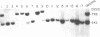Abstract
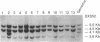
X chromosome-linked severe combined immunodeficiency (XSCID) is characterized by markedly reduced numbers of T cells, the absence of proliferative responses to mitogens, and hypogammaglobulinemia but normal or elevated numbers of B cells. To determine if the failure of the B cells to produce immunoglobulin might be due to expression of the XSCID gene defect in B-lineage cells as well as T cells, we analyzed patterns of X chromosome inactivation in B cells from nine obligate carriers of this disorder. A series of somatic cell hybrids that selectively retained the active X chromosome was produced from Epstein-Barr virus-stimulated B cells from each woman. To distinguish between the two X chromosomes, the hybrids from each woman were analyzed using an X-linked restriction fragment length polymorphism for which the woman in question was heterozygous. In all obligate carriers of XSCID, the B-cell hybrids demonstrated preferential use of a single X chromosome, the nonmutant X, as the active X. To determine if the small number of B-cell hybrids that contained the mutant X were derived from an immature subset of B cells, lymphocytes from three carriers were separated into surface IgM positive and surface IgM negative B cells prior to exposure to Epstein-Barr virus and production of B-cell hybrids. The results demonstrated normal random X chromosome inactivation in B-cell hybrids derived from the less mature surface IgM positive B cells. In contrast, the pattern of X chromosome inactivation in the surface IgM negative B cells, which had undergone further replication and differentiation, was significantly nonrandom in all three experiments [logarithm of odds (lod) score greater than 3.0]. These results suggest that the XSCID gene product has a direct effect on B cells as well as T cells and is required during B-cell maturation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Hovi T., Watts R. W., Webster A. D. Immunological observations on patients with Lesch-Nyhan syndrome, and on the role of de-novo purine synthesis in lymphocyte transformation. Lancet. 1975 Dec 13;2(7946):1179–1183. doi: 10.1016/s0140-6736(75)92661-6. [DOI] [PubMed] [Google Scholar]
- Aman P., Ehlin-Henriksson B., Klein G. Epstein-Barr virus susceptibility of normal human B lymphocyte populations. J Exp Med. 1984 Jan 1;159(1):208–220. doi: 10.1084/jem.159.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankhurst A. D., Lambert P. H., Miescher P. A. Studies on the thymic dependence of the immunoglobulin classes of the mouse (38570). Proc Soc Exp Biol Med. 1975 Feb;148(2):501–504. doi: 10.3181/00379727-148-38570. [DOI] [PubMed] [Google Scholar]
- Bloemmen J., Eyssen H. Immunoglobulin levels of sera of genetically thymusless (nude) mice. Eur J Immunol. 1973 Feb;3(2):117–118. doi: 10.1002/eji.1830030213. [DOI] [PubMed] [Google Scholar]
- Buckley R. H., Gilbertsen R. B., Schiff R. I., Ferreira E., Sanal S. O., Waldmann T. A. Heterogeneity of lymphocyte subpopulations in severe combined immunodeficiency. Evidence against a stem cell defect. J Clin Invest. 1976 Jul;58(1):130–136. doi: 10.1172/JCI108441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley R. H., Schiff S. E., Sampson H. A., Schiff R. I., Markert M. L., Knutsen A. P., Hershfield M. S., Huang A. T., Mickey G. H., Ward F. E. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol. 1986 Apr 1;136(7):2398–2407. [PubMed] [Google Scholar]
- Conley M. E. B cells in patients with X-linked agammaglobulinemia. J Immunol. 1985 May;134(5):3070–3074. [PubMed] [Google Scholar]
- Conley M. E., Bartelt M. S. In vitro regulation of IgA subclass synthesis. II. The source of IgA2 plasma cells. J Immunol. 1984 Nov;133(5):2312–2316. [PubMed] [Google Scholar]
