Abstract
The relationship between corticosteroids and osteonecrosis is well known. Limited data suggest that statins modulate cholesterol metabolism and may protect against osteonecrosis. We analyzed our prospective renal transplant database to determine if statin usage reduces the incidence of corticosteroid-related osteonecrosis. We identified 2,881 renal transplantation patients who met our entry criteria. Among 338 patients on statins, 15 (4.4%) developed osteonecrosis versus 180 of 2543 (7%) patients who were not on statins. Osteonecrosis-free survival was similar in patients with and without statin exposure.
Introduction
Osteonecrosis (ON) is a disabling disease and its pathogenesis is associated with corticosteroid exposure, ethanol usage, coagulapathies, and lupus erythematosus. It is an important orthopaedic complication of corticosteroid immunosuppression after solid organ transplantation occurring with a frequency of 3 to 41%.[1-3] Abnormalities of lipid metabolism, fat overload, and intraosseous hypertension have been cited frequently as important in the development of ON.[4-6] Previous studies suggest that ON develops in 5 to 11% of organ transplant patients within 1 year after the transplant, [7-9] but it is not possible to predict which patients receiving corticosteroids will develop ON. Aside from minimizing the exposure to corticosteroids or other risk factors, there are no definite measures available to prevent ON.[10-12]
Animal and limited clinical data have suggested that statins may have a protective role against ON. Wang and other authors [13-18] have shown that corticosteroids cause the marrow pluripotent cells to differentiate into fat cells through down-regulation of Cbfa1/Runx2 gene expression and osteocalcin promoter activity while increasing the expression of adipose-specific genes 422(aP2) and PPARγ2. Statins do the opposite, by decreasing adipogenic and stimulating osteogenic differentiation through suppressing PPARγ2 and increasing Cbfa1/Runx2 expression in bone marrow mesenchymal cells.
Our goal was to determine if statin usage, in renal transplantation patients, is associated with a reduction in incidence of osteonecrosis.
Materials and Methods
Study design
Our transplant recipient data is prospectively gathered and maintained in a computer data base with the funding provided by the National Institutes of Health (NIH, Bethesda, MD). Using this database [19, 20] we retrospectively identified 3399 patients who had a renal transplant between January 1985 and December 2003. This time period was selected to allow a minimum follow-up of 3 years after the transplantation. Entry criteria were patients greater than or equal to 16 years of age, first-time renal transplants, and no prior corticosteroid exposure. Statin usage was defined as being on a statin drug at the time of transplant or initiated within 31 days after transplant and continuing for at least 1 year duration. This time period was selected based upon the finding that ON commonly develops within the first year after transplantation. [7-9] The indication for statin treatment was hypercholesterolemia. Dosages were adjusted by the treating physician until the cholesterol level was reduced to the clinically appropriate target range. The most commonly used statin drugs were Advicor® (niacin 500 mg + lovastatin 20 mg; Kos Pharmaceuticals, Inc, Cranbury, NJ), atorvastatin (Lipitor®, Pfizer Inc, New York, NY), crivastatin (Baycol®, Bayer AG, Leverkusen, Germany), rosuvastatin (Crestor®, AstraZeneca PLC, London, UK), fluvastatin (Lescol®, Novartis AG, Basel, Switzerland), lovastatin (Mevacor®, Merck and Co, Inc, Whitehouse Station, NY), pravastatin (Pravachol®, Bristol-Myers Squibb Co, New York, NY), and simvastatin (Zocor®, Merck and Co, Inc).
Power analysis
We performed a power analysis to determine what potential difference in ON-free survivorship we would have been able to detect, given the size of our patient cohort, at five and ten years after transplantation. At power = 95% and level of significance p = 0.05, our data were sufficient to detect a difference in ON-free survival, if it was present, of 3% at 5 years (statin exposure group 91% versus non-statin exposure group 94%) and 5% at 10 years (statin exposure group 88% versus non-statin exposure group 93%).
