Abstract
An operant conditioning situation for the blow fly (Protophormia terrae novae) is described. Individual flies are trained to enter and reenter a hole as the operant response. Only a few sessions of contingent reinforcement are required to increase response rates. When the response is no longer followed by food, the rate of entering the hole decreases. Control procedures revealed that rate of responding is not a simple overall result of feeding or of aging. The flies entered into the hole only if the response was required to obtain the food.
Keywords: associative learning, operant conditioning, blow flies, apparatus
The majority of studies on learning in dipterans have been done in the context of classical conditioning of proboscis extension where one or more pairings of a conditioned stimulus (CS) with an unconditioned stimulus (US) subsequently leads to an extension of the proboscis to the CS in anticipation of the US (Chabaud, Devaud, Pham-Delègue, Preat & Kaiser, 2006; Nelson, 1971). Work has also been done on olfactory avoidance by pairing an odor with electric shock, this odor being subsequently avoided by the flies (Tully & Quinn, 1985; Waddell & Quinn, 2001). Species of Musca, Drosophila and Phormia have all been used as model systems to study various aspects of behavior including learning, memory (Davis, 2005; Fukushi, 1976, 1979; McGuire, 1984), and motivation (Dethier, 1976).
In contrast to classical conditioning, there are few studies of operant conditioning in dipterans (Brembs, 2003). Operant conditioning would require the insect to operate some manipulandum or use a behavior in a new or arbitrary way to obtain reinforcement (Abramson, 1994). The various techniques used previously include mazes (Bicker & Spatz, 1976; Fukushi, 1985, 1989), shuttle boxes (Diegelmann, Zars, & Zars, 2006; Leeming, 1985; Leeming & Little, 1977; Putz & Heisenberg, 2002; Wustmann, Rein, Wolf, & Heisenberg, 1996) and situations where leg position or force exerted in stationary flight is punished (Booker & Quinn, 1981; Brembs & Heisenberg, 2000; Gong, Xia, Liu, Feng, & Guo, 1998; Mariath, 1985; Wolf & Heisenberg, 1991).
Operant chambers are available for several invertebrate species including crustaceans (Abramson & Feinman, 1990), stingless bees (Pessotti, 1972), honey bees (Grossmann, 1973; Sigurdson, 1981), snails (Balaban & Chase, 1989), Aplysia (Downey & Jahan-Parwar, 1972) and cockroaches (Rubadeau & Conrad, 1963). The only procedure we know where a dipteran used a behavior in an arbitrary way was developed by Wolf, Voss, Hein and Heisenberg (1992). In this study, a drosophila successfully controlled the angular position of a visual panorama by choosing the orientation of its posture.
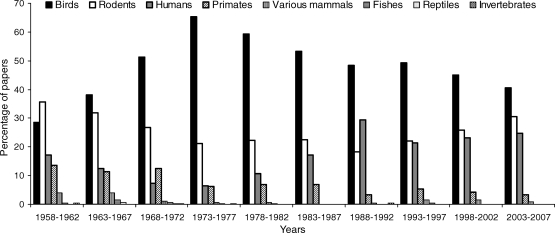
Since the beginning of JEAB, most published experimental articles about conditioning concerned a narrow list of species. Figure 1 shows the range of species covered by the journal from 1958 to 2008. The vast majority of articles concern birds (97.4% of these were pigeons), rodents (97% were rats), humans, and various primates. These species make up 97.9% of the total published articles. Mammals such as horses, cats, dogs or porpoises comprise 1.42% of the total articles, fishes and reptiles 0.5% of the total articles, and finally invertebrates 0.13% of the total articles. Despite the importance of insects in the animal kingdom and what they can tell us about the evolution of learning, we found only two articles with insects, one with the honey bee (Grossman, 1973) and the other with cockroaches (Rubadeau & Conrad, 1963). The cockroach article contained no data on which to evaluate its effectiveness.
Fig 1.
Range of species covered by JEAB from 1958 to 2008. Species from 5 successive years have been combined. Only empirical articles with data have been considered in the analysis.
The present article describes an operant conditioning paradigm developed for an invertebrate, the blow fly Protophormia terrae novae. An individual blow fly enters a small hole to receive food. We decided to focus our attention on P. terrae novae because of its importance as a myiasis pest of livestock such as sheep (Wall & Shearer, 1997) and its use as an object for life history studies (Collatz, 1997). We were also interested in whether lawful data can be obtained from a traditional operant situation using a nontraditional organism. We were aided in our design by the large size of P. terrae novae that can approach 7–12 mm in length and by the large amount of information on its natural history and feeding preferences (Norris, 1965; Whitworth, 2006).
