Abstract
Objectives
Determine whether the association of interleukin 6 (IL-6) with lower-extremity function in the year after hip fracture is explained by an effect on muscle mass or strength.
Design
Analysis on data from a longitudinal cohort study.
Setting
Two Baltimore area hospitals.
Participants
Community-dwelling women aged ≥65 years, admitted to one of two hospitals in Baltimore with a new, non-pathological fracture of the proximal femur between 1992 and 1995.
Measurements
At 2, 6 and 12 months post fracture, serum IL-6, appendicular lean muscle mass (aLM), and grip strength were measured and the Lower Extremity Gain Scale (LEGS), a summary measure of performance of 9 lower extremity tasks was calculated. Generalized estimating equations (GEE) were used to model the longitudinal relationship between IL-6 tertile and LEGS. We examined whether muscle mass or strength explained the relationship between IL6 and LEGS by adding measures aLM, grip strength or both into the model.
Results
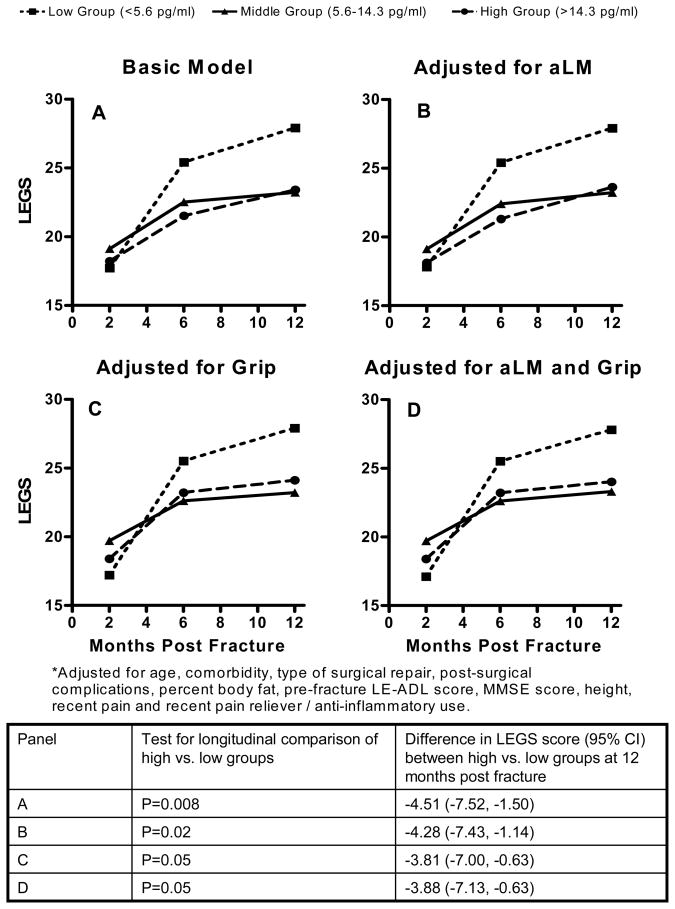
Subjects in the lowest IL-6 group performed better on the LEGS than those in the highest tertile by 4.51 (95% CI: 1.50, 7.52) points at 12 months post fracture. Adjusting for aLM, and grip strength this difference was 4.28 points (95% CI: 1.14, 7.43) and 3.81 points (95% CI: 0.63, 7.00) respectively. Adjusting for both aLM and grip strength the mean difference in LEGS score was 3.88 points (95% CI: 0.63, 7.13).
Conclusions
In older women after hip fracture, the diminished recovery of lower extremity function observed with higher levels of the inflammatory marker IL-6 is better explained by reduced muscle strength, rather than reduced muscle mass.
Introduction
Low muscle mass or sarcopenia is an important factor in the development of functional disability in older adults. Recent studies have suggested that loss of muscle mass does not fully explain the decreased muscle strength seen with advanced age;1, 2 and changes in muscle strength, in disproportion to changes in muscle mass, have been observed.
Serum markers of chronic inflammation have been associated with sarcopenia, and decreased muscle strength;3–7 and inflammation is believed to contribute to muscle weakness by both accelerated protein loss and contractile dysfunction.8
We have found that in older women recovering from hip fracture, the serum level of the inflammatory cytokine interleukin 6 (IL-6) is associated with functional recovery in the 12 months post fracture.9 Although a substantial loss of muscle mass (5 to 6%) occurs in women during the year after hip fracture,10, 11 there is evidence to suggest that a decline in muscle strength to a greater degree than a decline in muscle mass is associated with worse recovery of lower extremity function.12–14 Whether the association of IL-6 level with lower extremity function in the year post hip fracture is explained by an effect on muscle mass or strength is not known.
