Abstract
The first morphological sign of vertebrate postcranial body segmentation is the sequential production from posterior paraxial mesoderm of blocks of cells termed somites. Each of these embryonic structures is polarized along the anterior/posterior axis, a subdivision first distinguished by marker gene expression restricted to rostral or caudal territories of forming somites. To better understand the generation of segment polarity in vertebrates, we have studied the zebrafish mutant fused somites (fss), because its paraxial mesoderm lacks segment polarity. Previously examined markers of caudal half-segment identity are widely expressed, whereas markers of rostral identity are either missing or dramatically down-regulated, suggesting that the paraxial mesoderm of the fss mutant embryo is profoundly caudalized. These findings gave rise to a model for the formation of segment polarity in the zebrafish in which caudal is the default identity for paraxial mesoderm, upon which is patterned rostral identity in an fss-dependent manner. In contrast to this scheme, the caudal marker gene ephrinA1 was recently shown to be down-regulated in fss embryos. We now show that notch5, another caudal identity marker and a component of the Delta/Notch signaling system, is not expressed in the paraxial mesoderm of early segmentation stage fss embryos. We use cell transplantation to create genetic mosaics between fss and wild-type embryos in order to assay the requirement for fss function in notch5 expression. In contrast to the expression of rostral markers, which have a cell-autonomous requirement for fss, expression of notch5 is induced in fss cells at short range by nearby wild-type cells, indicating a cell-non-autonomous requirement for fss function in this process. These new data suggest that segment polarity is created in a three-step process in which cells that have assumed a rostral identity must subsequently communicate with their partially caudalized neighbors in order to induce the fully caudalized state.
Keywords: Fused somites, tbx24, T-box, Segment polarity, Somitogenesis, Paraxial mesoderm, Induction, Community effect
Introduction
Somitogenesis is the serial production, from anterior to posterior along the embryonic axis, of epithelial blocks of mesodermal cells, termed somites, from the morphologically unsegmented presomitic mesoderm (PSM) in the growing vertebrate embryo (reviewed in Pourquie, 2001). Somites are bilaterally symmetrical, and differentiate into the muscle, skin, and axial skeleton. Positioning of boundaries, or furrows, between each successive somite is thought to be controlled in part by a biochemical oscillator active in cells of the tailbud and posterior PSM. This appears to consist of a feedback loop involving genes and proteins of the Delta/Notch signaling system and its Her repressor gene targets (reviewed in Rida et al., 2004; Weinmaster and Kintner, 2003). In addition, the Wnt and Fgf signaling systems may modulate the action of the oscillator (Aulehla et al., 2003; Dubrulle et al., 2001; Sawada et al., 2001). Genes that display the distinctive dynamic, wavelike expression domains characteristic of the segmentation oscillator are known as cyclic genes (Pourquie and Tam, 2001), and the posterior region of the PSM and tailbud that exhibits these periodic changes will be here termed the oscillatory zone.
Each of the somites is clearly polarized along its rostral/caudal axis, as evidenced by differential permissiveness to neural crest and sensory nerve axon outgrowth in rostral and caudal halves (reviewed in Pourquie, 2001). In addition, skeletal elements derived from sclerotomal cells of the somite project from the vertebral body, or centra, in a specific, polarized manner. Even prior to somite boundary formation, the prospective somitic cells within the anterior end of the PSM exhibit striped expression of many genes, indicating that the prepatterning of these cells into a polarized array prefigures the morphological aspects of segment polarity. The region in the anterior PSM in which these stable, polarized stripes of gene expression are first seen will here be termed the segment polarity zone. Despite the importance of this segmental polarity to the functional form of the animal, the mechanism of its generation remains unresolved.
One strategy to understand the generation of segment polarity is to isolate and characterize mutants that fail in one or more aspects of this process. The zebrafish recessive viable mutant fused somites (fss) does not form embryonic somites, and the striped expression of marker genes in the segment polarity zone of fss mutant embryos is lost (van Eeden et al., 1996). Genes usually found restricted to the caudal halves of prospective and formed somites, such as myoD, snail1 (van Eeden et al., 1996), ephrinB2 (Durbin et al., 2000), and deltaC (Jiang et al., 2000), are instead expressed ubiquitously throughout the paraxial mesoderm of fss, whereas rostral marker genes such as fgf8, ephA4, deltaD, mespa, mespb, papc, and lfng (Durbin et al., 2000; Jiang et al., 2000; Prince et al., 2001; Sawada et al., 2000) are either absent or dramatically down-regulated. These molecular marker data indicate that the paraxial mesoderm of fss embryos is profoundly caudalized (Durbin et al., 2000), suggesting a simple 2-step model for the generation of segment polarity. In the first step, paraxial mesoderm is formed during gastrulation with a default caudal identity. In the second step, the fss gene is required to generate regions of rostral identity, in some manner, from a tissue that otherwise has a uniform caudal state. However, the recent finding that the caudal segment polarity marker ephrinA1 is down-regulated in fss/tbx24 PM (Barrios et al., 2003) suggests that this 2-step model may be incomplete.
The fused somites gene has recently been cloned and encodes a novel transcription factor of the T-box family, tbx24, which is expressed throughout the anterior oscillatory and segment polarity zones and the two most recently formed somites of the zebrafish embryo (Nikaido et al., 2002). Importantly, tbx24 is ubiquitously expressed in the PSM, leaving open the question of how fss/tbx24 might act to generate segment polarity, and conflicting evidence exists regarding whether fss/tbx24 is required cell-autonomously for expression of rostral identity (Barrios et al., 2003; Durbin et al., 2000). One possibility is that a cryptic segmental pattern exists at the protein level, either by differential abundance, sub-cellular localization, or by interaction with some other localized factor. Alternatively, the fss/tbx24 phenotype may result from a simple defect in the maturation of PSM cells (Holley and Takeda, 2002; Nikaido et al., 2002).
