Abstract
Current methods used to measure protein expression on microarrays, such as labeled fluorescent imaging, are not well suited for real-time, diagnostic measurements at the point of care. Studies have shown that microelectrical sensors utilizing silica nanowire, impedimetric, surface acoustic wave, magnetic nanoparticle and microantenna technologies have the potential to impact disease diagnosis by offering sensing characteristics that rival conventional sensing techniques. Their ability to transduce protein binding events into electrical signals may prove essential for the development of next-generation point-of-care devices for molecular diagnostics, where they could be easily integrated with microarray, microfluidic and telemetry technologies. However, common limitations associated with the microelectrical sensors, including problems with sensor fabrication and sensitivity, must first be resolved. This review describes governing technical concepts and provides examples demonstrating the use of various microelectrical sensors in the diagnosis of disease via protein biomarkers.
Keywords: biomarker, impedimetric, magnetic nanoparticle, microantenna, point of care, surface acoustic wave, telemedicine
Recent advancements in biomarker identification provide the possibility for the clinical diagnosis of many diseases at the point of care (POC). Biomarker technology has been applied for the diagnosis of a wide variety of conditions, including cancers, cardiac diseases, autoimmune diseases, and acute events, such as stroke, cardiac ischemia, head injury and pathogen detection [1–4]. Molecular diagnostic techniques utilizing biomarkers may be suitable as detection platforms for POC diagnostic devices when coupled with microarray and microfluidic technologies [5]. Clinical benefits of POC testing would include faster turnaround time, reduced dependence on central laboratory facilities and the availability of results at the time of physician consultation [6]. This would enable more timely treatment and decreased hospital length of stay, leading to lower overall costs and increased patient satisfaction [7].
A major challenge with biomarker technology at the POC is the limitation associated with the current techniques used to measure protein expression. Currently, many molecular methods label the target protein, and utilize fluorescent imaging techniques to determine expression. Such techniques typically require advanced optical imaging systems, including lasers, which can be bulky and expensive. These systems function well in central diagnostic laboratories, but would be more difficult to miniaturize and make portable for applications at POC.
A system involving microelectrical sensors could be used to measure the physical characteristics of the proteins or an attached label. The measuring process may eventually allow for real-time analysis, assuming that techniques for sample preparation improve. The measurements could be digitized and transmitted for the purpose of telemedicine. As a result of these properties, microelectrical sensors may permit biomarker detection in POC devices.
This paper will review promising reports and potential further developments of silica nanowire (SiNW), impedimetric, surface acoustic wave (SAW), magnetic nanoparticle and microantenna technologies. These approaches involve protein binding events affecting a propagating electrical signal, which may be translated into the amount of protein interactions.
Nanowires
Advancements in nanotechnology have enabled the fabrication of electrochemical sensors that are comparable in size to those of the biological and chemical species being sensed [8,9]. Such sensors could function as primary transducers that interface with macroscopic instruments [8]. A variety of biomolecules can be used in microelectrical sensors, including proteins (receptor proteins as well as antibodies), bacteriophages and aptamers. For the purpose of this report, the focus will be on the use of proteins or antibodies in microelectrical sensors [10–12]. One type of nanoscale biosensor is constructed with semiconducting SiNWs, which have the ability to bind analytes on their surface [8]. These SiNWs may be configured as field-effect transistors (FETs). The binding of a charged antigen to an antibody immobilized on a SiNW can act as a field-effect gate upon each individual SiNW, thereby changing the conductance through the wire [13]. This change in conductance of the SiNW can be measured electronically and correlated to the amount of molecular activities that have occurred on the sensing surface. In principle, SiNWs could be fabricated onto a relatively small sensing area using photolithography and metal deposition to form a microarray, allowing for simultaneous detection of different biomarkers in a given sample [9].
Cui and colleagues demonstrated the use of SiNW devices configured to function as a FET for the real-time detection of protein interactions [14]. In the experiment, biotin was immobilized to the oxide surface of the SiNWs and used as a binding receptor. The conductance of the SiNWs increased when solutions of streptavidin were delivered to the nanowire sensor devices. Similarly, Zheng and colleagues utilized SiNW devices functionalized with distinct surface receptors that were incorporated in an array pattern for the purpose of multiplexed electrical detection of cancer markers [15]. Simultaneous conductance measurements from multiple SiNWs were recorded as different protein solutions were sequentially delivered. Concentration-dependent conductance changes were only observed for the individual SiNW with the corresponding surface receptors for the specific protein solution [15].
Impedimetrics
Impedance, the ratio of voltage to current, reflects the amount of hindrance to current flow that exists between two electrodes. Impedance has real and imaginary components known as resistance and capacitance [16]. The impedance of a charged aqueous solution can be determined by measuring the current caused by a sinusoidal voltage applied across the electrodes. The binding of bacteria or proteins to the electrodes of an impedimetric sensor should result in a measurable change in impedance, and may thus be a suitable strategy for measuring biomarker levels at the POC. Studies have shown that these binding events do affect the measured impedance of a sensor [17–20].