We examined a cohort of women admitted to two Baltimore area hospitals between 1992 and 1995 who were enrolled in the Baltimore Hip Studies. Women 65 years and older were followed for a year post hip fracture, in order to evaluate whether the association of IL-6 levels with lower extremity function in the year post hip fracture is explained by an effect on muscle mass or strength.
Methods
Subjects were drawn from the third cohort in the Baltimore Hip Studies (BHS-3). Between 1992 and 1995, 205 Caucasian women, aged at least 65 years and community dwelling, admitted to one of two hospitals in Baltimore with a new, non-pathological fracture of the proximal femur, agreed to participate in a prospective study. Subjects were evaluated at 3 or 10 days (baseline) and at 2, 6, and 12 months post hospitalization for fracture (follow-up). The study protocol was approved by the Institutional Review Boards of both hospitals, and of the University of Maryland Baltimore. A more detailed description of this cohort may be found elsewhere.9, 10
Measure of Function
At the 2, 6 and 12 month evaluations, timed performance of 9 lower extremity tasks was assessed at each evaluation: [1] reach for an item on the ground from a sitting position; [2] put a sock on the fractured-side foot; [3] put a shoe on the fractured-side foot; [4] rise from an armless chair; [5] walk ten feet; [6] step up four steps; [7] step down four steps; [8] get on the toilet; and [9] get off the toilet. Performance of these tasks was scored on a scale of 0 to 4 to create a summary score of lower extremity function, the Lower Extremity Gain Scale (LEGS).15 This score ranges from 0–36 with higher scores indicating better performance. It has been shown to be a reliable measure and the domains that it assesses have been identified by hip fracture patients and their caregivers as important aspects of functioning. A difference of approximately 2 points on this scale is believed to be clinically meaningful.16
IL-6 measurement
The methods for collection and storage of serum samples have been described in detail elsewhere.17 Briefly, sera obtained at the 2, 6 and 12 month time points were stored at −70C. When stored at these temperatures IL-6 is believed to remain stable in serum for many years.18 IL-6 levels were measured in serum that had not been previously thawed by a two-antibody ELISA using biotin-strepavidin-peroxidase detection, and commercially available human antibodies (Pierce/Endogen, Rockford, IL). Samples from these time points were analyzed in the same batch to minimize laboratory variability. The linear range for the IL-6 assay was 1.5 to 100 pg/ml. The inter-assay CV was 3.6% and the intra-assay CV% was 8%. Cytokine measurements above the upper detection limit of 100 pg/ml were converted to the highest level of the assay and measurements below the lower detection limit of 1.5 pg/ml were scored as zero. At the 2-month, 6-month and 12-month examinations 7%, 5% and 5% respectively of the IL-6 measurements were above the assay limit and 1%, 3% and 5% were below the detection limit for the assay.
Measures of Body Composition
At the 2, 6 and 12 month evaluations, dual-energy x-ray absorptiometry (DXA) (Hologic, Inc., Waltham, MA) was used to measure whole and regional body composition, using a method described in detail elsewhere.10 Since the presence of post-operative swelling and metallic implants may affect DXA readings,19 appendicular lean mass (aLM) was calculated as the sum of the lean mass in the arms and twice the lean mass in the unfractured leg, as has been done in other studies of hip fracture patients.14 Total body fat was calculated similarly and percent body fat was calculated based on the DXA results at each of the 2, 6 and 12-month evaluations.
Measure of Strength
Due to the proximity of the hip fracture event, measures of proximal lower extremity strength were not obtained in this cohort. Grip strength, which has been shown to be a strong predictor of lower extremity function 20, 21 was measured at each of the 2, 6 and 12-month evaluations using a handheld dynamometer (Jamar, Clifton, NJ). The maximum strength results (in kilograms) of four grip strength trials (two in each arm) with patients seated were used.