METHOD
Subjects
Six adult blow flies (Protophormia terrae novae), were reared from maggots obtained from a local supplier. When the larvae pupated, they were placed individually in their own “home cage.” The home cage was constructed from transparent plastic kitchen boxes (“Mepal moduul” 320 ml, 12.5 cm × 7.6 cm × 6.3 cm) with the inside dimensions reduced to 3 cm × 4.5 cm × 12 cm with two horizontal partitions. Twenty-four 2-mm diameter holes were drilled in either side of the cage for air circulation.
The home cage also served as the experimental chamber. The subject remained in the home cage throughout the remainder of its life. When the cages were not connected to the training apparatus they were stored between 20 and 25°C. Because young adults do not feed before 2 days old, the experiment began when the flies were 3 days old. The flies stayed alive for approximately 2 weeks.
Apparatus
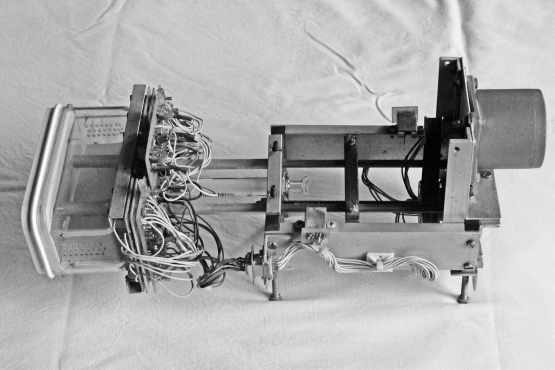
Figure 2 provides an overview of the apparatus. The experimental chamber with its associated circuitry is shown on the left with the 5-ml glass syringe and stepper motor used to deliver sucrose reinforcement shown on the right. The experimental chamber is easily connected to, and removed from, the reinforcement mechanism with two brass screws. Circuit diagrams are not provided in this article because they are standard circuits used, for example, to detect when an infrared light beam is broken and to turn on various lights and the stepper motor.
Fig 2.
Photograph of the experimental device. A plastic box containing the fly (to the left) is screwed to a stepping motor mechanism linked to a glass syringe. The box with the fly can be easily screwed in place.
The operant response consisted of moving in and out of a 7-mm hole. The hole was created from a larger hole 1.3 cm diameter × 1.5 cm long which was reduced to the required size by two plastic plates. The rationale behind reducing the size was to allow the fly to enter the hole only in the horizontal plane thereby minimizing variations in topography that could influence performance. Moving in and out of the hole was detected with two infrared emitter and detector pairs (Honeywell SEP8736 and SDP8436). The two infrared lights were positioned in the form of a cross to ensure that all entrances into the hole were detected.
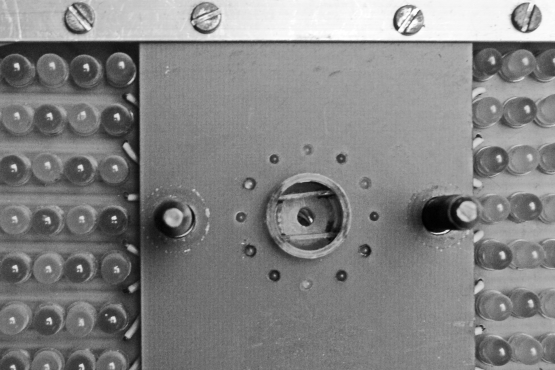
Entry into the hole was signalled with twelve 1.8-mm diameter light emitting diodes (six yellow and six green, in alternation) placed equal distance around the circumference of the circle (2.5 cm diameter, see Figure 3). On each side of the circle, seven lines of six 5-mm light-emitting diodes were arranged in alternation of green and yellow (but not used in this experiment). The purpose of the lights was to provide discriminative stimuli. Figure 3 shows the response hole and discriminative stimuli.
Fig 3.
Photograph of the hole that constitutes the operant response. Note the two plastic plates that restrict the orientation into the hole. Entry into the hole is detected by photocells. Twelve light-emitting diodes (yellow and green) are arranged around the hole and serve as discriminative stimuli.
