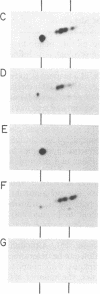
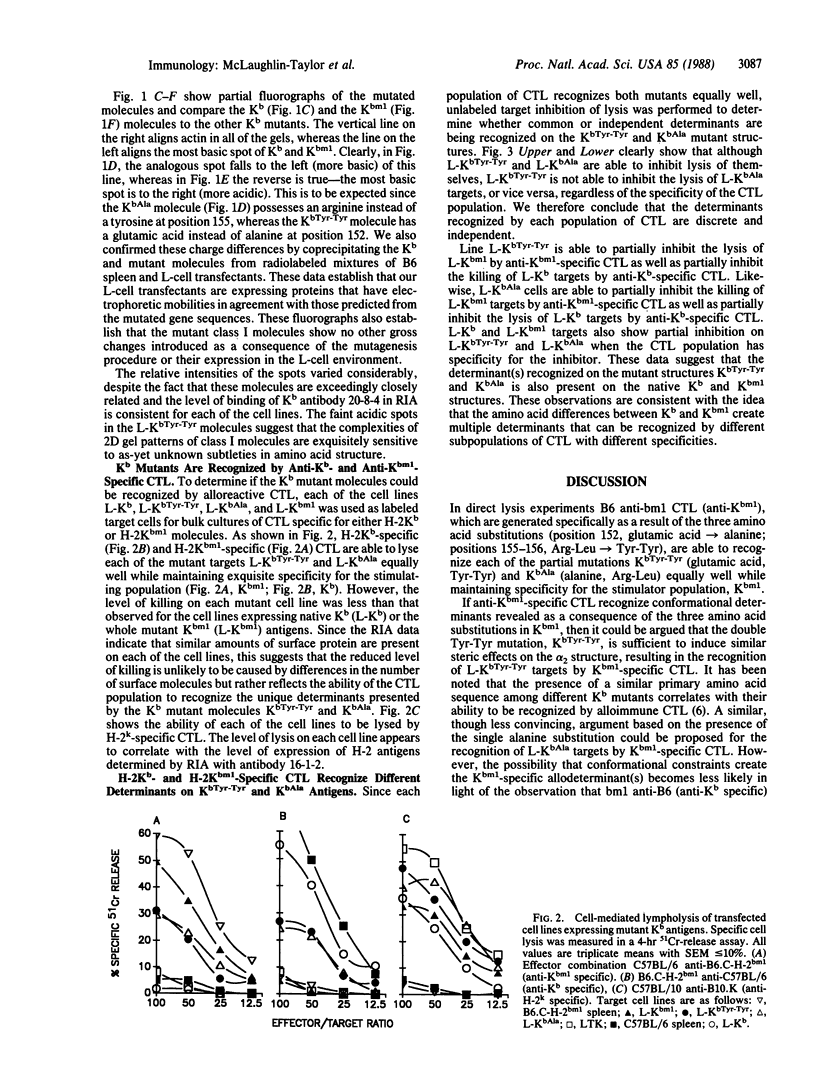
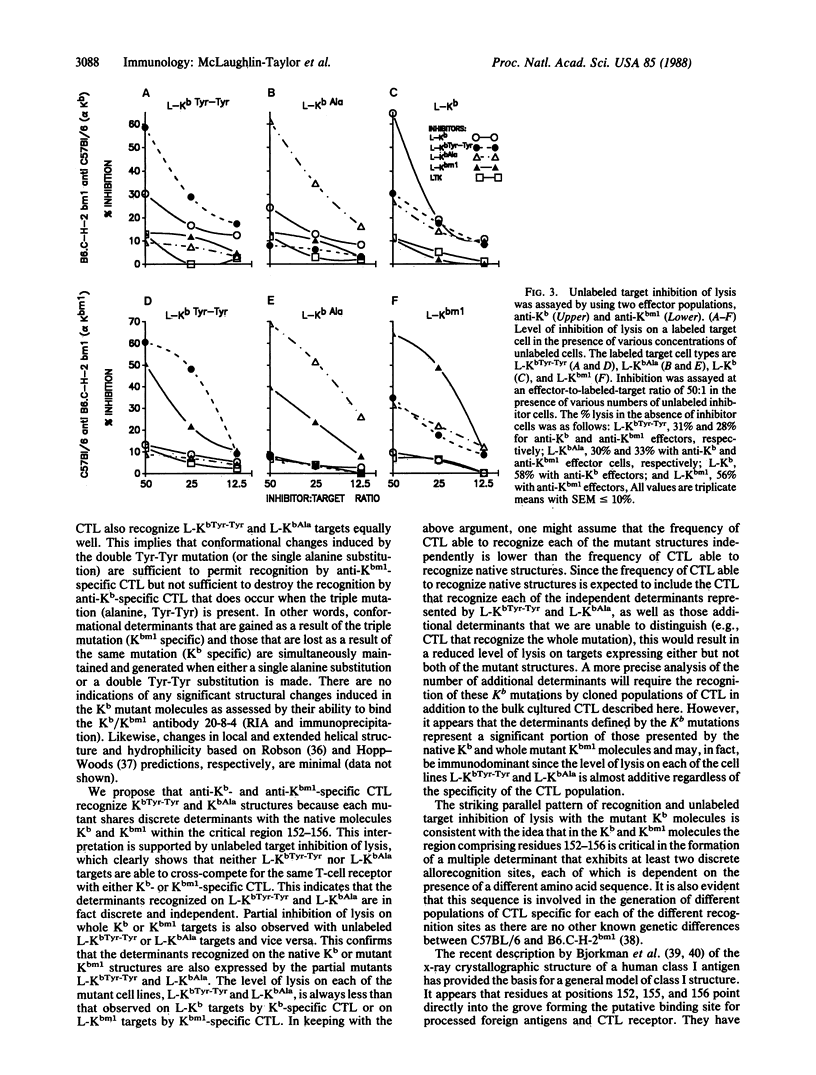
Abstract
To identify critical amino acid residues recognized by alloreactive cytolytic T lymphocytes (CTL) generated between H-2Kb and H-2Kbm1, we have derived a series of cloned L-cell lines expressing the following mutant H-2Kb class I genes. Cell line L-KbTyr-Tyr expresses a mutant gene in which positions 155-156 of the Kb molecule have been changed from Arg-Leu to Tyr-Tyr, leaving position 152 unchanged. Cell line L-KbAla expresses the reciprocal mutant gene that has position 152 of the Kb molecule mutated from glutamic acid to alanine, leaving positions 155-156 unchanged. Electrophoretic mobilities of the mutant Kb molecules reflect only those changes predicted by the mutations. Mutant-specific (anti-Kbm1) and native-specific (anti-Kb) CTL lyse L-KbTyr-Tyr and L-KbAla target cells equally well. Unlabeled target inhibition of lysis revealed a pattern of recognition and inhibition that suggests that the amino acid differences between Kbm1 and Kb create at least two discrete determinants that can be recognized by different populations of CTL. The results suggest that these determinants consist, at least in part, of a linear amino acid sequence from which critical amino acid residues can be identified.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Campen T. J., Royer H. D., Hussey R. E., Poole C. B., Reinherz E. L. Molecular analysis of T cell receptor (Ti) variable region (V) gene expression. Evidence that a single Ti beta V gene family can be used in formation of V domains on phenotypically and functionally diverse T cell populations. J Exp Med. 1985 Jun 1;161(6):1326–1343. doi: 10.1084/jem.161.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen H., Wraith D., Pala P., Askonas B., Flavell R. A. Domain interactions of H-2 class I antigens alter cytotoxic T-cell recognition sites. Nature. 1984 May 17;309(5965):279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- Arnold B., Horstmann U., Kuon W., Burgert H. G., Hämmerling G. J., Kvist S. Alloreactive cytolytic T-cell clones preferentially recognize conformational determinants on histocompatibility antigens: analysis with genetically engineered hybrid antigens. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7030–7034. doi: 10.1073/pnas.82.20.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D. W., Kohn H. I. Inherited histocompatibility changes in progeny of irradiated and unirradiated inbred mice. Genet Res. 1965 Nov;6(3):330–340. doi: 10.1017/s0016672300004225. [DOI] [PubMed] [Google Scholar]