Covariates
Age, the type of surgical repair (internal fixation, hemi-arthroplasty or arthroplasty), and the presence or absence of 20 non-infectious and 3 infectious in- hospital complications were collected from the hospital record at baseline. Since infection is associated with elevated inflammatory cytokine levels,22 infectious and non- infectious in-hospital complications were tabulated separately. The list of potential complications appears in detail elsewhere.9 The presence, prior to the fracture, of osteoarthritis, coronary artery disease (history of either angina pectoris, cardiovascular disease or myocardial infarction), congestive heart failure, stroke, dementia, diabetes, chronic obstructive pulmonary disease, and peripheral vascular disease was also collected at baseline from the hospital record. These conditions were chosen because they have been observed to be associated with an adverse effect on functional recovery in elders.23 Details on physical therapy treatment was assessed by a review of the medical record at the acute inpatient, rehabilitation center and outpatient settings and the number of physical therapy sessions received by the participants was tabulated. At each of the 2, 6 and 12-month evaluations, mental Status was assessed using the Mini-Mental State Examination (MMSE).24 At the baseline evaluation, subjects reported on their pre-fracture limitations with eleven activities of Daily Living requiring lower extremity functioning (LE-ADLs). A score of LE-ADL function was calculated as the count of the number of activities in which the person was impaired (required either human or equipment assistance or both or completely unable to perform). The eleven activities included were Walking ten feet or across a room; Walking one block on a level sidewalk; Climbing five stairs; Getting into a car; Getting into and out of bed; Rising from an armless chair; Putting on pants; Putting socks and shoes on both feet; Getting in/out of a bath or shower; Taking a bath, shower, or sponge bath; and Getting on and off the toilet. The scale thus ranges from zero to eleven with higher scores representing greater pre-fracture dependency. Height was collected by self report at the baseline examination. Proxy data were used whenever patient data were unavailable or the patient was cognitively impaired (MMSE score less than 17). At each visit, participants were asked about pain symptoms and the use of medications during the previous week. The presence of pain was defined as a positive response to the question “within the past week have you had any pain or discomfort whatsoever.” Use of pain reliever/anti-inflammatory agents was defined as the report of use during the previous week of aspirin, non-aspirin based pain relievers or corticosteroids.
Statistical Analyses
Due to the proximity of the baseline evaluation to the hip fracture surgery, only 56 participants attempted the LEGS measurements at baseline (data not shown). Also, the serum levels of IL-6 at the baseline evaluation are more likely to reflect a normal physiological response to the trauma of hip fracture and subsequent surgical repair, and not a prolonged inflammatory process.25, 26 Consistent with our previous work,9 we therefore restricted the current analyses to the 2, 6 and 12 month follow-up time points. Subjects were divided into groups based on their serum IL-6 levels over the 2, 6 and 12 month time points of the follow-up period (low group: IL-6 ≤ 5.63, middle group 5.63 <IL-6≤ 14.30, and high group IL-6 > 14.30 pg/ml). These cut-points represent the IL-6 tertile levels of all the observations over the 2 to 12 month follow-up period, therefore ensuring that approximately equal numbers of observations are included in each IL-6 group over the follow-up period.
Generalized estimating equations (GEE) 27 were used to model the longitudinal relationship between IL-6 category at the 2, 6 and 12 month follow-up evaluations and grip strength and aLM, using a normal working model and robust standard errors in order to account for correlations across time within individuals. Generalized F tests were used to compare grip strength and aLM trajectories between those in the mid-level and high IL-6 categories to the lowest (reference) IL-6 group. The models were adjusted for age, comorbidity, and percent body fat.
Generalized estimating equations (GEE) 27 were similarly used to model the longitudinal relationship between IL-6 category at the 2, 6 and 12 month follow-up evaluations and LEGS. Again, generalized F tests were used to compare LEGS trajectories between those in the mid-level and high IL-6 categories to the lowest (reference) IL-6 group, and time-specific t-tests were performed to compare IL-6 group scores at each time point. The models were adjusted for age, comorbidity, the type of surgical repair (internal fixation versus arthroplasty repairs), post-surgical complications, the number of physical therapy sessions received by 2 months post-fracture, percent body fat, pre-fracture LE-ADL score, MMSE score, height, pain and medication use.
We examined whether muscle mass, muscle strength or both mediated (according to the definition of Baron and Kenny28), in part, the relationship between serum IL6 levels and lower extremity function by adding the measures of aLM, grip strength or both into the model. We examined differing effects of muscle mass and strength over time by including time-by-predictor interaction terms into the models. We also examined for interaction between muscle mass and strength through the inclusion of interaction terms in the model.