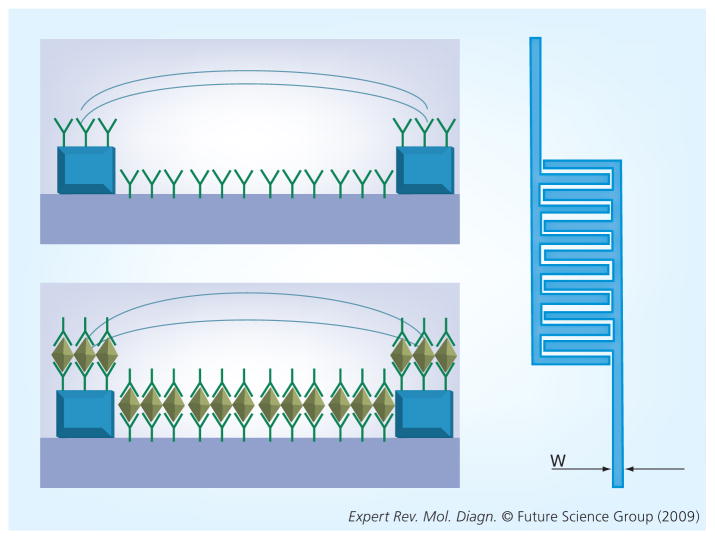
An impedimetric sensor for biomarker detection would require an immobilized analyte, such as antibodies, on a dielectric substrate, which can specifically bind to target molecules present in a sample solution; for simplicity, we will describe an impedimetric sensor that utilizes antibodies as the immobilized analyte (Figure 1). A sample solution containing the target molecule(s), in this case proteins, would then be incubated on the sensor. Here, the target proteins will be captured by their corresponding antibodies. The unbound antigens would then be washed away, and the electrodes covered by a charged aqueous solution, such as phosphate-buffered saline. Ideally, an impedimetric sensor would utilize an interdigitated transducer (IDT) because it increases surface area, resulting in an amplification of the signal. The use of an IDT may also increase the sensitivity of an impedimetric sensor [16]. In addition to using an IDT, an impedimetric sensor would also utilize two sensing lines that work in parallel. These sensing lines would consist of a reference line containing only the immobilized analyte, in this case antibodies, and the experimental line, which may have antigens bound to the immobilized antibodies. These two lines can be compared with each other to determine whether there are protein interactions in the experimental line. The addition of molecules such as proteins or bacteria would influence both the resistance and capacitance of the active surfaces of the sensor [18]. A change in capacitance would reflect a change in the effective surface area of the electrodes because of the bound antigens [16].
Figure 1. The top impedance sensor has antibodies immobilized on top of and between the gold electrodes, while the bottom sensor also has bound antigens.
Both sensors will have an aqueous solution covering the active region (interdigitated region) of the sensor. The bound antigens will change the resistance and capacitance of the sensor. The image to the right represents a top view of a sensor, which is an interdigitated circuit.
This approach has been used to detect 103 cfu/ml salmonella in which anti-salmonella antibodies were immobilized on the electrode surfaces [20]. Escherichia coli bacteria (106 cfu/ml) have also been detected by measuring changes in conductance, which is related to impedance due to the binding of E. coli to the electrode surface [17]. Growth-based detection of bacteria captured on the active surface of impedimetric sensors has been demonstrated by multiple investigators [11,21]. In addition to the detection of bacteria, several groups have reported on the use of impedimetric sensors for the detection of protein–protein (antibody–antigen) interactions [18,19,22]. Specifically, researchers have demonstrated the ability to detect cardiac markers by measuring the change in the impedance of a sensor when antimyoglobin antibodies (100 ng/ml) bound to myoglobin proteins immobilized on a sensing surface [19]. Similarly, researchers were able to detect the stroke marker neuron-specific enolase (NSE) by measuring the change in impedance when electrodes containing immobilized antiNSE antibodies were incubated with solutions containing various concentrations of the protein NSE. These researchers were able to detect NSE as low as 0.5 pg/ml and calculated that nonspecific binding accounted for approximately 10% of the sensor response [22]. These findings demonstrate the potential for the development of impedimetric sensors for the diagnosis of disease.
Surface acoustic wave sensors
Surface acoustic waves are mechanical vibrations that propagate just below the surface of piezoelectric solids when excited by an electrical signal at the resonant frequency. The velocity of a SAW is sensitive to changes in the mass applied to the active area, the viscosity of the material applied to the active area and the temperature of the surface [23]. A typical SAW delay-line consists of two IDTs that are an electrode pair fabricated on a piezoelectric substrate via photolithography. A sinusoidal voltage applied to the input IDT is translated into oscillating mechanical strain, forming a SAW that propagates along the surface of the piezoelectric material. The SAW is then converted back into a sinusoidal voltage of different frequency (phase) and amplitude at the output IDT. These differences are related to changes in the velocity of the SAW and can be correlated to changes in the mass loading, viscosity and temperature on the surface of the substrate. The effect of temperature on the substrate can be accounted for by using a dual delay-line configuration with both lines at the same temperature.