The perturbation of segment polarity in fss/tbx24 embryos could simply reflect an earlier disorganization of segmental prepatterning as a whole, but this appears unlikely to be the case. The hemal and neural arches of the axial skeleton, which normally project only from the rostral half of each vertebral centrum, grow also from the caudal half and are severely distorted in fss/tbx24 (van Eeden et al., 1996). However, the vertebral bodies themselves appear normally segmented, implying that underlying segmental information is still generated in the fss/tbx24 embryo. Consistent with this notion, examination of the wavelike expression domains of the cyclic genes her1, her7, and deltaC (dlc) in the oscillatory zone of fss/tbx24 embryos reveals an essentially normal sequence of stripes, suggesting that the segmentation oscillator is still functional (Gajewski et al., 2003; Holley et al., 2000; Jiang et al., 2000). In the anterior PSM of fss/tbx24 embryos, however, instead of increasing in level and arresting at the site of the future somite furrow as expected in wild-type embryos, the wavelike expression domains of her1, her7, and dlc grow weaker and disappear (Gajewski et al., 2003; Holley et al., 2000; Jiang et al., 2000; van Eeden et al., 1998). Thus, the somitogenic defect in fss/tbx24 does not appear to be at the level of basic spatial subdivision, but rather at some later point between the oscillator and morphogenesis. Since embryological experiments in chick have shown the importance of juxtaposing somite halves with different polar identities for morphological boundary formation (Aoyama and Asamoto, 1988; Sato et al., 2002; Stern and Keynes, 1987), the lack of polarity was postulated to be the underlying cause of the failure to form epithelial boundaries seen in the fss/tbx24 mutant embryo (Durbin et al., 2000).
A link between segment polarity and somite morphogenesis is provided by the Eph and ephrin families of cell-contact repulsion receptors and ligands, which are expressed in rostrally and caudally polarized stripes in the wild-type PSM (Barrios et al., 2003; Durbin et al., 1998, 2000; Xu et al., 1994). This juxtaposition of fields of receptor and ligand-bearing cells is lost in fss/tbx24 (Durbin et al., 2000). Elegant transplantation studies indicate that the direct cause of the failure of somite furrow formation in fss/tbx24 embryos is likely defective Eph/ephrin signaling (Barrios et al., 2003; Durbin et al., 2000). Indeed, this signaling process can drive the mesenchymal to epithelial transition of somite boundary formation in fss/tbx24 host tissue without generating segment polarity, suggesting that Eph/ephrin signaling directly mediates the morphogenetic changes of somitogenesis without affecting cell fate in the PSM (Barrios et al., 2003). Thus, current evidence suggests that the segmentation defect in fss/tbx24 mutant embryos stems from a failure to establish segment polarity; downstream of the segmentation oscillator, and upstream of the Eph/ephrin signaling system.
In this paper, we start by confirming that the paraxial mesoderm of fss/tbx24 mutants is not truly caudalized, as was previously thought, because, like ephrinA1 (Barrios et al., 2003), the caudal half-segment marker gene notch5 (Westin and Lardelli, 1997) is severely down-regulated in the segment polarity zone and somites. We have used cell transplantation together with confocal microscopy and fluorescent gene probes to better characterize the fss/tbx24 phenotype. We show by analysis of mosaic embryos that fss/tbx24 is responsible for acquisition of rostral half-segment identity in a cell autonomous manner, and that notch5 expression can be induced in neighboring fss/tbx24 host cells by wild-type grafts. Thus, our results are the first evidence of inductive patterning during the generation of segment polarity in the zebrafish, and lead us to propose an additional step in segment polarity in the zebrafish, in which a ground state of partial caudal identity in the PSM must be further patterned by rostral cells in order for a complete caudal cell state, and thus complete segment polarity, to be established.
Materials and methods
Maintenance of fish and mutant strains
Zebrafish were maintained according to standard conditions (http://www.zfin.org) on a 14-h light, 10-h dark cycle. Embryos were collected by natural spawning, raised at 28.5°C and staged according to Kimmel et al. (1995). Mutant allele used was fused somites/tbx24 (fsste314a), first described by van Eeden et al. (1996).
Cell transplantation
Single and double blastoderm cell transplantations were carried out according to Ho and Kane (1990). Briefly, donor embryos were labeled at the one- or two-cell stage with 5% fluorescein-labeled 40 kDa fixable dextran (Molecular Probes, Oregon), and grown to sphere stage, whereupon a forged micropipette was used to remove cells from a donor embryo, and place varying numbers of cells at the margin of an unlabelled host embryo. The resulting chimeric embryo was grown until segmentation stages and the location of labeled donor cells within the paraxial mesoderm, as well as the development of morphologically distinct boundaries was monitored under a fluorescent dissection microscope (Leica, New York). Embryos with features of interest were mounted in 3% methylcellulose and examined at higher magnification with a Zeiss Axioscop. Images were captured using a Nikon D1 digital SLR, and stored as Adobe Photoshop files for manipulation and analysis. Embryos were fixed in 4% paraformaldehyde and used in subsequent in situ hybridization steps.
In situ hybridization and microscopy
In situ hybridization was according to Prince et al. (1998) with modifications according to Oates et al. (2000). Probes to mespb, deltaC, deltaD, papc, notch5, and fgf8 have been previously described (Dornseifer et al., 1997; Furthauer et al., 1997; Oates and Ho, 2002; Westin and Lardelli, 1997; Yamamoto et al., 1998). After color development, some embryos were counterstained with 1 μg/mL Hoechst 43222 for 30 min, then washed 2× in PBT/10 mM EDTA before equilibrating in PBS/80% glycerol/10 mM EDTA. Embryos were either photographed on a Leica dissecting microscope with a Nikon D1 digital camera in whole mount, or, after deyolking, and flat mounting, photographed on a Zeiss Axiophot with a Nikon D1 digital camera. After transplantation and in situ hybridization, all embryos were examined and the position of transplanted cells and gene expression were recorded by confocal microscopy on a Zeiss Axiovert 100 M LSM510. Images were imported into Adobe Photoshop and adjusted for contrast in parallel before building into figures.