The validity of GEE results are contingent on missing data being missing completely at random (MCAR) as defined by Rubin.29 A sensitivity analysis of the MCAR assumption using weighted estimating equations (WEE) was performed.30 The weights were calculated by performing a logistic regression of observing both IL-6 and LEGS (yes/no) on the covariates used in the analysis model for each visit.
Results
Characteristics of the study sample are summarized in Table 1. Mean (SD) age was 79.66 (7.83) years. All subjects were white. At the 2, 6 and 12 month evaluations, serum was collected and analyzed for IL-6 levels on 91, 96 and 77 participants, grip strength was measured on 85, 91 and 73 participants, DXA evaluations for body composition were performed on 78, 82 and 70 participants, and the LEGS test was completed by 62, 75 and 54 participants, respectively. Of these subjects, 51, 63 and 51 subjects had complete information required for the analyses presented here at 2, 6 and 12 months, respectively and 68 subjects were included at least once in the primary analyses. Subjects who were included in the 2 month analyses, but had incomplete information at either the 6 or 12 month evaluations did not differ significantly with respect to age, comorbidity, post-fracture surgical complications, or the number of physical therapy sessions received, but they reported limitations with a greater number of lower extremity tasks pre-fracture (4.59 versus 1.67, p<0.001).
Table 1.
Characteristics of the study sample at two months post hip fracture.
| IL-6 Group N=68 |
||||
|---|---|---|---|---|
| Variable | Low (< 5.63 pg/ml) | Middle (5.63–14.30 pg/ml) | High (> 14.30 pg/ml) | P value |
| N = 19 | N=28 | N=21 | ||
| Baseline Age (Years) | ||||
| Mean (SD) | 78.89 (8.88) | 79.35 (7.21) | 80.76 (7.88) | 0.54 |
| BMI Count (%) | ||||
| <18.5 kg/m2 | 1 (5.3) | 4 (14.3) | 3 (14.3) | 0.22 |
| 18.5–24.9 kg/m2 | 11 (57.9) | 14(50.0) | 8 (38.1) | |
| 25–29.9 kg/m2 | 7 (36.8) | 4 (14.3) | 9 (42.9) | |
| ≥ 30.0 kg/m2 | 0 (0.0) | 6 (21.4) | 1 (4.8) | |
| Baseline Comorbidities Count(%) | ||||
| 0 | 7 (36.8) | 7 (25.0) | 6 (28.6) | 0.85 |
| 1 | 6 (31.6) | 10 (35.7) | 9 (42.9) | |
| ≥ 2 | 6 (31.6) | 11 (39.3) | 6 (28.6) | |
| Type of Surgical Repair Count (%) | ||||
| Internal fixation | 10 (52.6) | 16 (57.1) | 17 (85.0) | 0.06 |
| Arthroplasty | 9 (47.4) | 12 (42.9) | 3 (15.0) | |
| In Hospital | ||||
| Complications Count (%) | ||||
| 0 | 16 (84.2) | 22 (78.6) | 14 (70.0) | 0.56 |
| ≥ 1 | 3 (15.8) | 6 (21.4) | 6 (30.0) | |
| Self Report of Pain week prior to the 2-month evaluation Count (%) | ||||
| Yes | 16 (84.2) | 23 (82.1) | 16 (76.2) | 0.79 |
| No | 3 (15.8) | 5 (17.9) | 5 (23.8) | |
| Used anti-inflammatory drugs or other pain relievers in the week prior to the 2-month evaluation Count (%) | ||||
| Yes | 13 (84.6) | 21 (75.0) | 15 (71.4) | 0.88 |
| No | 6 (15.4) | 7 (25.0) | 6 (28.6) | |
| Pre-fracture limitations in LE-ADL function Mean Number of Limitations (SD) | 3.56 (3.05) | 4.27 (3.06) | 3.36 (2.48) | 0.92 |
| MMSE at 2 months post fracture Mean (SD) | 27.43 (2.94) | 26.70 (3.73) | 25.57 (3.89) | 0.12 |
| Number of physical therapy sessions completed by 2 months Mean (SD) | 21.95 (13.47) | 24.93 (13.37) | 27.30 (18.93) | 0.60 |
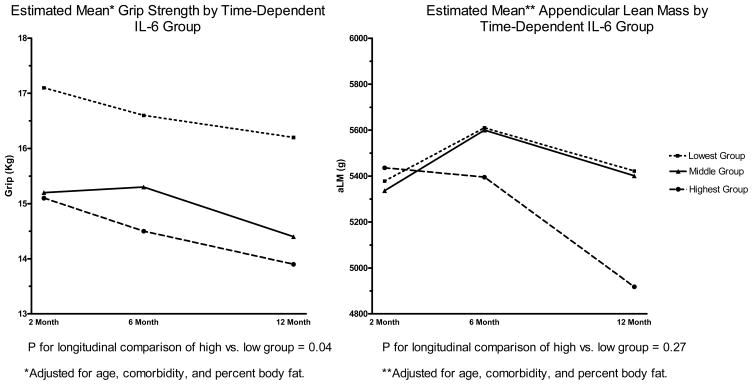
In longitudinal analyses, subjects in the low Il-6 group displayed greater aLM (p=0.07) and grip strength (p=0.12) during the follow-up period. At 12 months post fracture those in the low Il-6 group had, on average 0.56 kg (95% CI: 0.07 kg, 1.1 kg) greater aLM and 2.4 kg greater grip strength (95% CI: 0.14 kg, 4.7 kg) compared to those in the highest group. (Figure 1).