Demographics
There were 2881 patients who met the entry criteria. There were 1752 (61%) male and 1129 (39%) female patients. Among the 2881 patients, 1619 had varying levels of exposure to statins; however, only 338 patients met our on-statins definition.
There were 338 (12%) patients in the statin cohort and 2543 (88%) in the non-statin cohort (Table 1). In the statin cohort, 180 (53%) were males and 158 (47%) were females. In the non-statin cohort 1572 (62%) were males and 971 (38%) were females. The mean age of the overall patient cohort was 43 years (range, 16–77 years) and mean follow-up was 128 months (range, 36–242 months). In the statin cohort, mean age was 47 years (range 23-74) and mean follow-up 91 months (range 43-229) and in the non-statin cohort the mean age was 42 years (range 16-247) and mean follow-up 136 months (range 43-247). The most common primary diagnosis that led to end stage renal failure was diabetic nephropathy. Patients were prospectively followed by regular clinic visits. We depended upon self-reporting of fractures, ON, joint pain, or arthritis (not otherwise specified) as noted in the charts. The medical record was reviewed for any patients reporting joint pain (not otherwise specified) to verify the diagnosis and look for other possible etiologies. Data gathered consisted of name, gender, age, indication and transplant type (living twin, living non-twin, cadaver), transplant date, preemptive transplant (transplant without prior dialysis), post-dialysis transplant, number of rejection episodes, statin drug usage (yes/no), No patients were contacted specifically for this study and only chart data was reviewed.
TABLE 1. Demographics of Study Cohort.
| Variable | Value |
|---|---|
| Total number of patients | 2881 |
| Male: female | 1752 (61%):1129 (39%) |
| Age (years)* | 43 ± 12.8 (16–77) |
| Follow-up (months)* | 128 ± 57.8 (36–242) |
| Statin usage: no statin usage | 338/2881 (12%):2543/2881 (88%) |
| Total number of patients with osteonecrosis | 195 (7%) |
| Total number of sites affected | 286 |
| Femoral head | 278 |
| Other | 8 |
Values are expressed as mean ± standard deviation, with range in parentheses
Statistical analysis
Multivariate Cox regression tests [21] were used to analyze ON-free survival on statins and other variables including gender, rejection episodes, and year of transplantation. Survivorship analysis was performed using Kaplan-Meier methods with the endpoint defined as occurrence of ON. [22] Log-rank and Wilcoxon tests were performed for comparison of data between the statins vs. non statin cohort to determine whether there was a relationship between the time course for development of ON and statin usage. The data were analyzed using SAS for Windows statistical software package (Version 9.1 2003; SAS Institute Inc, Cary, NC).
Results
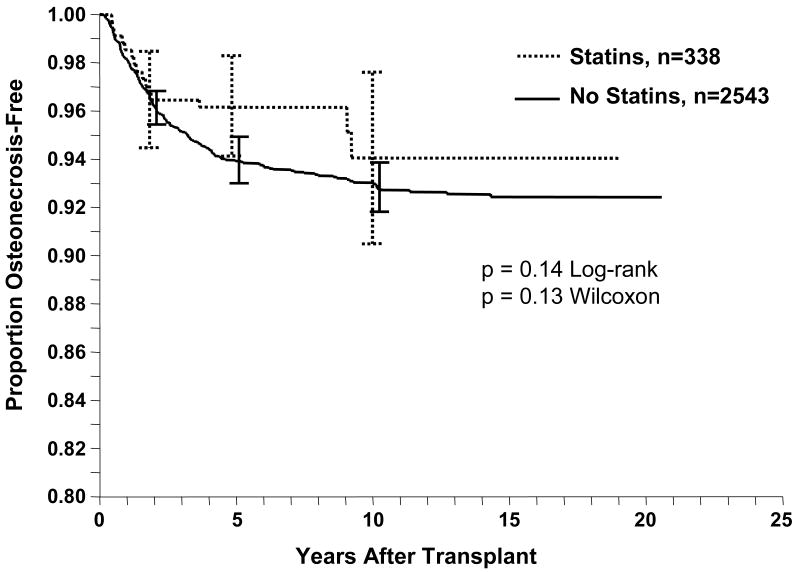