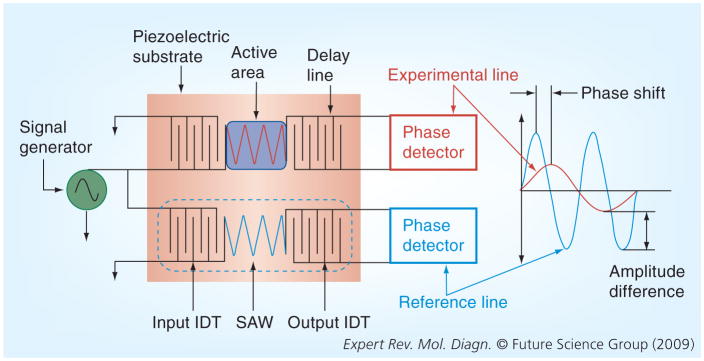
Figure 2 displays a typical SAW sensor in the ‘dual-delay-line’ configuration. Measurements comparing the experimental line with the reference line should minimize the effect of temperature [24,25].
Figure 2. A SAW delay line consists of an IDT, an output IDT and a piezoelectric substrate.
Two delay lines operate in parallel, with one line acting as a reference line and the other acting as an experimental line. A sinusoidal voltage is applied to the input IDT, which develops an alternating electric field that is translated into a mechanical SAW by the piezoelectric effect. The velocity of the SAW is affected by mass loading, fluid viscosity and temperature on the surface of the substrate. The reverse piezoelectric effect translates the SAW into an oscillating electric field at the output IDT. Any difference in velocity between the two delay lines would be reflected as a phase shift and amplitude difference.
IDT: Input interdigitated transducer; SAW: Surface acoustic-wave.
The addition of bound antigens to immobilized antibodies on the surface of the piezoelectric substrate may result in a detectable mass loading [23,26]. Welsch and colleagues demonstrated the ability of a SAW delay line that utilized a shear, horizontally polarized propagation mode, to detect antigen–antibody binding, reporting a sensitivity of approximately 6 pg/mm2 [27]. Dahint and colleagues developed a SAW sensor utilizing a different piezoelectric substrate with improved sensitivity of approximately 0.5 pg/mm2 corresponding to a concentration of approximately 0.5 μg/ml [28]. However, these reported sensitivities have to be improved for a SAW delay line to detect low-abundance proteins (pg/ml to ng/ml in patient serum), which would be useful biomarkers for many diseases.
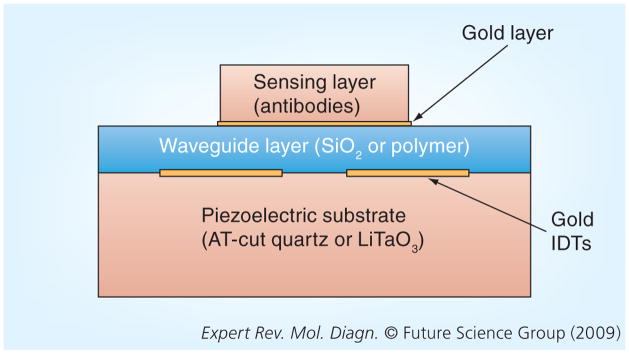
One subcategory of SAW, the Love propagation mode, appears especially promising for chemical and biosensing applications due to high sensitivity to mass loading and the ability to make measurements in liquid environments with minimal propagation losses [23]. A Love mode SAW sensor (Figure 3) contains the addition of a waveguide layer and makes use of special piezoelectric substrates, namely AT-cut quartz and 36°-Y-cut-X-propagating LiTaO3 [29,30]. The waveguide functions to trap the SAW along the surface of the piezoelectric substrate to minimize energy losses and to protect the IDTs from corrosion in a liquid-sensing environment [24,26]. The surface of the waveguide in contact with the sensing layer is often modified with a gold layer (~50 nm thick), which provides better adherence of the sensing layer (antibodies) and prevents the nonspecific binding of proteins to the active area. Nonspecific binding represents a major hurdle for implementation of microelectrical sensors for protein detection in general.
Figure 3. Cross-sectional view of a Love mode surface acoustic-wave sensor (layer thicknesses not to scale) displaying the piezoelectric substrate, two IDTs, the waveguide layer and the sensing layer.