Figure 1.
Association of Grip Strength and Appendicular Lean Mass with Time-Dependent IL-6 Group
Subjects in the low IL-6 group performed better on the LEGS than those in the high group over the 2, 6 and 12 months evaluations (p=0.007 in longitudinal analyses). Performance on the LEGS did not differ significantly between the middle and high IL-6 groups. The difference in LEGS score between the high and low groups was greatest at 12 months post-fracture when, adjusted for covariates only, on average those in the highest IL-6 group scored 4.51 (95% CI: 1.50, 7.52) points worse on the LEGS measure (Figure 2, panel A). Adjusting for covariates and aLM (Figure 2, panel B), the difference in LEGS scores at 12 months was 4.28 points (95% CI: 1.14, 7.43); adjusting for covariates and grip strength (Figure 2, panel C) the difference at 12 months was 3.81 points (95% CI: 0.63, 7.00). Adjusting for covariates and both aLM and grip strength (Figure 2, panel D) resulted in little change of the 12-month effect estimate over adjustment for covariates and grip strength alone; the mean difference at 12 months between the low and high groups of IL6 in this model was 3.88 points (95% CI: 0.63, 7.13).
Figure 2.
Estimated Mean* LEGS Scores by Time-Dependent IL-6 Group
Adjusted for covariates, and IL-6 group, for each standard deviation (SD) difference in aLM (SD=2.6 kg), LEGS scores differed by on average 0.3 points (p=0.62) while for each SD difference in grip strength (SD = 4.8 kg), LEGS scores differed by 2.3 points (p=0.005). With both aLM and grip strength in the model, for each SD difference in grip strength, LEGS scores differed by 2.4 points (p=0.007).
The sensitivity analysis yielded similar results and the same conclusions as the GEE analysis (data not shown).
Discussion
We previously reported that serum IL-6 levels are adversely associated with lower extremity function in the year following hip fracture.9 In the current analyses, we explored whether muscle mass, strength or both explained this association. We found that although the overall interpretation of the longitudinal association between IL-6 group and LEGS changed little with the different models, adjustment for muscle mass attenuated the 12-month effect estimate by 5%, from 4.51 to 4.28 points whereas adjustment for muscle strength resulted in a 16% attenuation of the 12-month estimated effect form 4.51 to 3.81 points. Adjustment for both muscle mass and strength did not alter the effect estimate substantially over adjustment for strength alone. These results would suggest that the association of IL-6 with lower extremity function following hip fracture is explained by muscle strength in disproportion to muscle mass.
In studies of older adults, including adults post hip fracture, muscle strength, in disproportion to muscle mass, has been associated with poor function.12–14, 31 Our results are consistent with previous studies that have found that, in older adults, levels of inflammatory markers are more strongly associated with muscle strength than muscle mass32 and that changes in muscle strength may explain the association between IL-6 with functional loss.33 To our knowledge, however, this is the first study to examine these associations longitudinally in a cohort of frail older adults recovering from a traumatic and disabling event, hip fracture.