Results
Segment polarity defects in presomitic mesoderm of fused somites/tbx24 mutant embryos
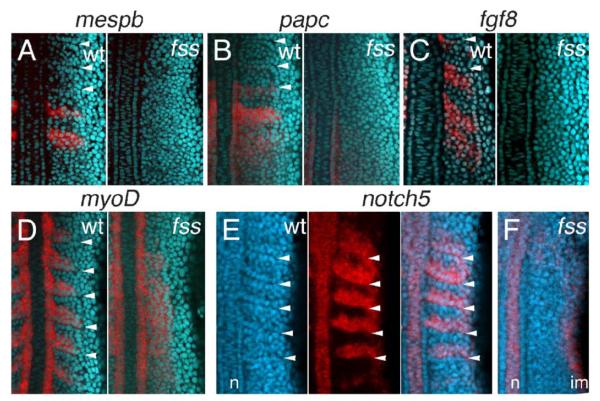
Previous reports have shown that all genetic markers of rostral segmental polarity that have been examined are absent or severely down-regulated in presomitic mesoderm (PSM) and somites of fss/tbx24 mutant embryos, whereas markers of caudal polarity are ubiquitously expressed (Durbin et al., 2000; Sawada et al., 2000; van Eeden et al., 1996). For example, previously examined rostral markers papc, mespb, and fgf8, which will be used below to assay transplantation experiments, are severely or completely down-regulated in fss/tbx24 mutants (Figs. 1A, B, C), whereas caudal marker myoD is expressed widely throughout the paraxial mesoderm (PM) (Fig. 1D). We have extended these results to include 3 additional markers of rostral (artl, tbx18, and robo2) and 4 of caudal identity (fgfr4, uncx4, slit2b, and fkh6) that are consistent with a caudalized state in fss/tbx24 (data not shown). Barrios et al. (2003) previously saw down-regulation of the caudal marker ephrinA1 in fss/tbx24 PM. Strikingly, we now find that notch5 (Westin and Lardelli, 1997), which in wild-type embryos is expressed in the caudal halves of the formed somites and in presumptive segments of the segment polarity zone (Fig. 1E, arrowheads), is also absent from the PM of fss/tbx24 mutant embryos during early segmentation (Fig. 1F). Thus, the PM of fss/tbx24 mutants is not completely caudalized, as was previously thought (Durbin et al., 2000), indicating that fss/tbx24 function is required for some aspects of caudal, as well as rostral identity. To determine the role of fss/tbx24 in the generation of rostral and caudal identities, we used cell transplantation to create genetic mosaics between wild-type and fss/tbx24 cells in an attempt to recreate the generation of segment polarity lacking in the mutant.
Fig. 1.
Gene expression defects in the paraxial mesoderm of fused somites/tbx24 mutant embryos. Expression of (A) mespb, (B) papc, (C) fgf8, and (D) myoD mRNA in paraxial mesoderm and tailbud of wild-type (left panel) and fss/tbx24 mutant (right panel) embryos at 5–6 (A, B) and 10 (C, D) somite stages. Embryos are deyolked and flat mounted with anterior up. Arrowheads indicate somitic boundaries. (E–F) Comparison of notch5 expression in wild type and fss/tbx24 backgrounds at the 5 somite stage. (E) Expression of notch5 mRNA in caudal half of PM segments. From left to right, panels show cell nuclei, notch5 expression, and a merge of the two. (F) Paraxial notch5 expression is absent from fss/tbx24 mutant embryos, although retained in the notochord and intermediate mesoderm.
Morphological furrow formation in fused somites/tbx24 mutant hosts is rescued by high density of wild-type cells
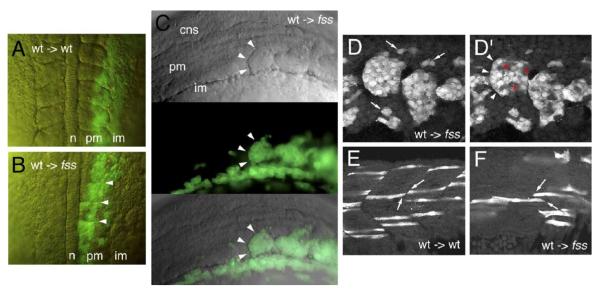
Using pre-gastrula cell transplantation (Ho and Kane, 1990), we found that transplantation of varying numbers (10–100) of wild-type donor cells into the blastoderm margin of a wild-type host gave rise to regions of low and high cell density within the PSM and PM, and did not disrupt somitogenesis (Fig. 2A). Under these conditions in 15/32 fss/tbx24 hosts, wild-type cells at high densities tended to compact together in the PSM, and were able to cause local formation of furrows, both within the wild-type donor clones, and between the edge of a wild-type clone and neighboring fss/tbx24 cells (arrowheads, Fig. 2B). In doing so, the wild-type cells formed varying length rows of somite-like blocks, each with a sharp rostral border (arrowheads, Fig. 2C). The internal organization of 5 of these donor cell blocks, or clusters in the host embryos was investigated with confocal microscopy. Figs. 2D and D’ show neighboring confocal sections through two such groups. The lateral surfaces of the clusters were completely donor derived (Fig. 2D), as were the internal rostral borders (arrowheads, Fig. 2D’), whereas the cells in the caudal half of the cluster interdigitated with their fss/tbx24 host neighbors (asterisks). This arrangement indicates that the clusters were polarized across their rostral–caudal axes. Wild-type donor cells that were scattered at low density were not associated with furrow formation and remained indistinguishable from their fss/tbx24 host neighbors by morphology, both during the time of somitogenesis (arrows, Fig. 2D), and later after differentiation into muscle fibers (Fig. 2F). Control wild-type donor cells always aligned with endogenous boundaries in wild-type hosts (Fig. 1E). Thus, transplantation of wild-type cells into fss/tbx24 hosts recapitulates the overt morphological features of wild-type somitogenesis, and we next examined whether aspects of segment polarity were associated with these wild-type donor cells, or the surrounding fss/tbx24 host cells.
Fig. 2.
Rescue of morphological boundary formation by wild-type cells in fused somites/tbx24 mutant hosts. Formation of morphological boundaries in PM of fss/tbx24 host embryos after transplantation of wild-type cells (green), shown in live embryos: dorsal view in panels A–B, anterior up; lateral views in panels C–F, anterior left. (A) Normal segmentation in 6-somite stage wild-type embryo after transplantation of wild-type cells into PM. (B) Morphological boundary formation (arrowheads) in sibling fss/tbx24 host associated with wild-type donor cell clusters. (C) Appearance of somite-like wild-type donor cell clusters at A/P level of somite 6 in paraxial mesoderm of fss/tbx24 host at 12-somite stage, showing strong boundary morphology (arrowheads). Top panel is DIC image, middle panel is fluorescent image of green transplanted wild-type cells and bottom panel is a merge. (D–F) Confocal sections through PM of wild-type (E) and fss/tbx24 (D, D’, F) embryos at 24 hpf. (D, D’) Arrangement of wild-type cells at high-density forming compact cell clusters in fss/tbx24 host embryos. (D) Section through lateral surface of cluster. Arrangement of wild-type cells at low density is indicated with arrows. (D’) More medial section through center of cluster, showing distinctive rostral morphological boundary (arrowheads), and interdigitation of wild-type with fss/tbx24 cells on caudal side of cluster (asterisks). (E) Ends of wild-type muscle fibers at low density align to segmental boundaries in the trunk of wild-type hosts (arrows). (F) Ends of wild-type muscle fibers at low density do not align in the trunk PM of fss/tbx24 host embryos (arrows). cns = central nervous system, pm = paraxial mesoderm, im = intermediate mesoderm, n = notochord.