The waveguide layer consists of either an oxide or polymer layer, which functions to trap the acoustic energy of the SAW at the interface between the waveguide and the substrate to minimize propagation losses. It also serves to protect the IDTs from corrosion, thus providing for measurements to be made in a liquid environment. The sensing layer provides for the capture and immobilization of the biological or chemical agent of interest to the active surface of the sensor in order to develop a detectable mass loading effect.
IDT: Input interdigitated transducer.
A Love wave SAW sensor with a sensing layer of anti-Bacillus antibodies was used to detect low levels of Bacillus thuringiensis in aqueous conditions [31]. Tests using bovine serum albumin (BSA) in place of B. thuringiensis spores indicated a detection limit of 0.187 ng BSA [31]. E. coli bacteria were also detected using Love mode SAW sensors [32]. The low-level detection of bacteria spores by Love mode SAW sensors is promising in regard to future protein biomarker detection using SAW technologies.
Magnetic labeling
Magnetic nanobeads are magnetic nanoparticles, usually iron oxide, coated with a material such as a biocompatible polymer [33]. The nanoparticles can be synthesized, using several techniques, to be smaller than hundreds of nanometers, a size comparable with cells, viruses, genes and proteins [34,35]. The surface of these nanoparticles can be functionalized with certain ligands to selectively bind to target proteins [33,36]. They have also been used as immunoassay labels [37]. In a microelectrical sensor application, magnetic nanoparticles could be used to label antigens or antibodies, and have been used to detect CA-125, an ovarian cancer biomarker; specifically, magnetic nanoparticles carrying anti-CA-125 antibodies were able to detect the protein at low concentrations (1–10 fmol) [38].
Magnetic nanoparticles are stable in that they are not affected by reagent chemistry, and they can be detected with minimal noise in a biological environment [33]. In the presence of an external magnetic field, the nanoparticles poles align [36]; when the field is removed, a relaxation occurs as a result of Brownian and Neel relaxations [33]. Magnetorelaxometry is a method that can be used to detect this relaxation time, which can be correlated to the presence of nanoparticles, such as those labeling antigens or antibodies [35,36]. This decaying magnetic field has been measured by several instruments, including hall sensors, giant magnetoresistive (GMR) sensors, anisotropic magnetoresistive sensors (AMR), and superconducting quantum interference detectors (SQUIDs) [33,37].
A magneto-impedance-based sensor could also be used to detect the presence of magnetic nanoparticle labels attached to antibodies immobilized on a substrate. An external magnetic field must still be applied to cause the nanoparticles to polarize. The impedance of the substrate would be affected by the resulting magnetic field of the nanoparticles [33].
Furthermore, there are techniques that utilize magnetic nanoparticles for sorting biological entities using microfluidics [39,40]. Osterfeld and colleagues believe that through the use of microfluidics, it should be possible to reduce magnetic nanoparticle label-based assay times to 30 min [41]. These results are promising for further development of POC devices utilizing this technology.
Microantennas
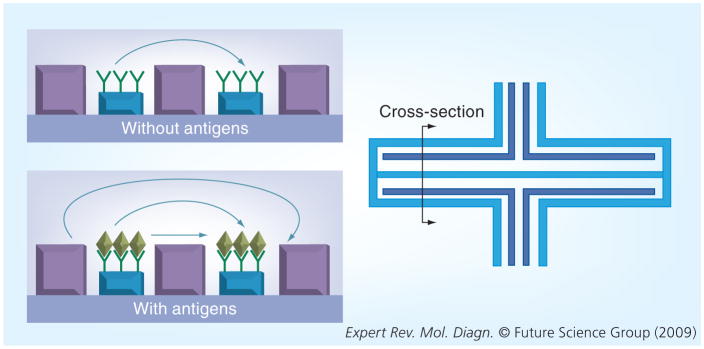
A theoretical approach to detecting protein–protein interactions (antigen–antibody interactions) is the use of planar microantennas. Antennas are devices that are used to transmit or receive electromagnetic waves, where the transmission path length between a pair of antennas affects the strength of a radiofrequency (RF) signal [42]. Biological microantennas could be constructed by printing a conductive material using photolithography, and subsequently attaching antibodies to this conductive layer (Figure 4) [43]. When an RF signal is applied to the transmitter microantenna, a measurable signal should be received by the receiver antenna; the binding of antigens by the antibodies printed on the microantennas should result in a change in the signal, relative to a reference signal. In this manner, the changed signal may be able to detect protein–protein interactions. In addition, the design of a microantenna sensor may also require the use of a shielded trace for better signal transmission between antennas [44]. Similarly, the additional presence of the antibody–antigen complex may increase the effective antenna height and reduce the transmission path length, thus resulting in a stronger signal. If the antigens alone fail to produce a noticeable increase in the effective height of the antenna, additional magnetic nanoparticle labels may have a pronounced effect.
Figure 4. The image to the right represents a top view of a microantenna sensor with a line representing a cross-section shown on the left.