In a less disabled cohort of older adults, inflammatory cytokine levels have been found to be associated with low levels of muscle mass32 and, in turn, low muscle mass was associated with poor lower extremity function.34 In contrast, in this and other cohorts of elders post hip fracture, an association between aLM and lower extremity function was not found.12, 14 Although aLM is considered a measure of sarcopenia, its utility may be limited in the study of frail elders such as those recovering from hip fracture, where many may have low muscle mass. For example, in the cohort studied here, the mean (SD) aLM at the baseline examination was 13.9 (3.0) kg compared to published values of 16.1 (2.5) kg in older white women from the Health Aging and Body Composition (Health ABC) Study.32 Previously published analyses from the BHS-3 cohort have demonstrated that lean body mass decreases rapidly post hip fracture with, compared to the immediately post-fracture measurement, a decline of 6.4% noted by 2 months post fracture and a persistent decrease of 6.0% noted at one year. In contrast, fat mass was found to decrease by only 1.6% by 2 months and increase by 3.6% by 12 months post fracture, suggesting that the loss of lean tissue exceeds that of overall body mass.10 Low muscle mass is believed to be central to the frailty syndrome35 and therefore in frail elders, such as those who suffer hip fracture, changes in muscle mass may be small. Processes intrinsic to the muscle itself, however, such as alterations of contractile protein structure with resulting changes in muscle contractile properties may explain the observed declines in strength that are associated with inflammation.
Although the specific mechanisms that explain declines in muscle strength in older adults are not completely understood, factors such as age-associated decreases in the proportion of myosin heavy chain type II fibers,36 increased connective tissue, fatty infiltration, 37 altered muscle metabolism,38 and changes to the muscle spindle39 are believed to play a role. Single muscle fibers taken from older subjects have been found to have significantly lower maximal force compared with similar fibers from younger individuals, suggesting that the intrinsic ability of muscle fibers to generate force is lower in older subjects.40 Although single muscle fibers from old subjects are frequently smaller in cross sectional area (CSA) than those taken from younger individuals,37 the decline in force observed with aging exceeds this decrease in CSA.41 This may be explained, in part, by myosin concentration levels in muscle fibers from older and younger individuals as myosin concentration has been found to have a linear relationship with specific force (force/CSA) generation in single muscle fibers and these concentrations are lower in fibers taken from the skeletal muscle of older, compared to younger subjects.41 Inflammation can also affect muscle contractile properties. In addition to the catabolic effect of chronic inflammatory states,42, 43 tumor necrosis factor alpha (TNF-α) is believed to depress contractile force at the myofilament level, by a mechanism mediated by reactive oxygen species (ROS) and nitric oxide (NO) derivative generation.44
These results must be viewed with the following limitations in mind. As we have previously reported, a proportion of study subjects had cytokine levels beyond the range of the assays employed and, due to selective forces, serum samples at later evaluations were obtained from younger women with less comorbidity, compared to the cohort as a whole at baseline.9 Also, all of the subjects included in these analyses were white women and thus we are unable to assess whether similar associations exist in men or in women of other ethnicities. Finally, the association of IL-6 with both muscle strength and muscle mass observed here explained 19% of the relationship between this inflammatory cytokine and recovery of lower extremity function post hip fracture. This would suggest that these associations, although interesting, represent but one piece of a more complex mechanism and other processes, beyond the scope of study of these analyses, are also likely to be involved.
In summary, we have found that muscle strength in disproportion to muscle mass explains the association of serum levels of the inflammatory cytokine IL-6 with lower extremity function in the year after hip fracture. These results should be confirmed in studies where direct measures of muscle composition are available.
Acknowledgments
Financial Disclosures: Supported by grants from the NIH: R37 AG09901, R37 AG009901, R01 AG029315, R01 AR47711, K23 AG019161 to A.R.C., P30 AG028747 and P60 AG12583 (Claude D. Pepper Older Americans Independence Center (OAIC) Junior Faculty Award to R.R.M.). Cytokine assay analyses were supported by a University of Maryland School of Medicine Intramural Award.
Sponsor’s Role: The sponsor had no role in the conduct of the study; the collection, management, analyses or interpretation of the data; or the preparation or approval of the manuscript.