- Barth R. K., Kim B. S., Lan N. C., Hunkapiller T., Sobieck N., Winoto A., Gershenfeld H., Okada C., Hansburg D., Weissman I. L. The murine T-cell receptor uses a limited repertoire of expressed V beta gene segments. Nature. 1985 Aug 8;316(6028):517–523. doi: 10.1038/316517a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Bluestone J. A., Foo M., Allen H., Segal D., Flavell R. A. Allospecific cytolytic T lymphocytes recognize conformational determinants on hybrid mouse transplantation antigens. J Exp Med. 1985 Jul 1;162(1):268–281. doi: 10.1084/jem.162.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Uehara H., Martinko J., Nathenson S. G. Primary structure of a murine transplantation antigen. Nature. 1981 May 7;291(5810):35–39. doi: 10.1038/291035a0. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- De Waal L. P., Nathenson S. G., Melief C. J. Direct demonstration that cytotoxic T lymphocytes recognize conformational determinants and not primary amino acid sequences. J Exp Med. 1983 Nov 1;158(5):1720–1726. doi: 10.1084/jem.158.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Bennink J. R., Wettstein P. J. Negatively selected H-2bml and H-2b cells stimulated with vaccinia virus completely discriminate between mutant and wild-type H-2K alleles. J Immunol. 1981 Jan;126(1):131–133. [PubMed] [Google Scholar]
- Epstein S. L., Ozato K., Sachs D. H. Blocking of allogeneic cell-mediated lympholysis by monoclonal antibodies to H-2 antigens. J Immunol. 1980 Jul;125(1):129–135. [PubMed] [Google Scholar]
- Harmon R. C., Stein N., Frelinger J. A. Monoclonal antibodies reactive with H-2 determinants. Immunogenetics. 1983;18(5):541–545. doi: 10.1007/BF00364395. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Chaouat G., Rabourdin-Combe C., Claverie J. M. Working principles in the immune system implied by the "peptidic self" model. Proc Natl Acad Sci U S A. 1987 May;84(10):3400–3404. doi: 10.1073/pnas.84.10.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. B., Ozato K., Richardson J. C., Bluestone J. A. Molecular localization of allogeneic and self determinants recognized by bulk and clonal populations of cytotoxic T cells. J Immunol. 1985 Feb;134(2):677–683. [PubMed] [Google Scholar]