The upper sensor has antibodies immobilized on top of the active region of the antennas, while the lower sensor also has captured antigens. The shielded traces are on both sides of the transmitting and receiving antennas. Their presence should prevent signals from traveling in a direct path between antennas. The bound antigens should change the attenuation of the sensor because of the increased affected antenna height.
Expert commentary
Studies have shown that the discussed microelectrical sensors have the potential to impact disease diagnosis by offering sensing characteristics that rival conventional sensing techniques. The ability to convert chemical and biological binding events into electronic and digital signals suggests the potential for an interface between these sensors and microprocessors, which would further the development of next-generation POC devices for molecular diagnostics. However, common limitations associated with the microelectrical sensing techniques, including problems with sensor fabrication and sensitivity, need to be resolved [8,15,18,19,27,28].
The manufacture of microelectrical sensors requires technologies that have not yet matured sufficiently for widespread application. For example, photolithography, the technique commonly used to construct microcircuits on substrates, is currently limited to a line-width resolution [45]. The sensitivity of impedimetric and SAW-based sensors are limited by this line-width resolution [45]. Higher resolution allows for the construction of IDTs with improved signal amplification and higher operating frequencies. This would allow for higher sensitivity of the impedimetric and SAW techniques, respectively [20,23].
The biochemical nature of protein interactions presents challenges that affect measurement sensitivity. The sensitivity of nanowire-based sensors depends on the ionic strength of the analyte. As is the case with blood serum samples, diagnostics will require a standard desalting step before analysis to achieve the highest sensitivity [15]. The sensitivity of magnetic nanoparticle-based sensors is limited by dissimilarity in the size of the particles. Sensitivity could be improved if future technologies allow for the synthesis of homogenous particles [37]. Nonspecific binding of proteins to the sensing platform also represents a major hurdle for implementation of microelectrical sensors for protein detection in general. Finally, the signal-to-noise ratio of microelectrical sensors must be further improved in order to better differentiate between actual antigen-binding events and sample noise. This can be achieved through the development of more advanced sample-preparation strategies and optimization of the chemistry occurring at the active area of the sensor.
An additional strategy would improve the sensitivity of protein detection in sensing devices through electrochemical means. Current electrochemical immunosensors (EIS) utilize a variety of strategies for biomarker detection, including but not limited to: amperometric devices utilizing chemical reactions to generate a current on the sensing electrode, sandwich EIS devices that use magnetic gold nanoparticles to enhance enzyme labeling, and macrocantilever devices that produce measurably sharp changes in electrical impedance [46–48]. To date, EIS devices have been used for the multiplex measurement of cancer biomarkers (including AFP, ferritin, CEA, hCG-β, CA15-3, CA125 and CA19-9), for the quantification of levels of the colorectal cancer biomarker carcinoembryonic antigen in solution, and for the quantification of the prostate cancer biomarker AMCAR from patient urine samples [46–48]. For more detail on these devices and detection strategies, we suggest the following references [46–49]. EIS devices are well-suited for incorporation in lab-on-a-chip devices due to the fact that they can be economically mass produced and miniaturized, and have excellent detection limits necessary to find the low-abundance biomarkers present in small volume samples [48].
Upon resolution of these limitations, microelectrical sensors would be suitable for application in hand-held POC diagnostic devices, where their small physical size and high sensitivity would be useful in the multiplexed, simultaneous detection of an array of disease biomarkers.
Five-year view
Healthcare reform requires a paradigm shift from clinic-based to in-home care in order to relieve the stresses of soaring health-care costs. POC diagnostics will play a critical role in decreasing healthcare costs by providing quicker diagnoses, resulting in more efficient treatment and reduced time spent by patients in the clinic. Microelectrical sensors may be used in conjunction with micro-fluidic and telemetry technologies to aid the development of POC diagnostic devices to be implemented in the reformed healthcare system. These developments could be supported with both public and private investments, including the multibillion dollar allocation from the American Recovery and Reinvestment Act of 2009 for health information technology, including telemedicine [50].
Key issues
Current methods used to measure protein expression on microarrays are not well suited for making real-time, diagnostic measurements at the point of care.
Microelectrical sensors have been shown to be capable of measuring protein-binding events.
Studies indicate that silica nanowire biosensors are capable of highly sensitive and selective real-time detection of protein interactions.
Impedimetric sensors utilizing interdigitated transducer electrodes have been shown to be capable of detecting protein interactions.
Investigations into the performance of Love Mode surface acoustic-wave sensors indicate that they may be promising for use in biosensing applications.
Magnetic nanoparticle labels are used for biomarker detection and, together with microfluidics, may be developed for point-of-care devices.
Common limitations associated with the microelectrical sensing techniques include problems with sensor fabrication and sensitivity.
Microelectrical sensors may be integrated with microarray, microfluidic and telemetry technologies to aid in the development of point-of-care diagnostic devices.