In the overall patient cohort of 2881 patients, 195 (7%) developed ON in 286 joints. In the femoral head, 96 patients developed ON unilaterally and 91 bilaterally. Eight patients had involvement of other bones. Among the 338 patients in the statin cohort, 15 patients (4.4%) developed ON at 23 sites (all involving the femoral head). In the non-statin cohort of 2543 patients, 180 patients (7%) developed ON at 263 sites (255 femoral heads and eight other sites). ON-free survival stratified by statin usage did not show a relationship between statin exposure and development of ON (p = 0.14, log-rank) (Fig 1). At 5 years, the ON-free survivorship for those patients on statins versus not on statins was 96% ± 2.1% (95% confidence interval) versus 94% ± 1.0% (95% C.I.). Cox regression revealed that statin usage did not predict (p = 0.8) ON-free survival. Other variables (Table 2) that were associated with a higher incidence of ON were (1) male gender (p = 0.008), (2) higher number of rejection episodes (p = 0.009), and (3) earlier year of transplant (p = 0.01).
Fig 1.
A univariate Kaplan-Meier life table analysis of ON-free survival of kidney transplant patients on statins identified no trend in ON risk reduction for patients taking statins (p = 0.14, log-rank). Error bars = 95% confidence intervals.
TABLE 2. Cox Multivariate Analysis Table of Predictors with Relative Risk.
| Predictor | Adjusted Relative Risk (95% Confidence Interval) |
p Value |
|---|---|---|
| Gender (as male) | 1.52 (1.11–2.09) | 0.008 |
| Number of rejection episodes | 1.17 (1.04–1.32) | 0.009 |
| Year of transplant | 0.96 (0.93–0.99) | 0.01 |
| Statin use | 1.08 (0.61–1.91) | 0.8 |
Discussion
Osteonecrosis is an important orthopaedic complication of corticosteroid immunosuppression after solid organ transplantation with poorly understood pathogenesis. Aside from minimizing the exposure to corticosteroids or other risk factors, there are no definite measures available to prevent ON. Our goal was to determine if statin usage, in renal transplantation patients, is associated with a reduction in incidence of osteonecrosis.
Prior reports suggest a relationship between statins and ON. Wang and co-authors [15, 18] have shown corticosteroids cause the marrow pluripotent cells to differentiate into fat cells through down-regulation of Cbfa1/Runx2 gene expression and osteocalcin promoter activity while increasing the expression of adipose-specific genes 422(aP2) and PPARγ2. Statins do the opposite by decreasing adipogenic and stimulating osteogenic differentiation through suppressing PPARγ2 and increasing Cbfa1/Runx2 expression in bone marrow mesenchymal cells.[13, 14, 16, 23] This may or may not be related to their known mechanism of action in lowering cholesterol via inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase. The effect of corticosteroids and statins is concentration and time dependent.[13-16, 23-26] Corticosteroids are a clear risk factor for ON, and therefore, with these animal and laboratory findings in mind, we investigated whether or not statins have a protective role against ON in humans.
Pritchett's study in 2001 [17] reported a protective role of statins against ON in humans. He reported the incidence of ON of 1% in 284 patients on statins, which appears less than the historical incidence of 5% to 11% previously reported in the literature. [7-9] He concluded statins may protect against the development of ON in patients receiving corticosteroid treatment. However, his study was retrospective with poorly controlled entry criteria. There were no controls other than historical controls. In addition, the study group was a heterogeneous group of patients with respect to corticosteroid indication.