The reinforcer was 0.086 µl of 5% sucrose solution delivered at the bottom of the hole by the 0.1-mm diameter needle of a 5-ml glass syringe. The tip of the needle was cut to be flush with the bottom of the hole. After each session, the hole was cleaned with alcohol and water. The syringe was activated automatically with a stepper motor controlled by ITC Comstep and Powerstep interface connected to a 486DX2-50 PC computer through the printer port. The software was written in Borland Pascal (MS DOS).
Procedure
Sessions were conducted daily for each fly. A session began by carrying a subject's home container to the conditioning device and screwing it in place. Figure 4 shows the home cage connected to the training device. Each animal received one session per day throughout its lifetime. Each session ended after 15 responses or 1 hr. Our principal purpose in limiting the session length was to obtain some baseline data on the longevity of the flies when confined to the apparatus and to collect data on continuous reinforcement (CRF) schedules that can be used later for experiments on schedules of reinforcement. A session began by turning on the yellow and green lights that were positioned around the response hole. When the animal entered the hole the lights were turned off.
Fig 4.
A photograph of the fly chamber connected to operant device.
In operant conditioning studies it is standard practice to study a few animals over a long period of time (Sidman, 1960). This type of design focuses on a detailed analysis of an individual's behavior, and is the most popular design in operant conditioning. We used an AB design with two flies (flies F3 and F4), with A corresponding to the baseline level in which each response was not followed by reinforcement, and B corresponding to the conditioning phase in which each response was reinforced. For flies F3 and F4, we stayed 6 days at the baseline level before switching to the B phase, which continued until the fly died. For two other flies (F5 and F6), we used an ABA design. For fly F5, the first two phases were performed over 6 days, with one day devoted to a return to baseline. For fly F6, we alternated the phase every 3 days. We used two additional control flies (F1 and F2), which remained under baseline conditions throughout their life. The rationale behind the use of these two animals was to determine if entering the hole increased in the absence of reinforcement and as the fly ages.
Phase A consisted of the baseline condition. During this phase, each entrance to the hole activated the syringe, but it was not filled, and so the fly received the same amount of vibration from the stepper motor as in phase B, but without food. At the beginning of the sessions, a drop of 1.29 µl sucrose solution (equivalent to the total amount received per session in phase B) was manually put inside the box at a random place. As we know that sugar may elicit walking (Dethier, 1957), the goal of this procedure was to measure the rate of hole entering elicited by sucrose solution alone. The yellow and green lights were on except during a 3-s timeout following each exit from the hole.
During phase B, each access to the hole was followed by a reinforcer (CRF schedule). After the reinforcer was consumed, and the fly backed out of the hole, a 3-s time-out (yellow and green lights off) was scheduled with no responses being reinforced. This procedure was instigated to prevent small movements of the fly being recorded as a response. In addition, the delay had the benefit of requiring the animal to back out and then reenter the hole thereby providing response feedback. Following the training session no additional food or water was provided.
RESULTS AND DISCUSSION
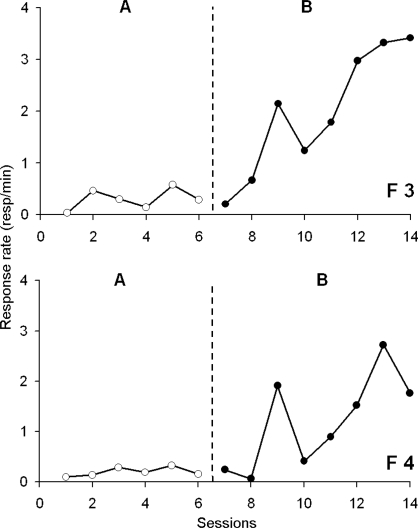
Figure 5 shows the rate of hole entering for two flies that received no reinforcement for entering the hole during the first phase but whose hole entry was reinforced during the second phase of training (i.e., AB design). The two flies showed similar patterns of behavior. A very low rate of responding was observed during the unreinforced baseline condition, which substantially increased during the second phase of the experiment when food was contingent upon entering the hole. Note that the change appeared only after several sessions, suggesting that the contingency took some time to be effective. Whether this was due to some inhibitory conditioning learned during the first phase is not known at this time. As the contingent reinforcement sessions continued, the rate of responding increased until the end of the animal's life.
Fig 5.
Response rate (responses/min) as a function of experimental sessions for 2 flies F3 and F4 involved in the AB design. The vertical dotted line shows the switch from the baseline level (“A”, open circles) to the continuous reinforcement schedule (“B”, closed circles). These 2 flies worked for 14 consecutive days.