Acknowledgments
The authors thank Ryan T Noonan for his assistance with copyediting the manuscript.
Footnotes
Financial & competing interests disclosure
This work was supported in part by grants DK063665, DK066020, DK075566 from the NIH to Brian C-S Liu. Additional funding was supported by the Interstitial Cystitis Association and the Fishbein Family Foundation. Robert J Caiazzo Jr serves as a consultant for Inanovate, Inc. Brian C-S Liu serves on the Board of Scientific Advisors for Inanovate, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
David L Arruda, Email: arruda.david@gmail.com, Wentworth Institute of Technology, 550 Huntington Avenue, Boston, MA 02115, USA, Tel.: +1 508 269 2031, Fax: +1 617 989 4591.
William C Wilson, Email: wilsonw2@wit.edu, Wentworth Institute of Technology, 550 Huntington Avenue, Boston, MA 02115, USA, Tel.: +1 857 284 3015, Fax: +1 617 989 4591.
Crystal Nguyen, Email: crystal.nguyen@gmail.com, Wentworth Institute of Technology, 550 Huntington Avenue, Boston, MA 02115, USA, Tel.: +1 860 306 4391, Fax: +1 617 989 4591.
Qi W Yao, Email: yao_qiwei@msn.com, Wentworth Institute of Technology, 550 Huntington Avenue, Boston, MA 02115, USA, Tel.: +1 617 319 0551, Fax: +1 617 989 4591.
Robert J Caiazzo, Jr, Email: rcaiazzo@partners.org, Molecular Urology Laboratory, Brigham and Women’s Hospital, Harvard Medical, School, 221 Longwood Avenue, LMRC-610, Boston, MA 02115, USA, Tel.: +1 617 732 4804, Fax: +1 617 582 6191.
Ilie Talpasanu, Email: talpasanui@wit.edu, Assistant Professor, Department of Electronics and Mechanical, Wentworth, Institute of Technology, 550 Huntington Avenue, Boston, MA 02115, USA, Tel.: +1 617 989 4226, Fax: +1 617 989 4591.
Douglas E Dow, Email: dowd@wit.edu, Assistant Professor, Department of Electronics and Mechanical, Wentworth Institute of Technology, 550 Huntington Avenue, Boston, MA 02115, USA, Tel.: +1 617 989 4134 Fax: +1 617 989 4591.
Brian C-S Liu, Email: bliu@partners.org, Director, Translational Research. Molecular Urology Laboratory, Brigham and Women’s Hospital, Harvard Medical School, 221 Longwood Avenue, LMRC-610, Boston, MA 02115, USA, Tel.: +1 617 732 4973, Fax: +1 617 582 6191.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4(4):1123–1133. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein EA, Kaplan LA. Serum enzymes, isoenzymes, myoglobin, and contractile proteins in acute myocardial infarction. Cardiovasc Clin. 1983;13(3):355–369. [PubMed] [Google Scholar]
- 3.Notkins AL. New predictors of disease. Sci Am. 2007;296(3):72–79. [PubMed] [Google Scholar]
- 4.Reynolds MA, Kirchick HJ, Dahlen JR, et al. Early biomarkers of stroke. Clin Chem. 2003;49(10):1733–1739. doi: 10.1373/49.10.1733. [DOI] [PubMed] [Google Scholar]
- 5.Hall DA, Ptacek J, Snyder M. Protein microarray technology. Mech Ageing Dev. 2007;128(1):161–167. doi: 10.1016/j.mad.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St-Louis P. Status of point-of-care testing: promise, realities, and possibilities. Clin Biochem. 2000;33(6):427–440. doi: 10.1016/s0009-9120(00)00138-7. [DOI] [PubMed] [Google Scholar]
- 7.Price CP. Point of care testing. BMJ. 2001;322(7297):1285–1288. doi: 10.1136/bmj.322.7297.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Patolsky F, Zheng G, Lieber CM. Nanowire-based biosensors. Anal Chem. 2006;78(13):4260–4269. doi: 10.1021/ac069419j. Describes the use of silicon nanowire devices for the detection and quantification of biological and chemical species such as proteins and viruses. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 10.Xu D, Yu X, Liu Z, He W, Ma Z. Label-free electrochemical detection for aptamer-based array electrodes. Anal Chem. 2005;77(16):5107–5113. doi: 10.1021/ac050192m. [DOI] [PubMed] [Google Scholar]
- 11.Shabani A, Zourob M, Allain B, et al. Bacteriophage-modified microarrays for the direct impedimetric detection of bacteria. Anal Chem. 2008;80(24):9475–9482. doi: 10.1021/ac801607w. [DOI] [PubMed] [Google Scholar]
