission of asthma susceptibility. J Immunol. 2003;170(4):1683–9. doi: 10.4049/jimmunol.170.4.1683. [DOI] [PubMed] [Google Scholar]
- (16).Herz U, Joachim R, Ahrens B, Scheffold A, Radbruch A, Renz H. Allergic sensitization and allergen exposure during pregnancy favor the development of atopy in the neonate. Int Arch Allergy Immunol. 2001;124(1–3):193–6. doi: 10.1159/000053708. [DOI] [PubMed] [Google Scholar]
- (17).Melkild I, Groeng EC, Leikvold RB, Granum B, Lovik M. Maternal allergen immunization during pregnancy in a mouse model reduces adult allergy-related antibody responses in the offspring. Clin Exp Allergy. 2002;32(9):1370–6. doi: 10.1046/j.1365-2745.2002.01458.x. [DOI] [PubMed] [Google Scholar]
- (18).Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008;14(2):170–5. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- (19).Institute of Laboratory Animal Resources Commission of Life Sciences NRC . Guide for the Care and Use of Laboratory Animals. National Academy Press; 1996. [Google Scholar]
- (20).Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106(1 Pt 1):150–8. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- (21).Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009;123(2):443–51. doi: 10.1016/j.jaci.2008.12.1107. [DOI] [PubMed] [Google Scholar]
- (22).Burks AW, Williams LW, Helm RM, Thresher W, Brooks JR, Sampson HA. Identification of soy protein allergens in patients with atopic dermatitis and positive soy challenges; determination of change in allergenicity after heating or enzyme digestion. Adv Exp Med Biol. 1991;289:295–307. doi: 10.1007/978-1-4899-2626-5_22. 295–307. [DOI] [PubMed] [Google Scholar]
- (23).Li XM, Zhang TF, Huang CK, Srivastava K, Teper AA, Zhang L, et al. Food Allergy Herbal Formula-1 (FAHF-1) blocks peanut-induced anaphylaxis in a murine model. J Allergy Clin Immunol. 2001;108(4):639–46. doi: 10.1067/mai.2001.118787. [DOI] [PubMed] [Google Scholar]
- (24).Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28(4):581–9. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- (25).Qu C, Srivastava K, Ko J, Zhang TF, Sampson HA, Li XM. Induction of tolerance after establishment of peanut allergy by the food allergy herbal formula-2 is associated with up-regulation of interferon-gamma. Clin Exp Allergy. 2007;37(6):846–55. doi: 10.1111/j.1365-2222.2007.02718.x. [DOI] [PubMed] [Google Scholar]
- (26).Li XM, Srivastava K, Huleatt JW, Bottomly K, Burks AW, Sampson HA. Engineered recombinant peanut protein and heat-killed Listeria monocytogenes coadministration protects against peanut-induced anaphylaxis in a murine model. J Immunol. 2003;170(6):3289–95. doi: 10.4049/jimmunol.170.6.3289. [DOI] [PubMed] [Google Scholar]
- (27).Lee SY, Huang CK, Zhang TF, Schofield BH, Burks AW, Bannon GA, et al. Oral Administration of IL-12 Suppresses Anaphylactic Reactions in a Murine Model of Peanut Hypersensitivity. Clin Immunol. 2001;101(2):220–8. doi: 10.1006/clim.2001.5122. [DOI] [PubMed] [Google Scholar]
- (28).Morafo V, Srivastava K, Huang CK, Kleiner G, Lee SY, Sampson HA, et al. Genetic susceptibility to food allergy is linked to differential TH2-TH1 responses in C3H/HeJ and BALB/c mice. J Allergy Clin Immunol. 2003;111(5):1122–8. doi: 10.1067/mai.2003.1463. [DOI] [PubMed] [Google Scholar]
- (29).Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120(3):506–15. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- (30).Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358(1):28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- (31).Li XM, Huang CK, Schofield BH, Burks AW, Bannon GA, Kim KH, et al. Strain-dependent induction of allergic sensitization caused by peanut allergen DNA immunization in mice. J Immunol. 1999;162:3045–52. [PubMed] [Google Scholar]
- (32).Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358(1):28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- (33).Khodoun M, Strait R, Orekov T, Hogan S, Karasuyama H, Herbert DR, et al. Peanuts can contribute to anaphylactic shock by activating complement. J Allergy Clin Immunol. 2009;123(2):342–51. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics. 1987;79:683–8. [PubMed] [Google Scholar]
- (35).Niggemann B, Reibel S, Roehr CC, Felger D, Ziegert M, Sommerfeld C, et al. Predictors of positive food challenge outcome in non-IgE-mediated reactions to food in children with atopic dermatitis. J Allergy Clin Immunol. 2001;108(6):1053–8. doi: 10.1067/mai.2001.120192. [DOI] [PubMed] [Google Scholar]
- (36).Clark AT, Islam S, King Y, Deighton J, Anagnostou K, Ewan PW. Successful oral tolerance induction in severe peanut allergy. Allergy. 2009 doi: 10.1111/j.1398-9995.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- (37).Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007;120(3):491–503. doi: 10.1016/j.jaci.2007.07.015. [DOI] [PubMed] [Google Scholar]
- (38).Oh MSHLX. Food Allergy: Adverse Reactions to Foods and Food Additives. 2rd Ed Blackwell Publishing; Baltimore: 2009. Therapeutic Approaches Under Development. [Google Scholar]
- (39).van WF, Nierkens S, de JW, Wehrens EJ, Boon L, van KP, et al. The CD28/CTLA-4-B7 signaling pathway is involved in both allergic sensitization and tolerance induction to orally administered peanut proteins. J Immunol. 2007;178(11):6894–900. doi: 10.4049/jimmunol.178.11.6894. [DOI] [PubMed] [Google Scholar]
- (40).Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80(5):477–83. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]