Our large patient cohort was powered sufficiently (significance level p = 0.05, 95% power) to detect a reduction in risk as small as 3% at 5 years and 5% at 10 years in the incidences of ON. We did not identify any risk reduction, therefore, given our definition of statin exposure, if a reduction in ON incidence exists it likely is less than 5% at 10 years. We believe a reduction of risk of 5% or less is of dubious clinical importance.
The number of rejection episodes, a surrogate of peak corticosteroid exposure, was associated with the development of ON. This would be expected given the body of knowledge identifying a relationship between corticosteroids and ON. As all our patients were on corticosteroid immunosuppression, we could not study if actual corticosteroid exposure was related to ON development. However, our data demonstrated that a higher number of rejection episodes was associated with a higher incidence of ON.
Our data also suggest that male gender is associated with an increased risk (34%) of ON compared to females. To our knowledge, this has not been reported previously. The explanation for this finding is unknown yet a gender difference related to differing fat metabolism may be a factor.[27, 28]
Our study has some limitations. The methodology is suboptimal because it is not a randomized prospective study, however, the large size of the patient cohort and the statistical significance achieved support the validity of the results and conclusion. Some of the patients assigned to the non-statin group actually had some limited exposure to statins, but not enough to meet the specific defined criteria for this study. The definition for statin usage was delineated to identify those patients who were taking statins at the time of highest risk for developing osteonecrosis. Therefore, although the non-statin group is not a pure non-statin group, its composition is justified biologically. Asymptomatic patients with ON were not captured as most of the cases were self-reported and prospective MRI screening was not done routinely. Although prevalence of asymptomatic ON has been reported between 6% to 9% in various studies [29-31] and the detection of asymptomatic disease is interesting, it is unlikely to change a patient's disease management. Fortunately, asymptomatic disease tends to have a benign course in majority of patients. [29, 30] In some rare cases, spontaneous resolution has been documented. [32]
This study included only renal transplant patients so the data may or may not be applicable to patients at risk for ON for other reasons. While it would have been useful to analyze the relationship between cholesterol levels and ON we did not have sufficient data on cholesterol levels to perform such an analysis, however, dosages were modified in principle to reduce cholesterol to clinically appropriate levels. All statins may not be the same. Nevertheless, we are not aware of data to suggest that therapeutic dosages of different statins have a variable influence on the pathophysiology of ON. Finally, as all our patients were on corticosteroid immunosuppression, we could not study if actual corticosteroid exposure was related to ON development. We believe that the large size and homogeneity of our patient cohort, from a single transplant center, with the prospective collection of data, partially offset these deficiencies. Despite the stated limitations of this study, the data and analysis provides important new information in our knowledge of ON and it is unlikely that a randomized, prospective study will be performed to address this study's goal.
We conclude that among renal transplant patients, statin usage does not appear to lower the risk of ON. Large scale, randomized trials might reveal a reduced incidence of ON related to statin usage but it is unlikely to be very large. The number of rejection episodes and male gender was associated with a higher risk.
Acknowledgments
The authors thank Paul Lender for all the figures and data management.
One or more of the authors has received funding from NIH Grant DK13083(AJM) and educational grants by Wright Medical Technology, Inc, Arlington, TN (EYC), and Biomet Inc, Warsaw, IN (EYC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Muhammad Ajmal, Email: Muhammad.Ajmal2@va.gov, Department of Orthopaedic Surgery, VA Hospital, Nashville, Affiliated with University of Vanderbilt, 1310 24th Ave. South, Nashville, TN 37212, Phone: 615-327-5356, Fax: 615-321-6342.
A.J. Matas, Email: Matas001@umn.edu, Department of Surgery, University of Minnesota, Minneapolis, MN 55457, Phone: 612-625-6460, Fax: 612-624-7168.
M. Kuskowski, Email: Michael.kuskowski@med.va.gov, Minneapolis VA Medical Center, 1 Veteran Drive, Minneapolis, MN 55417, Phone: 612-725-2000.