- 12.Koo OK, Liu Y, Shuaib S, et al. Targeted capture of pathogenic bacteria using a mammalian cell receptor coupled with dielectrophoresis on a biochip. Anal Chem. 2009;81(8):3094–3101. doi: 10.1021/ac9000833. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CJ, Zhukovsky N, Cass AE, Nagy JM. Proteomics, nanotechnology and molecular diagnostics. Proteomics. 2008;8(4):715–730. doi: 10.1002/pmic.200700665. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y, Wei Q, Park H, Lieber CM. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science. 2001;293(5533):1289–1292. doi: 10.1126/science.1062711. [DOI] [PubMed] [Google Scholar]
- 15•.Zheng G, Patolsky F, Cui Y, Wang WU, Lieber CM. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat Biotechnol. 2005;23(10):1294–1301. doi: 10.1038/nbt1138. Describes highly sensitive, label-free, multiplexed electrical detection of cancer markers using silicon nanowire devices configured in an array format. [DOI] [PubMed] [Google Scholar]
- 16•.Nilsson JW, Riedel SA. Electric Circuits. Pearson/Prentice Hall; NJ, USA: 2005. Explains impedance and other general circuit theory concepts. [Google Scholar]
- 17.Suehiro J, Noutomi D, Shutou M, Hara M. Selective detection of specific bacteria using dielectrophoretic impedance measurement method combined with an antigen–antibody reaction. J Electrostatics. 2003;58(3–4):229–246. [Google Scholar]
- 18.Yu X, Lv R, Ma Z, et al. An impedance array biosensor for detection of multiple antibody–antigen interactions. Analyst. 2006;131(6):745–750. doi: 10.1039/b517148b. [DOI] [PubMed] [Google Scholar]
- 19.Tweedie M, Subramanian R, Lemoine P, et al. Fabrication of impedimetric sensors for label-free point-of-care immunoassay cardiac marker systems, with passive microfluidic delivery. Conf Proc IEEE Eng Med Biol Soc. 2006;1:4610–4614. doi: 10.1109/IEMBS.2006.260742. [DOI] [PubMed] [Google Scholar]
- 20••.Kim G, Morgan M, Hahm BK, et al. Interdigitated microelectrode based impedance biosensor for detection of Salmonella enteritidis in food samples. J Physics: Conference Series. 2008;100(5):052044. Describes impedimetric sensors in biological applications with promising results. [Google Scholar]
- 21.Oliver LM, Dunlop PS, Byrne JA, et al. An impedimetric sensor for monitoring the growth of Staphylococcus epidermidis. Conf Proc IEEE Eng Med Biol Soc. 2006;1:535–538. doi: 10.1109/IEMBS.2006.260394. [DOI] [PubMed] [Google Scholar]
- 22.Barton AC, Davis F, Higson SP. Labeless immunosensor assay for the stroke marker protein neuron specific enolase based upon an alternating current impedance protocol. Anal Chem. 2008;80:9411–9416. doi: 10.1021/ac801394d. [DOI] [PubMed] [Google Scholar]
- 23••.Ballantine DS. Acoustic Wave Sensors: Theory, Design, and Physico-Chemical Applications. Academic Press; CA, USA: 1997. Describes the theory and design of surface acoustic wave sensors in detail and offers insight into many of their practical applications, including that as biosensors. [Google Scholar]
- 24.Hermann F, Weihnacht M. Sensors based on shear-horizontal surface acoustic waves in layered quartz/SiO2 and LiTaO3/SiO2 structures. Presented at: IEEE Ultrasonics Symposium; Lake Tahoe, CA, USA. 17–20 October 1999. [Google Scholar]
- 25.Gizeli E, Bender F, Rasmusson A, et al. Sensitivity of the acoustic waveguide biosensor to protein binding as a function of the waveguide properties. Biosens Bioelectron. 2003;18(11):1399–1406. doi: 10.1016/s0956-5663(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 26•.Lange K, Rapp BE, Rapp M. Surface acoustic wave biosensors: a review. Anal Bioanal Chem. 2008;391(5):1509–1519. doi: 10.1007/s00216-008-1911-5. Provides a review of the current state of surface acoustic wave biosensors with emphasis on Love mode technologies. [DOI] [PubMed] [Google Scholar]
- 27.Welsch W, Klein C, von Schickfus M, Hunklinger S. Development of a surface acoustic wave immunosensor. Anal Chem. 1996;68(13):2000–2004. doi: 10.1021/ac960198z. [DOI] [PubMed] [Google Scholar]
- 28.Dahint R, Bender F. A concentration dependent study of acoustic plate mode immunosensor response using antigen/antibody systems with different binding ability. IEEE Trans Ultrason Ferroelectr Freq Control. 1998;45(5):1216–1220. doi: 10.1109/58.726446. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Kazumi M, Shimizu H. SH-type and Rayleigh-type surface waves on rotated Y-cut LiTaO3; Presented at: IEEE Ultrasonics Symposium; Phoenix, AZ, USA. 26–28 October 1977. [Google Scholar]