- Conley M. E., Brown P., Pickard A. R., Buckley R. H., Miller D. S., Raskind W. H., Singer J. W., Fialkow P. J. Expression of the gene defect in X-linked agammaglobulinemia. N Engl J Med. 1986 Aug 28;315(9):564–567. doi: 10.1056/NEJM198608283150907. [DOI] [PubMed] [Google Scholar]
- Conley M. E., Chan M. A., Sigal N. H. In vitro regulation of IgA subclass production. III. Selective transformation of IgA1 producing cells by Epstein-Barr virus. J Immunol. 1987 Mar 1;138(5):1403–1407. [PubMed] [Google Scholar]
- Conley M. E., Koopman W. J. Serum IgA1 and IgA2 in normal adults and patients with systemic lupus erythematosus and hepatic disease. Clin Immunol Immunopathol. 1983 Mar;26(3):390–397. doi: 10.1016/0090-1229(83)90123-x. [DOI] [PubMed] [Google Scholar]
- Cowan M. J., Wara D. W., Weintrub P. S., Pabst H., Ammann A. J. Haploidentical bone marrow transplantation for severe combined immunodeficiency disease using soybean agglutinin-negative, T-depleted marrow cells. J Clin Immunol. 1985 Nov;5(6):370–376. doi: 10.1007/BF00915333. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. The origin and development of human tumors studied with cell markers. N Engl J Med. 1974 Jul 4;291(1):26–35. doi: 10.1056/NEJM197407042910109. [DOI] [PubMed] [Google Scholar]
- Forrester L. M., Ansell J. D., Micklem H. S. Development of B lymphocytes in mice heterozygous for the X-linked immunodeficiency (xid) mutation. xid inhibits development of all splenic and lymph node B cells at a stage subsequent to their initial formation in bone marrow. J Exp Med. 1987 Apr 1;165(4):949–958. doi: 10.1084/jem.165.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuscoe J. C., Fenwick R. G., Jr, Ledbetter D. H., Caskey C. T. Deletion and amplification of the HGPRT locus in Chinese hamster cells. Mol Cell Biol. 1983 Jun;3(6):1086–1096. doi: 10.1128/mcb.3.6.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand E. W., Dosch H. M. Diagnosis and classification of severe combined immunodeficiency disease. Birth Defects Orig Artic Ser. 1983;19(3):65–72. [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Epstein-Barr virus binding sites on lymphocyte subpopulations and the origin of lymphoblasts in cultured lymphoic cell lines and in the blood of patients with infectious mononucleosis. Clin Immunol Immunopathol. 1975 Mar;3(4):514–524. doi: 10.1016/0090-1229(75)90076-8. [DOI] [PubMed] [Google Scholar]
- Griscelli C., Durandy A., Virelizier J. L., Ballet J. J., Daguillard F. Selective defect of precursor T cells associated with apparently normal B lymphocytes in severe combined immunodeficiency disease. J Pediatr. 1978 Sep;93(3):404–411. doi: 10.1016/s0022-3476(78)81146-9. [DOI] [PubMed] [Google Scholar]
- Hansson M., Falk K., Ernberg I. Epstein-Barr virus transformation of human pre-B cells. J Exp Med. 1983 Aug 1;158(2):616–622. doi: 10.1084/jem.158.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R. Genetic deficiencies of adenosine deaminase and purine nucleoside phosphorylase: overview, genetic heterogeneity and therapy. Birth Defects Orig Artic Ser. 1983;19(3):73–81. [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamatani N., Yamanaka H., Nishioka K., Nakamura T., Nakano K., Tanimoto K., Mizuno T., Nishida Y. A new method for the detection of Lesch-Nyhan heterozygotes by peripheral blood T cell culture using T cell growth factor. Blood. 1984 Apr;63(4):912–916. [PubMed] [Google Scholar]