Wild-type cells autonomously express rostral polarity markers in fused somites/tbx24 mutant hosts
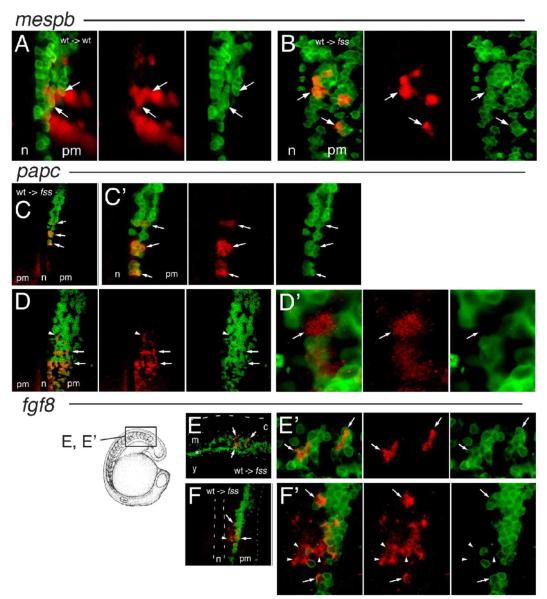
To determine whether fss/tbx24 function in wild-type donor cells was sufficient for adoption of rostral segment polarity fate in fss/tbx24 mutant host embryos, we assayed the expression of a number of rostral marker genes after transplanting fluorescently labeled wild-type cells into fss/tbx24 host embryos. A series of preliminary experiments using non-fluorescent detection of gene expression (NBT/BCIP, DAB) and DIC microscopy yielded data of insufficient accuracy to unambiguously determine which cells expressed a given gene (data not shown). However, using confocal microscopy to detect fluorescent labeling of both donor cells and expression of target genes enabled this distinction to be made at cellular resolution (Table 1). We first examined the ability of wild-type cells located in the fss/tbx24 mutant PSM to express the mespb gene. mespb is normally a marker of rostral segment polarity in the two presumptive segments in the segment polarity zone, and in the most recently formed somite (Fig. 1A) (Durbin et al., 2000; Sawada et al., 2000). Wild-type cells placed into wild-type hosts expressed mespb normally after transplantation (Fig. 3A). However, in fss/tbx24 hosts that possessed wild-type grafts at the same A/P level as the segment polarity zone, and so occupying the normal territory of mespb expression, we found examples of autonomous mespb expression only in the wild-type donor cells, and never in the mutant host cells (Fig. 3B, Table 1). Thus, fss/tbx24 function appears to be sufficient for autonomous mespb expression in the wild-type donor cells, but not sufficient to induce expression in neighboring fss/tbx24 cells.
Table 1.
Segmentation gene expression in wild-type–fss/tbx24 mosaic experiments
| Marker | n embryosa | n fss/tbx24b | Location of donor cellsc |
Location of expressiond |
||
|---|---|---|---|---|---|---|
| n somitic PM | n PSM | n donor (%) | n host (%) | |||
| papc | 45 | 25 | 17e | - | 0 | 0 |
| - | 21 | 21 (100) | 2 (9) | |||
| mespb | 51 | 27 | 16e | - | 0 | 0 |
| - | 10 | 3 (30) | 0 | |||
| fgf8 | 56 | 28 | 15 | - | 0 | 0 |
| - | 9 | 4 (44) | 1 (11) | |||
| notch5 | 128 | 72 | 47 | - | 24 (51) | 23 (49) |
| - | 19 | 3 (16) | 1 (5) | |||
Number of host embryos of all genotypes that received transplants.
Number of fss/tbx24 host embryos.
Number of embryos with donor wild-type cells in either more mature (the equivalent of somitic level) PM, or in PSM. Both situations can occur in a given embryo.
Location of gene expression with respect to wild-type donor or fss/tbx24 host cells. Percentage given is relative to number of embryos with wild-type donor cells in indicated area of PM (n somitic PM or n PSM).
Gene is not normally expressed in this region.
Fig. 3.
Expression of the rostral segment polarity marker genes mespb, papc, and fgf8 by wild-type cells in fused somites/tbx24 hosts. (A–B) Expression of mespb mRNA (red) in confocal sections of the right-hand side of the PM at the A/P level of the segment polarity zone in 8-somite stage embryos containing transplanted wild-type cells (green). (A) Normal mespb expression in wild-type embryos, arrows indicate transplanted cells expressing mespb. (B) Cell-autonomous mespb expression in wild-type donor cells in fss/tbx24 host PSM (arrows). (C–D’) Expression of papc mRNA (red) in confocal sections of the paraxial mesoderm at an A/P level spanning the segment polarity zone in 8-somite stage fss/tbx24 mutant host embryos containing transplanted wild-type cells (green). (C) papc expression associated with small wild-type donor cell clusters (arrows), in contrast to absence of papc on contralateral side. (C’) Higher magnification of C showing papc expression only in wild-type donor cells (arrows). (D) Striped expression of papc in large, high-density clone of wild-type cells (arrows) and location of papc expression in host cell (arrowhead). (D’) Higher magnification of region indicated by arrowhead in D showing papc expression in fss/tbx24 host cell (arrow). (E–F’) Expression of fgf8 mRNA (red) in confocal sections of the paraxial mesoderm at an A/P level spanning the segment polarity zone in an 18-somite (lateral view E, E’) and 6-somite stage (dorsal view F, F’) fss/tbx24 mutant host embryos containing transplanted wild-type cells (green). Location of E, E’ shown in diagrammatic form. (E) fgf8 expression in wild-type cells (arrows), dashed line indicates the dorsal extent of the embryo, the dotted lines delimit the paraxial mesoderm, and the asterisk marks the intermediate mesoderm. (E’) Higher magnification of fgf8 expressing region in panel C, arrows mark fgf8-positive wild-type donor cells. (F) Expression of fgf8 associated with wild-type donor cells in paraxial mesoderm, notochord delineated with dashed line and position of the lateral edge of embryo with a dotted line. (F’) Higher magnification of F, showing fgf8 expression in wild-type donor (arrows) and fss/tbx24 mutant host cells (arrowheads). c = central nervous system, m = paraxial mesoderm, y = yolk, n = notochord, pm = paraxial mesoderm.