Supported by grants from the NIH: R37 AG09901, R37 AG009901, R01 AG029315, R01 AR47711, K23 AG019161 to A.R.C., P30 AG028747 and P60 AG12583 (Claude D. Pepper Older Americans Independence Center (OAIC) Junior Faculty Award to R.R.M.). Cytokine assay analyses were supported by a University of Maryland School of Medicine Intramural Award.
Footnotes
Author Contributions: Ram R. Miller: Study concept, design, statistical analysis, analysis and interpretation of data, preparation of manuscript. Michelle D Shardell: Study design, statistical analysis, analysis and interpretation of data, preparation of manuscript. Gregory E Hicks; Study design, analysis and interpretation of data, preparation of manuscript. Anne R Cappola: Acquisition of subjects, analysis and interpretation of data, preparation of manuscript. William G Hawkes: Analysis and interpretation of data, preparation of manuscript. Janet A. Yu-Yahiro: Acquisition of subjects, analysis and interpretation of data, preparation of manuscript. Jay Magaziner: Acquisition of subjects, analysis and interpretation of data, preparation of manuscript.
References
- 1.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 2.Goodpaster BH, Park SW, Harris TB, et al. The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 3.Payette H, Roubenoff R, Jacques PF, et al. Insulin-like growth factor-1 and interleukin-6 predict sarcopenia in very old community-living men and women: The Framingham Study. J Am Geriatr Soc. 2003;51:1237–1243. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- 4.Cesari M, Penninx BWJH, Pahor M, et al. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59A(3):242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 5.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88(5):2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 6.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52(4):M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 8.Reid MB, Li YP. Cytokines and oxidative signalling in skeletal muscle. Acta Physiol Scand. 2001;171:225–232. doi: 10.1046/j.1365-201x.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- 9.Miller RR, Cappola AR, Shardell MD, et al. Persistent Changes in Interleukin-6 And Lower Extremity Function Following Hip Fracture. J Gerontol A Biol Sci Med Sci. 2006;61:1053–1058. doi: 10.1093/gerona/61.10.1053. [DOI] [PubMed] [Google Scholar]
- 10.Fox KM, Magaziner J, Hawkes WG, et al. Loss of bone density and lean body mass after hip fracture. Osteoporos Int. 2000;11:31–35. doi: 10.1007/s001980050003. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson M, Nilsson JA, Sernbo I, Redlund-Johnell I, Johnell O, Obrant KJ. Changes of bone mineral mass and soft tissue composition after hip fracture. Bone. 1996;18(1):19–22. doi: 10.1016/8756-3282(95)00422-x. [DOI] [PubMed] [Google Scholar]
- 12.Visser M, Harris TB, Fox KM, et al. Change in muscle mass and muscle strength after a hip fracture: Relationship to mobility recovery. J Gerontol: Med Sci. 2000;55A(8):M434–440. doi: 10.1093/gerona/55.8.m434. [DOI] [PubMed] [Google Scholar]
- 13.Wehren LE, Hawkes WG, Hebel JR, Orwig DL, Magaziner J. Bone mineral density, soft tissue body composition, strength, and functioning after hip fracture. J Gerontol A Biol Sci Med Sci. 2005;60(1):80–84. doi: 10.1093/gerona/60.1.80. [DOI] [PubMed] [Google Scholar]
- 14.Di Monaco M, Vallero F, Di Monaco R, Tappero R, Cavanna A. Muscle mass and functional recovery in women with hip fracture. Am J Phys Med Rehabil. 2006;85:209–215. doi: 10.1097/01.phm.0000200387.01559.c0. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman S, Hawkes WG, Hebel JR, Fox KM, Lydick E, Magaziner J. The Lower Extremity Gain Scale: A performance-based measure to assess recovery after hip fracture. Arch Phys Med Rehabil. 2006;87(3):430–436. doi: 10.1016/j.apmr.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Hawkes WG, Williams GR, Zimmerman S, et al. A clinically meaningful difference was generated for a performance measure of recovery from hip fracture. J Clin Epidemiol. 2004;57:1019–1024. doi: 10.1016/j.jclinepi.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 17.YuYahiro J, Michael RH, Dubin NH, et al. Serum and urine markers of bone metabolism during the year after hip fracture. J Amer Geriatr Soc. 2001;49(7):877–883. doi: 10.1046/j.1532-5415.2001.49177.x. [DOI] [PubMed] [Google Scholar]
- 18.Kenis G, Teunissen C, De Jongh R, Bosmans E, Steinbusch H, Maes M. Stability of interleukin 6, soluble interleukin 6 receptor, interleukin 10 and CC16 in human serum. Cytokine. 2002;19(5):228–235. [PubMed] [Google Scholar]