- Levy R. B., Shearer G. M. Cell-mediated lympholysis responses against autologous cells modified with haptenic sulfhydryl reagents. IV. Self-determinants recognized by wild-type anti-H-2Kb and H-2Db-restricted cytotoxic T cells specific for sulfhydryl and amino-reactive haptens are absent in certain H-2 mutant strains. J Immunol. 1982 Oct;129(4):1525–1529. [PubMed] [Google Scholar]
- Maryanski J. L., Pala P., Corradin G., Jordan B. R., Cerottini J. C. H-2-restricted cytolytic T cells specific for HLA can recognize a synthetic HLA peptide. Nature. 1986 Dec 11;324(6097):578–579. doi: 10.1038/324578a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin-Taylor E., Woodward J. G., McMillan M., Frelinger J. A. Distinct epitopes are recognized by cytolytic T lymphocyte clones on the same class I molecule: direct demonstration using DNA-transfected targets and long-term cytolytic T cell clones. Eur J Immunol. 1984 Nov;14(11):969–974. doi: 10.1002/eji.1830141102. [DOI] [PubMed] [Google Scholar]
- McMillan M., Frelinger J. A., Jones P. P., Murphy D. B., McDevitt H. O., Hood L. Structure of murine Ia antigens. Two dimensional electrophoretic analyses and high pressure liquid chromatography tryptic peptide maps of products of the I-A and I-E subregions and of an associated invariant polypeptide. J Exp Med. 1981 Apr 1;153(4):936–950. doi: 10.1084/jem.153.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief C. J., de Waal L. P., van der Meulen M. Y., Melvold R. W., Kohn H. I. Fine specificity of alloimmune cytotoxic T lymphocytes directed against H-2K. A study with Kb mutants. J Exp Med. 1980 May 1;151(5):993–1013. doi: 10.1084/jem.151.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabholz M., MacDonald H. R. Cytolytic T lymphocytes. Annu Rev Immunol. 1983;1:273–306. doi: 10.1146/annurev.iy.01.040183.001421. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Owen M. J., Crumpton M. J. The role of class I and II antigens in T cell recognition. Br Med Bull. 1987 Jan;43(1):228–240. doi: 10.1093/oxfordjournals.bmb.a072173. [DOI] [PubMed] [Google Scholar]
- Ozato K., Evans G. A., Shykind B., Margulies D. H., Seidman J. G. Hybrid H-2 histocompatibility gene products assign domains recognized by alloreactive T cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2040–2043. doi: 10.1073/pnas.80.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Ozato K., Takahashi H., Appella E., Sears D. W., Murre C., Seidman J. G., Kimura S., Tada N. Polymorphism of murine major histocompatibility Class I antigen: assignment of putative allodeterminants to distinct positions of the amino acid sequence within the first external domain of the antigen. J Immunol. 