- 30.Bender F, Cernosek RW, Josse F. Love-wave biosensors using cross-linked polymer substrates. Electron waveguides on LiTaO3. Lett. 2000;36(19):1672–1673. [Google Scholar]
- 31.Branch DW, Brozik SM. Low-level detection of a Bacillus anthracis simulant using Love-wave biosensors on 36 degrees YX. 19(8):849–859. doi: 10.1016/j.bios.2003.08.020. LiTaO3Biosens. Bioelectron. [DOI] [PubMed] [Google Scholar]
- 32.Moll N, Pascal E, Dinh DH, et al. A Love wave immunosensor for whole E. coli bacteria detection using an innovative two-step immobilisation approach. Biosens Bioelectron. 2007;22(9–10):2145–2150. doi: 10.1016/j.bios.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 33••.Varadan VK. Nanomedicine: Design and Applications of Magnetic Nanomaterials, –Nanosensors, and Nanosystems. Wiley; West Sussex, UK: Hoboken; NJ, USA: 2008. Explains current use of magnetic nanoparticles and several different applications for microfluidics. [Google Scholar]
- 34.Somasundaran P. Encyclopedia of Surface and Colloid Science. Taylor & Francis; NY, USA: 2006. [Google Scholar]
- 35.Wnek GE, Bowlin GL. Encyclopedia of Biomaterials and Biomedical Engineering. Informa Healthcare USA; New York, USA: 2008. [Google Scholar]
- 36.Schwarz JA, Contescu CI, Putyera K. Dekker Encyclopedia of Nanoscience and Nanotechnology. M Dekker; NY, USA: 2004. [Google Scholar]
- 37.Clarke J, Braginski AI. The SQUID Handbook. Wiley-VCH; Weinheim, Germany: 2004. [Google Scholar]
- 38.Bulte JWM, Modo MMJJ. Nanoparticles in Biomedical Imaging: Emerging Technologies and Applications. Springer; NY, USA: 2008. [Google Scholar]
- 39.Hardt S, Schönfeld F. Microfluidic Technologies for Miniaturized Analysis Systems. Springer; NY, USA: 2007. [Google Scholar]
- 40.Gomez FA. Biological Applications of Microfluidics. John Wiley; Hoboken, NJ, USA: 2008. [Google Scholar]
- 41•.Osterfeld SJ, Yu H, Gaster RS, et al. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc Natl Acad Sci USA. 2008;105(52):20637–20640. doi: 10.1073/pnas.0810822105. Promising application of magnetic nanoparticle labeling for biomarker detection including multiplexing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Radio Relay League. The ARRL Antenna Book; The American Radio Relay League, Inc; West Hartford, CT, USA. 2007. [Google Scholar]
- 43.Pozar DM, Schaubert D. Microstrip antennas: the Analysis and Design of Microstrip Antennas and Arrays. Institute of Electrical and Electronics Engineers; NY, USA: 1995. IEEE Antennas and Propagation Society. [Google Scholar]
- 44.Tong XC. Advanced Materials and Design for Electromagnetic Interference Shielding. CRC Press; Boca Raton, FL, USA: 2009. [Google Scholar]
- 45.Nonogaki S, Ueno T, Ito T. Microlithography Fundamentals in Semiconductor Devices and Fabrication Technology. Marcel Dekker; NY, USA: 1998. [Google Scholar]
- 46.Tang D, Yuan R, Chai Y. Ultrasensitive electrochemical immunosensor for clinical immunoassay using thionine-doped magnetic gold nanospheres as labels and horseradish peroxidase as enhancer. Anal Chem. 2008;80(5):1582–1588. doi: 10.1021/ac702217m. [DOI] [PubMed] [Google Scholar]
- 47.Maraldo D, Garcia FU, Mutharasan R. Method for quantification of a prostate cancer biomarker in urine without sample preparation. Anal Chem. 2007;79(20):7683–7690. doi: 10.1021/ac070895z. [DOI] [PubMed] [Google Scholar]
- 48.Wilson MS, Nie W. Multiplex measurement of seven tumor markers using an electrochemical protein chip. Anal Chem. 2006;78(18):6476–6483. doi: 10.1021/ac060843u. [DOI] [PubMed] [Google Scholar]
- 49.Stoeva SI, Lee JS, Smith JE, Rosen ST, Mirkin CA. Multiplexed detection of protein cancer markers with biobarcoded nanoparticle probes. J Am Chem Soc. 2006;128(26):8378–8379. doi: 10.1021/ja0613106. [DOI] [PubMed] [Google Scholar]
- 50.Bernert EJ, Ferris AM. CCH Tax Law Editors. American Recovery and Reinvestment Act of 2009: Conference Report CCH. Baker Hostetler; CCH Inc; IL, USA: 2009. [Google Scholar]