Expression of the cell adhesion gene papc is normally found in adaxial cells, in the rostral portions of two presumptive somites in the segment polarity zone, and along the rostral border of the most recently formed somite, but is absent from the segment polarity zone and more mature PM of fss/tbx24 mutants (Fig. 1B; Yamamoto et al., 1998). Because fss/tbx24 mutants retain papc expression in the adaxial cells, it is straightforward to determine the approximate A/P position of wild-type donor cells. We found that wild-type cells expressed papc readily in the segment polarity zone of the fss/tbx24 host, often in a striped pattern (arrows, Figs. 3C, D, Table 1). These stripes varied in their mediolateral width depending on the extent of the wild-type donor-derived clone and appeared remarkably well spaced along the A/P axis. Importantly, papc expression was restricted to the wild-type donor cells (Fig. 3C’, n = 21 hosts), indicating that fss/tbx24 is required cell-autonomously for papc expression. Indeed, even a single, isolated wild-type cell was capable of expressing papc (data not shown). However, in two cases, expression of papc was observed in a single fss/tbx24 host cell neighboring large wild-type grafts (Figs. 3D, D’, arrow in D’).
We also examined the expression of fgf8 in this assay (Figs. 3E–F’, Table 1), which in wild-type embryos is expressed in two broad stripes in the segment polarity zone, and subsequently in the rostral half of every somite (Fig. 1C; Furthauer et al., 1997). We observed instances of strictly donor cell autonomous expression in the posterior of the axis (Figs. 3E, E’), as well as infrequent expression of fgf8 in neighboring fss/tbx24 host cells (Figs. 3F, F’). Combined, the above results indicate that fss/tbx24 function is sufficient for expression of markers of rostral identity in the same cell, consistent with a cell-autonomous requirement. However, the striped papc expression patterns also indicate that some form of segment polarity can be generated within the larger clones of wild-type donor cells in an fss/tbx24 environment. Although nearly all cells expressing rostral markers in these experiments were wild type in origin, the existence of a few fss/tbx24 host cells able to express papc and fgf8 indicates that intercellular communication can overcome the fss/tbx24 block in some circumstances.
Wild-type cells at high density induce neighboring fused somites/tbx24 cells to express missing caudal polarity gene
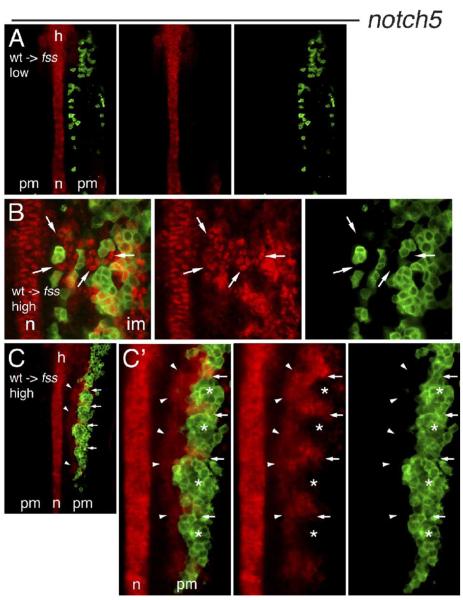
Given that the notch5 caudal polarity marker gene is not expressed in the fss/tbx24 PM (Fig. 1F), we next examined whether wild-type cells transplanted into a fss/tbx24 host could assume a complete caudal segment polarity state that included notch5 expression, and whether they could induce this state in neighboring fss/tbx24 host cells. In mutant embryos containing a low density of donor cells in the PM, we did not detect expression of notch5 in either donor or host cells (Fig. 4A). However, in fss/tbx24 embryos in which wild-type donor cell density was high, we observed strong notch5 expression in both host and donor cells, often in a series of stripes associated with the donor cells (Figs. 4B, C, Table 1). Examination of these embryos revealed that within the donor cell clusters themselves, notch5 expression was high in the caudal region and excluded from the rostral half (asterisks, Fig. 4C’). This indicates that wild-type cell clusters establish correct rostro-caudal polarity, recapitulating another aspect of normal somitogenesis. Strikingly, notch5 was also expressed in surrounding mutant host cells at a distance of up to 3 cell diameters from the wild-type donors (Figs. 4B arrows, C, C’ arrowheads), indicating that wild-type cells can induce their mutant fss/tbx24 neighbors to express a marker of caudal segment polarity normally missing in this genetic background. The induced expression of notch5 in fss/tbx24 cells did not always have a clearly striped pattern (e.g., Fig. 4C’), most likely because of close proximity (3 cell diameters) to wild-type cells and an inability of fss/tbx24 cells to actively repress caudal markers (see Discussion). This result demonstrates that PSM cells do not themselves require fss/tbx24 for direct expression of notch5, but rather for production of a signal that induces the expression of notch5 in neighboring cells.
Fig. 4.
Induction of notch5 expression in fss/tbx24 host cells by wild-type neighbors. Expression of notch5 mRNA (red) in confocal sections of the PM of 5–6 somite stage fss/tbx24 host embryos containing transplanted cells (green) from wild-type donors. Embryos are flat mounted, anterior up. (A) notch5 is not expressed in the PM of fss/tbx24 host embryo with low density of wild-type donor cells. (B) High-magnification view of PM of fss/tbx24 host embryo containing high density of wild-type donor cells. Autonomous notch5 expression is seen in wild-type cells, and notch5 induction in numerous fss/tbx24 host cells (arrows) up to three cell diameters from the wild-type cell clones. (C) Periodic stripes of notch5 expression in fss/tbx24 host embryo containing 5 somite-like clusters of wild-type donor cells in the right-hand PM. Contralateral side does not express notch5. Arrows indicate the boundaries between clusters, arrowheads mark fss/tbx24 host PM cells expressing notch5.(C’) High magnification of the region of notch5 expression. Arrows and arrowheads as in panel C, asterisks mark the rostral half of cell clusters. Note high-level notch5 expression in the caudal part of each somite-like cluster. n = notochord, h = hindbrain, pm = paraxial mesoderm, im = intermediate mesoderm.
Discussion
In this report, we address the role of the T-box gene fused somites/tbx24 in the generation of segment polarity in the paraxial mesoderm of the zebrafish embryo. Using cell transplantation between wild-type and mutant embryos combined with cellular-resolution analysis of gene expression, we present evidence that fss/tbx24 is required cell-autonomously for the expression of rostral segment identity. Further, we have uncovered a novel inductive event producing complete caudal half-segment identity, which requires fss/tbx24 in the sending cells. This induction likely takes place temporally downstream of the Delta/Notch-dependent somitogenesis oscillator, and upstream of the Eph/ephrin-mediated production of epithelialized somite boundaries.