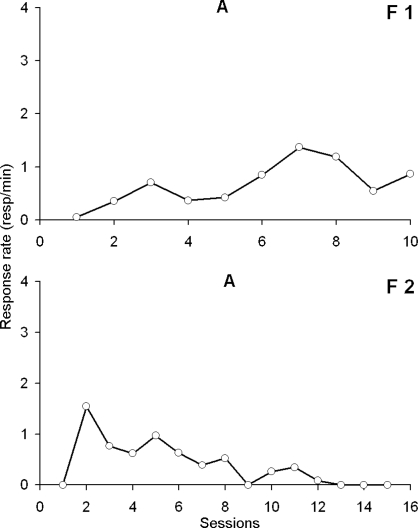
Two alternative explanations other than the effect of reinforcement for the increase in responding observed during the contingent condition are that 1) as the fly ages its hole entering behavior increases as a result of maturation and 2) hole entering behavior increases as a result of sucrose stimulation per se. Figure 6 shows the results for flies F1 and F2 who were only exposed to the baseline condition. No systematic or substantial increase in response rate was observed during the lifetime of these subjects nor did hole entering increase with repeated consumption of sugar solution. F1 showed a small increase from session 1 to session 10, but it was not comparable to the large one observed with experimental flies when the response was reinforced. Contrary to F1, F2 showed a regular decrease in response rate. The highest rate was obtained the second day, and a zero rate was obtained after 12 days.
Fig 6.
Response rate (responses/min) as a function of experimental sessions for the 2 control flies F1 and F2. No reinforcer was given to these flies (baseline condition written as “A”). F1 remained alive for 10 consecutive days, and F2 for 16 days.
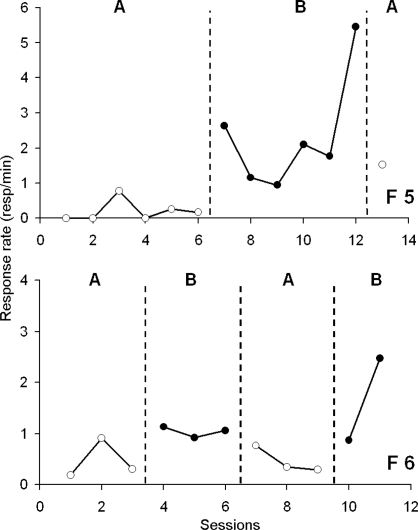
In order to obtain some information on the effect of extinction, we returned a fly to the baseline condition following contingent reinforcement (i.e., ABA design). Figure 7 shows that the behavior of fly F5 was similar to the behavior of flies F3 and F4 that received a baseline condition followed by contingent reinforcement. When fly F5 was returned to the baseline condition its rate of entering the hole decreased. Unfortunately, fly F5 died after only one extinction session.
Fig 7.
Response rate (responses/min) as a function of experimental sessions for the 2 flies F5 and F6 involved in the ABA design. The dotted vertical line shows the switch to the next condition (Baseline, open circles, written as “A” or CRF, closedcircles, written as “B”). F5 stayed 6 days in each condition before a switch, and F6 only 3 days before a change in reinforcer delivery.
To gather additional data on the effect of extinction we reduced the number of sessions within each condition. This needed to be done because the lifespan of the blow fly is short and we had no way of predicting how many sessions could be conducted, or conditions employed, before an individual fly died. Figure 7 shows the performance of fly F6 across conditions. Although the response rate was low during the first contingent phase (B), it was reduced further by extinction (A) and increased during the second contingent condition (B).
It has been suggested that the small brain and short life span precludes insects from learning a wide range of behavior (Dethier, 1962; Mayr, 1974). Our data show that dipterans are capable of a wider range of learned behavior than previously demonstrated (Brembs, 2003; Dukas, 2008). The development of an operant conditioning paradigm for Protophormia terrae novae is the first step to quantitatively study operant conditioning phenomena in this economically important species and opens a promising field for comparative investigations on learning phenomena traditionally studied using operant methodology (Skinner, 1956; Staddon & Cerutti, 2003).
The apparatus functioned very well. Only a few sessions of contingent reinforcement were required to increase response rates. When the response no longer produced sucrose, the rate of entering the hole decreased. Moreover, within the individual chambers serving also as conditioning boxes, we were able to detect these changes in individual behavior (Dunlap, 1935) without any direct manipulation of the flies. As the control flies revealed, rate of responding was not a simple overall result of feeding or of aging. The flies entered the hole only if the response was required to get the food.