- 19.Giangregorio LM, Webber CE. Effects of metal implants on whole-body dual-energy x-ray absorptiometry measurements of bone mineral content and body composition. Can Assoc Radiol J. 2003;54(5):305–309. [PubMed] [Google Scholar]
- 20.Visser M, Deeg DJH, Lips P, Harris TB, Bouter LM. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–386. doi: 10.1111/j.1532-5415.2000.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuh D, Bassey EJ, Butterworth S, Hardy R, Wadsworth ME Team. tMS. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: associations with physical activity, health status, and socioeconomic conditions. J Gerontol A Biol Sci Med Sci. 2005;60(2):224–231. doi: 10.1093/gerona/60.2.224. [DOI] [PubMed] [Google Scholar]
- 22.Scherer MA, Neumaier M, von Gumppenberg S. C-reactive protein in patients who had operative fracture treatment. Clin Orthop. 2001;393:287–293. doi: 10.1097/00003086-200112000-00033. [DOI] [PubMed] [Google Scholar]
- 23.Miller RR, Zhang Y, Silliman RA, et al. Effect of medical conditions on improvement in self-reported and observed functional performance of elders. J Am Geriatr Soc. 2004;52:217–223. doi: 10.1046/j.0002-8614.2004.52057.x. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental state” - A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Krabbe KS, Bruunsgaard H, Hansen CM, et al. Ageing is associated with a prolonged fever response in human endotoxemia. Clin Diagn Lab Immunol. 2001;8:333–338. doi: 10.1128/CDLI.8.2.333-338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruunsgaard H, Skinhoj P, Qvist J, Pedersen BK. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J Infect Dis. 1999;180:551–554. doi: 10.1086/314873. [DOI] [PubMed] [Google Scholar]
- 27.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 28.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 29.Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581–592. [Google Scholar]
- 30.Shardell MD, Miller RR. Weighted estimating equations for longitudinal studies with death and non-monotone missing time-dependent covariates and outcomes. Statistics in Medicine. 2007 doi: 10.1002/sim.2964. Published online 20 Jun 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visser M, Newman AB, Nevitt MC, et al. Reexamining the sarcopenia hypothesis: muscle mass versus muscle strength. Ann NY Acad Sci. 2000;904:456–461. [PubMed] [Google Scholar]
- 32.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57A:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 33.Ferrucci L, Penninx BWJH, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 34.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci Mar. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 35.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 36.Tauchi H, Yoshiokam T, Kobayashi H. Age change of skeletal muscles of rats. Gerontologia. 1971;17:219–227. doi: 10.1159/000211826. [DOI] [PubMed] [Google Scholar]
- 37.Lexell J. Human aging, muscle mass and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50A(Special Issue):11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 38.Carmieli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Experimental Gerontology. 2002;37:477–489. doi: 10.1016/s0531-5565(01)00220-0. [DOI] [PubMed] [Google Scholar]
- 39.Liu J-X, Eriksson P-O, Thornell L-E, Pedrosa-Domellof F. Fiber content and myosin heavy chain composition of muscle spindles in aged human biceps brachii. J Histochem Cytochem. 2005;53(4):445–454. doi: 10.1369/jhc.4A6257.2005. [DOI] [PubMed] [Google Scholar]
- 40.Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol. 2000;279(3):C611–C618. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- 41.D’Antona G, Pellegrino MA, Adami R, et al. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552(2):499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beutler B, Mahoney J, Le Trang N, Pekala P, Cerami A. Purification of cachectin, a lipoprotein lipase-supressing hormone secreted by endotoxin induced raw 264.7 cells. J Exp Med. 1985;161:984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotler DP. Cachexia. Ann Intern Med. 2000;133(8):622–634. doi: 10.7326/0003-4819-133-8-200010170-00015. [DOI] [PubMed] [Google Scholar]
- 44.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha. Am J Respir Crit Care Med. 2002;166:479–484. doi: 10.1164/rccm.2202005. [DOI] [PubMed] [Google Scholar]