Autonomy of fused somites/tbx24 action
Since in fss/tbx24 mutant embryos there is a profound loss of rostral segment identity, a primary question has been whether fss/tbx24 is responsible for this state in a cell-autonomous manner. Transplantation experiments by Durbin et al. (2000) led to the idea that the fss/tbx24 gene was acting non-cell-autonomously with respect to rostral segment polarity, since wild-type cells in fss/tbx24 hosts did not express the rostral marker fgf8. Consistent with this, fss/tbx24 cells in wild-type hosts expressed fgf8 when located in the rostral epithelial boundary of a somite, suggesting that the wild-type host environment rescues the defect caused by loss of fss/tbx24 function. In contrast, Barrios et al. (2003) have recently shown that rostral markers papc and dld are expressed within wild-type grafts in fss/tbx24 hosts, suggesting that in fact fss/tbx24 acts cell autonomously with respect to generation of rostral half-segment identity.
Our results using papc and dld (data not shown) expression as markers of rostral identity are in good accordance with those of Barrios, and in addition, we find that cell-autonomous fss/tbx24 function is sufficient for mespb and fgf8 expression. The differing results using fgf8 (Durbin et al., 2000) may be a simple consequence of our host embryos containing higher donor cell densities than previously examined. This explanation fits well with our observation of somite-like wild-type donor cell clusters with complete rostral boundaries and morphological and molecular internal polarity occurring only in fss/tbx24 hosts with high local densities of wild-type donor cells. Such structures were not seen after transplantation of wild-type cells into fss/tbx24 host embryos by Barrios et al. (2003), who found that over-expression of EphA4 or ephrinB2 was required to induce morphological boundary formation. These differences may highlight a role for a community effect in segmentation and/or somitogenesis (Buckingham, 2003; Gurdon, 1988). Indeed, the existence of a community effect modifying gene expression in the zebrafish PSM has been previously suggested by Holley et al. (2000), who showed that the autonomous dependence of her1 expression on fss/tbx24 function in wild-type cells transplanted into fss/tbx24 mutant hosts could be overcome in the reciprocal transplant when mutant donor cells were surrounded by wild-type host cells.
Genetic variation between wild-type donor strains seems unlikely to have a role in the differences between our transplants and previous studies, since we observed cluster formation from donor clones from several different wild-type laboratory and commercial lines. As we have not characterized the cell polarity or epithelial character of these cell clusters, their exact relationship to the Eph/ephrin-induced somite-like structures seen by Barrios et al. (2003) is not clear. We emphasize that even though somite-like clusters of cells were readily generated, their formation was not a prerequisite for cell-autonomous expression of rostral markers, or for the induction of notch5 in cells neighboring the graft. This observation is consistent with the findings of Barrios et al. (2003), who showed that Eph/ephrin-induced boundary morphogenesis and segment polarity can be uncoupled.
The role of mesp genes in segment polarity
The two members of the zebrafish Mesp family of bHLH genes are expressed in two or three thin stripes in anterior PSM, where they become restricted to the rostral-most cells of the prospective somites, suggesting that they may play an important role in establishing segment polarity (Durbin et al., 2000; Sawada et al., 2000). Mutation in the mouse Mesp2 gene results in a loss of rostral half-segment identity (Saga et al., 1997), essentially the same phenotype as produced by the fss/tbx24 mutation, in which expression of both mespa and mespb is dramatically reduced (Durbin et al., 2000; Sawada et al., 2000). Given that mespb is sufficient to cause widespread activation of rostral segment polarity markers (such as notch6, fgfrf1, and papc), at the expense of caudal markers (such as myoD and notch5)in over-expression experiments (Sawada et al., 2000), it has been proposed that the fss/tbx24 phenotype is due in large part to the failure to express mespb (Sawada et al., 2000). The relationship between mespb function and furrow formation is not yet clear, however, as over-expression of mespb does not rescue this aspect of the fss/tbx24 phenotype (Holley and Takeda, 2002; AO, unpublished). The cell-autonomous expression of mespb in grafted wild-type cells is likely an important feature of our assay system, suggesting that mespb is in fact a direct target of the Fss/Tbx24 transcription factor. Thus, donor wild-type cells in the PSM of an fss/tbx24 host expressing mespb may be positioned at the top of a regulatory cascade that leads to the adoption of the rostral identity, and repression of the caudal state.
Inductive activities of wild-type cells
Our demonstration that fss/tbx24 mutant embryos are missing notch5 PSM expression, and therefore are not fully caudalized, raises the question of the role of fss/tbx24 in the generation of caudal identity. The caudal marker ephrinA1 was recently shown to be absent from the PM of fss/tbx24 mutant embryos (Barrios et al., 2003), indicating that notch5 is not the only missing caudal marker gene, and that the deficiency in caudal identity could be more severe than previously appreciated. Whether ephrinA1 is responsive to the community effect-derived signal responsible for notch5 induction or whether it is controlled by some other activity is currently under investigation. At present, we do not know the function of notch5 itself in segment polarity, although over-expression of an activated form of the notch5 receptor (ICD) disrupts somitogenesis (AO, unpublished), suggesting that it may have an important role. Since fss/tbx24 is expressed in the segment polarity zone in both rostral and caudal halves of prospective somites (Nikaido et al., 2002), the failure to express notch5 in the caudal half could be in principle a result of the lack of a direct activation by fss/tbx24 in these cells. We show, however, that the fss/tbx24 gene is not required in PSM cells for notch5 expression, and that it is sufficient for fss/tbx24 to be present in a nearby cell. This non-autonomy of fss/tbx24 function indicates the existence of an fss/tbx24-dependent signal or interaction that is capable of inducing notch5 expression. Mutant fss/tbx24 PSM cells must therefore express the receptor and signal transduction proteins for this signal. These results imply that in the wild-type zebrafish, generation of complete segment polarity involves at least one inductive step.
The pattern of induced notch5 expression in the fss/tbx24 host was not always segmentally arranged, although autonomous notch5 expression in donor cell clusters often was (for example, Fig. 4). In these cases, a continuous band of notch5-expressing cells was found immediately medial or lateral to the wild-type donor cells. This is most likely a consequence of the three-cell diameter inductive range of the signal, and the mediolateral position of the wild-type donor clone. In wild-type embryos, notch5-inductive signals would be released from cells within a domain of rostral identity that spans the mediolateral extent of the PSM. Therefore, target cells of partial caudal identity would not normally be available laterally. The striped pattern of notch5 expression exhibited in wild-type embryos and wild-type cell clusters would be generated despite the inductive signal because rostral cells would themselves inhibit notch5 expression through a mesp-dependent mechanism (Sawada et al., 2000). It is important to note that the presence and details of the notch5-inductive events were only apparent when a cellular-level resolution was obtained using a combination of fluorescent gene expression detection and confocal microscopy.