1985 Mar;134(3):1749–1758. [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Itakura K., Wallace R. B. Isolation of a cDNA clone for the murine transplantation antigen H-2Kb. Proc Natl Acad Sci U S A. 1982 May;79(10):3270–3274. doi: 10.1073/pnas.79.10.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B. Analysis of code relating sequences to conformation in globular prtoeins. Theory and application of expected information. Biochem J. 1974 Sep;141(3):853–867. doi: 10.1042/bj1410853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schold M., Colombero A., Reyes A. A., Wallace R. B. Oligonucleotide-directed mutagenesis using plasmid DNA templates and two primers. DNA. 1984 Dec;3(6):469–477. doi: 10.1089/dna.1.1984.3.469. [DOI] [PubMed] [Google Scholar]
- Schulze D. H., Pease L. R., Geier S. S., Reyes A. A., Sarmiento L. A., Wallace R. B., Nathenson S. G. Comparison of the cloned H-2Kbm1 variant gene with the H-2Kb gene shows a cluster of seven nucleotide differences. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2007–2011. doi: 10.1073/pnas.80.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheil J. M., Bevan M. J., Sherman L. A. Immunoselection of structural H-2Kb variants: use of cloned cytolytic T cells to select for loss of a CTL-defined allodeterminant. Immunogenetics. 1986;23(1):52–59. doi: 10.1007/BF00376522. [DOI] [PubMed] [Google Scholar]
- Stroynowski I., Clark S., Henderson L. A., Hood L., McMillan M., Forman J. Interaction of alpha 1 with alpha 2 region in class I MHC proteins contributes determinants recognized by antibodies and cytotoxic T cells. J Immunol. 1985 Sep;135(3):2160–2166. [PubMed] [Google Scholar]
- Tan Z. K., Ikuta S., Huang T., Dugaiczyk A., Itakura K. Solid-phase synthesis of polynucleotides. VIII: A simplified synthesis of oligodeoxyribonucleotides. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):383–391. doi: 10.1101/sqb.1983.047.01.045. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein P. J. H-2 effects on cell-cell interactions in the response to single non-H-2 alloantigens. V. Effects of H-2Kb mutations on presentation of H-4 and H-3 alloantigens. J Immunol. 1982 Jun;128(6):2629–2633. [PubMed] [Google Scholar]
- Weyand C., Hämmerling G. J., Goronzy J. Recognition of H-2 domains by cytotoxic T lymphocytes. Nature. 1981 Aug 13;292(5824):627–629. doi: 10.1038/292627a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]