- Katamine S., Otsu M., Tada K., Tsuchiya S., Sato T., Ishida N., Honjo T., Ono Y. Epstein-Barr virus transforms precursor B cells even before immunoglobulin gene rearrangements. Nature. 1984 May 24;309(5966):369–372. doi: 10.1038/309369a0. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Moser H. W., Moser A. B., Axelman J., Sillence D., Norum R. A. Adrenoleukodystrophy: evidence for X linkage, inactivation, and selection favoring the mutant allele in heterozygous cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5066–5070. doi: 10.1073/pnas.78.8.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Scher I., Cossman J., Kessler S., Mongini P. K., Hansen C., Finkelman F. D., Paul W. E. Role of the thymus in directing the development of a subset of B lymphocytes. J Exp Med. 1982 Mar 1;155(3):924–936. doi: 10.1084/jem.155.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm M. H., Paslay J. W., Davie J. M. Unbalanced X chromosome mosaicism in B cells of mice with X-linked immunodeficiency. J Exp Med. 1983 Sep 1;158(3):920–931. doi: 10.1084/jem.158.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhan W. L., Bakay B., Connor J. D., Marks J. F., Keele D. K. Hemizygous expression of glucose-6-phosphate dehydrogenase in erythrocytes of heterozygotes for the Lesch-Nyhan syndrome. Proc Natl Acad Sci U S A. 1970 Jan;65(1):214–218. doi: 10.1073/pnas.65.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa S. G., Pahwa R. N., Good R. A. Heterogeneity of b lymphocyte differentiation in severe combined immunodeficiency disease. J Clin Invest. 1980 Sep;66(3):543–550. doi: 10.1172/JCI109886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck J. M., Nussbaum R. L., Conley M. E. Carrier detection in X-linked severe combined immunodeficiency based on patterns of X chromosome inactivation. J Clin Invest. 1987 May;79(5):1395–1400. doi: 10.1172/JCI112967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Frank A., Henderson E., Schweitzer J., Miller G. Surface markers and size of lymphocytes in human umbilical cord blood stimulated into deoxyribonucleic acid synthesis by Epstein-Barr Virus. Infect Immun. 1979 Oct;26(1):225–231. doi: 10.1128/iai.26.1.225-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher I., Ahmed A., Strong D. M., Steinberg A. D., Paul W. E. X-linked B-lymphocyte immune defect in CBA/HN mice. I. Studies of the function and composition of spleen cells. J Exp Med. 1975 Apr 1;141(4):788–803. [PMC free article] [PubMed] [Google Scholar]
- Scher I., Berning A. K., Kessler S., Finkelman F. D. Development of B lymphocytes in the mouse; studies of the frequency and distribution of surface IgM and IgD in normal and immune-defective CBA/N F1 mice. J Immunol. 1980 Oct;125(4):1686–1693. [PubMed] [Google Scholar]
- Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Robins R. A., Stevens R. H., Klein R. B., Waldman D. J., Zeltzer P. M., Kessler S. W. Severe combined immunodeficiency with B lymphocytes: in vitro correction of defective immunoglobulin production by addition of normal T lymphocytes. Clin Exp Immunol. 1976 Oct;26(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Shearer W. T., Ritz J., Finegold M. J., Guerra I. C., Rosenblatt H. M., Lewis D. E., Pollack M. S., Taber L. H., Sumaya C. V., Grumet F. C. Epstein-Barr virus-associated B-cell proliferations of diverse clonal origins after bone marrow transplantation in a 12-year-old patient with severe combined immunodeficiency. N Engl J Med. 1985 May 2;312(18):1151–1159. doi: 10.1056/NEJM198505023121804. [DOI] [PubMed] [Google Scholar]
- Sprent J., Bruce J. Physiology of B cells in mice with X-linked immunodeficiency (xid). III. Disappearance of xid B cells in double bone marrow chimeras. J Exp Med. 1984 Sep 1;160(3):711–723. doi: 10.1084/jem.160.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. D., Ledgley C. J., Sigal N. H. Patterns of isotype commitment in human B cells: limiting dilution analysis of Epstein Barr virus-infected cells. J Immunol. 1983 Apr;130(4):1640–1645. [PubMed] [Google Scholar]
- Tosato G., Blaese R. M., Yarchoan R. Relationship between immunoglobulin production and immortalization by Epstein Barr virus. J Immunol. 1985 Aug;135(2):959–964. [PubMed] [Google Scholar]
- Wortis H. H., Burkly L., Hughes D., Roschelle S., Waneck G. Lack of mature B cells in nude mice with X-linked immune deficiency. J Exp Med. 1982 Mar 1;155(3):903–913. doi: 10.1084/jem.155.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]