Nature of the inductive signal
The spatial distribution of induced notch5 expression may reveal some properties of the signaling process itself. Since notch5 can be induced at a distance of three cell diameters, one hypothesis is that an inducing molecule released in the segment polarity zone is active only over short ranges. Instability or binding to extracellular matrix components might restrict the range of a diffusible molecule. A cell-contact-dependent signal could also act in a relay, and thus spread the induction of notch5 to a distance of three cell diameters. Alternatively, if a cell–cell signal was delivered in the oscillatory zone, potential cell mixing occurring during the transit of cells through the PSM could scatter notch5-expressing cells that had been in direct contact with the wild-type donor clusters at some earlier point, giving the appearance of a three-cell range. A large number of signaling molecules from different families are expressed in the PSM of zebrafish, including members of the Fgf, Delta, and Notch families. In chick and mouse embryos, inductive activity of Delta/Notch signaling is thought to mediate some aspects of segment polarity (Sato et al., 2002; Takahashi et al., 2000, 2003). In zebrafish, loss of fgf8 function in the acerebellar mutant gives a somitogenic phenotype, but without strong segment polarity defects (Reifers et al., 1998), although functional redundancy with other fgf genes (Reifers et al., 2000) may mask an effect. Clearly, direct functional tests must be made in zebrafish before conclusions can be drawn.
3-step model for sequential generation of segment polarity
The current hypothesis for the generation of segment polarity in zebrafish PM can be termed the “two-step” model. In the first step, PM is produced through gastrulation with a default and complete caudal state (Durbin et al., 2000). The next step requires action of the fss/tbx24 gene, which produces regions of rostral identity from within the field of caudal cells (Durbin et al., 2000; Sawada et al., 2000). The spatial patterning information for this step may derive from the site of arrest of the segmentation oscillator in the segment polarity zone (Henry et al., 2002; Holley et al., 2000, 2002; Oates and Ho, 2002; Sawada et al., 2001). Subsequently, morphological inter-somitic furrows are developed from the juxtaposition of cells with rostral and caudal identity using Eph/ephrin signaling (Barrios et al., 2003; Durbin et al., 1998, 2000). Our findings now indicate that this model needs revision. In the first step, the default state of PM produced by gastrulation is an incomplete or partial caudal identity, as shown by the absence of notch5 expression from the PM of fss/tbx24 mutant embryos. In the second step, the production of rostral identity is effected by fss/tbx24 in an almost entirely cell-autonomous manner. We now add a third step in which notch5 is induced in the caudal region of the forming somite by fss/tbx24-expressing cells of the neighboring rostral half-segment, thus completing segment polarization.
Acknowledgments
The authors thank Claudia Mueller for expert technical assistance, Tracy Roskoph, Jennifer Wiseman, Devon Mann, JoAnn Jonie Garcia, Jana Stolyichnaya, and Rebecca Bielang and the MPI-CBG fish facility for excellent fish care, Flavia Lega for help with in situ hybridization, Carl-Philipp Heisenberg, Ashley Bruce, and Girish Deshpande for insightful discussion and the members of the Oates and Ho labs for their technical advice and support. This work was funded by a Ludwig Institute for Cancer Research Postdoctoral Fellowship to ACO, and by NIH RO1 GM067714, MOD 1-FY01-623 to RKH.
References
- Aoyama H, Asamoto K. Determination of somite cells: independence of cell differentiation and morphogenesis. Development. 1988;104:15–28. doi: 10.1242/dev.104.1.15. [DOI] [PubMed] [Google Scholar]
- Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, Herrmann BG. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev. Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr. Biol. 2003;13:1571–1582. doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Buckingham M. How the community effect orchestrates muscle differentiation. BioEssays. 2003;25:13–16. doi: 10.1002/bies.10221. [DOI] [PubMed] [Google Scholar]
- Dornseifer P, Takke C, Campos-Ortega JA. Overexpression of a zebrafish homologue of the Drosophila neurogenic gene Delta perturbs differentiation of primary neurons and somite development. Mech. Dev. 1997;63:159–171. doi: 10.1016/s0925-4773(97)00037-3. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, McGrew MJ, Pourquie O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell. 2001;106:219–232. doi: 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- Durbin L, Brennan C, Shiomi K, Cooke J, Barrios A, Shanmugalingam S, Guthrie B, Lindberg R, Holder N. Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 1998;12:3096–3109. doi: 10.1101/gad.12.19.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin L, Sordino P, Barrios A, Gering M, Thisse C, Thisse B, Brennan C, Green A, Wilson S, Holder N. Anteroposterior patterning is required within segments for somite boundary formation in developing zebrafish. Development. 2000;127:1703–1713. doi: 10.1242/dev.127.8.1703. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Thisse C, Thisse B. A role for FGF-8 in the dorsoventral patterning of the zebrafish gastrula. Development. 1997;124:4253–4264. doi: 10.1242/dev.124.21.4253. [DOI] [PubMed] [Google Scholar]
- Gajewski M, Sieger D, Alt B, Leve C, Hans S, Wolff C, Rohr KB, Tautz D. Anterior and posterior waves of cyclic her1 gene expression are differentially regulated in the presomitic mesoderm of zebrafish. Development. 2003;130:4269–4278. doi: 10.1242/dev.00627. [DOI] [PubMed] [Google Scholar]
- Gurdon JB. A community effect in animal development. Nature. 1988;336:772–774. doi: 10.1038/336772a0. [DOI] [PubMed] [Google Scholar]
- Henry CA, Urban MK, Dill KK, Merlie JP, Page MF, Kimmel CB, Amacher SL. Two linked hairy/Enhancer of split-related zebrafish genes, her1 and her7, function together to refine alternating somite boundaries. Development. 2002;129:3693–3704. doi: 10.1242/dev.129.15.3693. [DOI] [PubMed] [Google Scholar]
- Ho RK, Kane DA. Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature. 1990;348:728–730. doi: 10.1038/348728a0. [DOI] [PubMed] [Google Scholar]