Edward Y. Cheng, Email: cheng002@umn.edu, Department of Orthopaedic Surgery, Mairs Family Professor, University of Minnesota, 2512 South 7th Street, Suite 200, Minneapolis, MN 55454, Phone: 612-273-7951, Fax: 612-273-7959.
References
- 1.Harrington KD, Murray WR, Kountz SL, Belzer FO. Avascular necrosis of bone after renal transplantation. J Bone Joint Surg Am. 1971 Mar;53(2):203–215. [PubMed] [Google Scholar]
- 2.Ibels LS, Alfrey AC, Huffer WE, Weil R., 3rd Aseptic necrosis of bone following renal transplantation: experience in 194 transplant recipients and review of the literature. Medicine (Baltimore) 1978 Jan;57(1):25–45. doi: 10.1097/00005792-197801000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Julian BA, Quarles LD, Niemann KM. Musculoskeletal complications after renal transplantation: pathogenesis and treatment. Am J Kidney Dis. 1992 Feb;19(2):99–120. doi: 10.1016/s0272-6386(12)70118-x. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho AC, Lees RS, Vaillancourt RA, Cabral RB, Weinberg RM, Colman RW. Intravascular coagulation in hyperlipidemia. Thromb Res. 1976 Jun;8(6):843–857. doi: 10.1016/0049-3848(76)90013-x. [DOI] [PubMed] [Google Scholar]
- 5.Jones JP., Jr Fat embolism, intravascular coagulation, and osteonecrosis. Clin Orthop Relat Res. 1993 Jul;(292):294–308. [PubMed] [Google Scholar]
- 6.Saito S, Ohzono K, Ono K. Early arteriopathy and postulated pathogenesis of osteonecrosis of the femoral head. The intracapital arterioles. Clin Orthop Relat Res. 1992 Apr;(277):98–110. [PubMed] [Google Scholar]
- 7.Hedri H, Cherif M, Zouaghi K, et al. Avascular osteonecrosis after renal transplantation. Transplant Proc. 2007 May;39(4):1036–1038. doi: 10.1016/j.transproceed.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Le Parc JM, Andre T, Helenon O, Benoit J, Paolaggi JB, Kreis H. Osteonecrosis of the hip in renal transplant recipients. Changes in functional status and magnetic resonance imaging findings over three years in three hundred five patients. Rev Rhum Engl Ed. 1996 Jun;63(6):413–420. [PubMed] [Google Scholar]
- 9.Marston SB, Gillingham K, Bailey RF, Cheng EY. Osteonecrosis of the femoral head after solid organ transplantation: a prospective study. J Bone Joint Surg Am. 2002 Dec;84-A(12):2145–2151. doi: 10.2106/00004623-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Arlet J. Nontraumatic avascular necrosis of the femoral head. Past, present, and future. Clin Orthop Relat Res. 1992 Apr;(277):12–21. [PubMed] [Google Scholar]
- 11.Cruess RL. Osteonecrosis of bone. Current concepts as to etiology and pathogenesis. Clin Orthop Relat Res. 1986 Jul;(208):30–39. [PubMed] [Google Scholar]
- 12.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995 Mar;77(3):459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Cui Q, Wang GJ, Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J Bone Joint Surg Am. 1997 Jul;79(7):1054–1063. doi: 10.2106/00004623-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Cui Q, Wang GJ, Su CC, Balian G. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997 Nov;(344):8–19. [PubMed] [Google Scholar]
- 15.Li X, Jin L, Cui Q, Wang GJ, Balian G. Steroid effects on osteogenesis through mesenchymal cell gene expression. Osteoporos Int. 2005 Jan;16(1):101–108. doi: 10.1007/s00198-004-1649-7. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Cui Q, Kao C, Wang GJ, Balian G. Lovastatin inhibits adipogenic and stimulates osteogenic differentiation by suppressing PPARgamma2 and increasing Cbfa1/Runx2 expression in bone marrow mesenchymal cell cultures. Bone. 2003 Oct;33(4):652–659. doi: 10.1016/s8756-3282(03)00239-4. [DOI] [PubMed] [Google Scholar]
- 17.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001 May;(386):173–178. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Wang GJ, Cui Q, Balian G. The Nicolas Andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000 Jan;(370):295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Humar A, Johnson EM, Gillingham KJ, et al. Venous thromboembolic complications after kidney and kidney-pancreas transplantation: a multivariate analysis. Transplantation. 1998 Jan 27;65(2):229–234. doi: 10.1097/00007890-199801270-00015. [DOI] [PubMed] [Google Scholar]
- 20.Almond PS, Matas A, Gillingham K, et al. Risk factors for chronic rejection in renal allograft recipients. Transplantation. 1993 Apr;55(4):752–756. 756–757. doi: 10.1097/00007890-199304000-00013. discussion. [DOI] [PubMed] [Google Scholar]
- 21.Cox D. The analysis of exponentially distributed life-times with two types of failures. (B).J Royal Stat Society. 1958;21:411–421. [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 23.Maritz FJ, Conradie MM, Hulley PA, Gopal R, Hough S. Effect of statins on bone mineral density and bone histomorphometry in rodents. Arterioscler Thromb Vasc Biol. 2001 Oct;21(10):1636–1641. doi: 10.1161/hq1001.097781. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs B. Epidemiology of traumatic and nontraumatic osteonecrosis. Clin Orthop Relat Res. 1978 Jan-Feb;(130):51–67. [PubMed] [Google Scholar]
- 25.Ono K, Tohjima T, Komazawa T. Risk factors of avascular necrosis of the femoral head in patients with systemic lupus erythematosus under high-dose corticosteroid therapy. Clin Orthop Relat Res. 1992 Apr;(277):89–97. [PubMed] [Google Scholar]
- 26.Zizic TM, Marcoux C, Hungerford DS, Dansereau JV, Stevens MB. Corticosteroid therapy associated with ischemic necrosis of bone in systemic lupus erythematosus. Am J Med. 1985 Nov;79(5):596–604. doi: 10.1016/0002-9343(85)90057-9. [DOI] [PubMed] [Google Scholar]
- 27.Lima JJ, Mauras N, Kissoon N, Wang J, Wiltrout SA, Sylvester JE. Influence of sex and beta2 adrenergic receptor haplotype on resting and terbutaline-stimulated whole body lipolysis. Metabolism. 2005 Apr;54(4):492–499. doi: 10.1016/j.metabol.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Mittendorfer B. Sexual dimorphism in human lipid metabolism. J Nutr. 2005 Apr;135(4):681–686. doi: 10.1093/jn/135.4.681. [DOI] [PubMed] [Google Scholar]
- 29.Fordyce MJ, Solomon L. Early detection of avascular necrosis of the femoral head by MRI. J Bone Joint Surg Br. 1993 May;75(3):365–367. doi: 10.1302/0301-620X.75B3.8496201. [DOI] [PubMed] [Google Scholar]
- 30.Mulliken BD, Renfrew DL, Brand RA, Whitten CG. Prevalence of previously undetected osteonecrosis of the femoral head in renal transplant recipients. Radiology. 1994 Sep;192(3):831–834. doi: 10.1148/radiology.192.3.8058956. [DOI] [PubMed] [Google Scholar]
- 31.Tervonen O, Mueller DM, Matteson EL, Velosa JA, Ginsburg WW, Ehman RL. Clinically occult avascular necrosis of the hip: prevalence in an asymptomatic population at risk. Radiology. 1992 Mar;182(3):845–847. doi: 10.1148/radiology.182.3.1535906. [DOI] [PubMed] [Google Scholar]
- 32.Cheng EY, Thongtrangan I, Laorr A, Saleh KJ. Spontaneous resolution of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2004 Dec;86-A(12):2594–2599. doi: 10.2106/00004623-200412000-00002. [DOI] [PubMed] [Google Scholar]