- Holley SA, Takeda H. Catching a wave: the oscillator and wavefront that create the zebrafish somite. Semin. Cell Dev. Biol. 2002;13:481–488. doi: 10.1016/s1084952102001015. [DOI] [PubMed] [Google Scholar]
- Holley SA, Geisler R, Nusslein-Volhard C. Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity. Genes Dev. 2000;14:1678–1690. [PMC free article] [PubMed] [Google Scholar]
- Holley SA, Julich D, Rauch GJ, Geisler R, Nusslein-Volhard C. her1 and the notch pathway function within the oscillator mechanism that regulates zebrafish somitogenesis. Development. 2002;129:1175–1183. doi: 10.1242/dev.129.5.1175. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Aerne BL, Smithers L, Haddon C, Ish-Horowicz D, Lewis J. Notch signalling and the synchronization of the somite segmentation clock. Nature. 2000;408:475–479. doi: 10.1038/35044091. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Kawakami A, Sawada A, Furutani-Seiki M, Takeda H, Araki K. Tbx24, encoding a T-box protein, is mutated in the zebrafish somite-segmentation mutant fused somites. Nat. Genet. 2002;31:195–199. doi: 10.1038/ng899. [DOI] [PubMed] [Google Scholar]
- Oates AC, Ho RK. Hairy/E(spl)-related (Her) genes are central components of the segmentation oscillator and display redundancy with the Delta/Notch signaling pathway in the formation of anterior segmental boundaries in the zebrafish. Development. 2002;129:2929–2946. doi: 10.1242/dev.129.12.2929. [DOI] [PubMed] [Google Scholar]
- Oates AC, Bruce AE, Ho RK. Too much interference: injection of double-stranded RNA has nonspecific effects in the zebrafish embryo. Dev. Biol. 2000;224:20–28. doi: 10.1006/dbio.2000.9761. [DOI] [PubMed] [Google Scholar]
- Pourquie O. Vertebrate somitogenesis. Annu. Rev. Cell Dev. Biol. 2001;17:311–350. doi: 10.1146/annurev.cellbio.17.1.311. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Tam PP. A nomenclature for prospective somites and phases of cyclic gene expression in the presomitic mesoderm. Dev. Cell. 2001;1:619–620. doi: 10.1016/s1534-5807(01)00082-x. [DOI] [PubMed] [Google Scholar]
- Prince VE, Joly L, Ekker M, Ho RK. Zebrafish hox genes: genomic organization and modified colinear expression patterns in the trunk. Development. 1998;125:407–420. doi: 10.1242/dev.125.3.407. [DOI] [PubMed] [Google Scholar]
- Prince VE, Holley SA, Bally-Cuif L, Prabhakaran B, Oates AC, Ho RK, Vogt TF. Zebrafish lunatic fringe demarcates segmental boundaries. Mech. Dev. 2001;105:175–180. doi: 10.1016/s0925-4773(01)00398-7. [DOI] [PubMed] [Google Scholar]
- Reifers F, Bohli H, Walsh EC, Crossley PH, Stainier DY, Brand M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain– hindbrain boundary development and somitogenesis. Development. 1998;125:2381–2395. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- Reifers F, Adams J, Mason IJ, Schulte-Merker S, Brand M. Overlapping and distinct functions provided by fgf17, a new zebrafish member of the Fgf8/17/18 subgroup of Fgfs. Mech. Dev. 2000;99:39–49. doi: 10.1016/s0925-4773(00)00475-5. [DOI] [PubMed] [Google Scholar]
- Rida PC, Le Minh N, Jiang YJ. A Notch feeling of somite segmentation and beyond. Dev. Biol. 2004;265:2–22. doi: 10.1016/j.ydbio.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Saga Y, Hata N, Koseki H, Taketo MM. Mesp2: a novel mouse gene expressed in the presegmented mesoderm and essential for segmentation initiation. Genes Dev. 1997;11:1827–1839. doi: 10.1101/gad.11.14.1827. [DOI] [PubMed] [Google Scholar]
- Sato Y, Yasuda K, Takahashi Y. Morphological boundary forms by a novel inductive event mediated by Lunatic fringe and Notch during somitic segmentation. Development. 2002;129:3633–3644. doi: 10.1242/dev.129.15.3633. [DOI] [PubMed] [Google Scholar]
- Sawada A, Fritz A, Jiang Y, Yamamoto A, Yamasu K, Kuroiwa A, Saga Y, Takeda H. Zebrafish Mesp family genes, mesp-a and mesp-b are segmentally expressed in the presomitic mesoderm, and mesp-b confers the anterior identity to the developing somites. Development. 2000;127:1691–1702. doi: 10.1242/dev.127.8.1691. [DOI] [PubMed] [Google Scholar]
- Sawada A, Shinya M, Jiang YJ, Kawakami A, Kuroiwa A, Takeda H. Fgf/MAPK signalling is a crucial positional cue in somite boundary formation. Development. 2001;128:4873–4880. doi: 10.1242/dev.128.23.4873. [DOI] [PubMed] [Google Scholar]
- Stern CD, Keynes RJ. Interactions between somite cells: the formation and maintenance of segment boundaries in the chick embryo. Development. 1987;99:261–272. doi: 10.1242/dev.99.2.261. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Koizumi K, Takagi A, Kitajima S, Inoue T, Koseki H, Saga Y. Mesp2 initiates somite segmentation through the Notch signalling pathway. Nat. Genet. 2000;25:390–396. doi: 10.1038/78062. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Inoue T, Gossler A, Saga Y. Feedback loops comprising Dll1, Dll3 and Mesp2, and differential involvement of Psen1 are essential for rostrocaudal patterning of somites. Development. 2003;130:4259–4268. doi: 10.1242/dev.00629. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Granato M, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, et al. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–164. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Holley SA, Haffter P, Nusslein-Volhard C. Zebrafish segmentation and pair-rule patterning. Dev. Genet. 1998;23:65–76. doi: 10.1002/(SICI)1520-6408(1998)23:1<65::AID-DVG7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Weinmaster G, Kintner C. Modulation of notch signaling during somitogenesis. Annu. Rev. Cell Dev. Biol. 2003;19:367–395. doi: 10.1146/annurev.cellbio.19.111301.115434. [DOI] [PubMed] [Google Scholar]
- Westin J, Lardelli M. Three novel Notch genes in zebrafish: implications for vertebrate Notch gene evolution and function. Dev. Genes Evol. 1997;207:51–63. doi: 10.1007/s004270050091. [DOI] [PubMed] [Google Scholar]
- Xu Q, Holder N, Patient R, Wilson SW. Spatially regulated expression of three receptor tyrosine kinase genes during gastrulation in the zebrafish. Development. 1994;120:287–299. doi: 10.1242/dev.120.2.287. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Amacher SL, Kim SH, Geissert D, Kimmel CB, De Robertis EM. Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm. Development. 1998;125:3389–3397. doi: 10.1242/dev.125.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]