However, at this stage of research, these first results need to be considered carefully. At the beginning of the session during the control phase (phase A) we gave in the chamber, but outside the response hole, an amount of sucrose equivalent to the total amount consumed by flies per session in the experimental condition (phase B). Although this procedure provides equal access to the solution across experimental and control phases, it does not exactly reproduce the periodic access to the reinforcer in the experimental condition. The possibility that hole entering might be a byproduct of periodic consumption cannot be completely ruled out. A yoked control could be used, but as Church (1964) has shown, the comparison of different subjects can be a source of additional problems if there are individual differences in the effectiveness of the reinforcer to elicit the response. A better control procedure may consist of providing the fly with a choice between two holes, only one providing reinforcement, with the response rate expected to be higher for the active hole. However, this procedure requires substantial changes in the hardware and software. As our preliminary data are encouraging, these developments will be the next goal in our fly learning analysis.
Contrary to most dipteran instrumental conditioning studies where negative reinforcement or punishment procedures are common (Booker & Quinn, 1981; Brembs & Heisenberg, 2000; Gong et al., 1998; Mariath, 1985; Wolf & Heisenberg, 1991), we used positive reinforcement with sucrose as the reinforcer. Our use of a nutritional positive reinforcer suggests that life history studies using operant conditioning can now be performed with the blow fly. Operant learning with food reinforcement may increase food consumption and fitness but at a cost relative to energy consumption and neural activity. Such a cost produced by an ability to learn may ironically decrease lifespan (Laughlin, 2001; Mery & Kawecki, 2004). It is known that mating shortens the lifespan in flies and the question naturally arises whether learned behavior also influences life history traits (Collatz & Wilps, 1985). Since it is relatively easy to study the behavior of a fly during its lifetime, our apparatus also opens the door to new studies of life history trade-offs.
Finally, as Phormia have been used for a number of years as a model insect for the study of aging (Collatz, 1997) our situation is ideal for such studies because now it is possible to study the maintenance of learned behavior (and specially of operant behavior) throughout its aging process (Fresquet & Medioni, 1993).
Acknowledgments
This research was supported by a grant from the Société Picarde pour l'Etude du Comportement Animal (http://speca.free.fr). This article was written during the completion by the first author of a Fulbright scholarship program at Oklahoma State University. Thanks to François Tonneau and John Kraft for their encouragement. Thanks to three reviewers for their helpful comments.
REFERENCES
- Abramson C.I. A primer of invertebrate learning: The behavioral perspective. Washington D.C: American Psychological Association; 1994. [Google Scholar]
- Abramson C.I, Feinman R.D. Lever-press conditioning in the crab. Physiology & Behavior. 1990;48:267–272. doi: 10.1016/0031-9384(90)90311-q. [DOI] [PubMed] [Google Scholar]
- Balaban P.M, Chase R. Self-stimulation in snails. Neuroscience Research Communications. 1989;4:139–146. [Google Scholar]
- Bicker G, Spatz H.C. Maze-learning ability of Drosophila melanogaster. Nature. 1976;260:371. [Google Scholar]
- Booker R, Quinn W.C. Conditioning of leg position in normal and mutant Drosophila. Proceedings of the National Academy of Sciences. 1981;78:3940–3944. doi: 10.1073/pnas.78.6.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembs B. Operant conditioning in invertebrates. Current Opinion in Neurobiology. 2003;13:710–717. doi: 10.1016/j.conb.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Brembs B, Heisenberg M. The operant and the classical in conditioned orientation of Drosophila melanogaster at the flight simulator. Learning & Memory. 2000;7:104–115. doi: 10.1101/lm.7.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud M.A, Devaud J.M, Pham-Delègue M.H, Preat T, Kaiser L. Olfactory conditioning of proboscis activity in Drosophila melanogaster. Journal of Comparative Physiology A. 2006;192:1335–1348. doi: 10.1007/s00359-006-0160-3. [DOI] [PubMed] [Google Scholar]
- Church R.M. Systematic effect of random error in the yoked control design. Psychological Bulletin. 1964;62:122–131. doi: 10.1037/h0042733. [DOI] [PubMed] [Google Scholar]
- Collatz K.G. Fifteen years of Phormia – on the value of an insect for the study of aging. Archives of Gerontology and Geriatrics. 1997;25:83–90. doi: 10.1016/s0167-4943(96)00773-x. [DOI] [PubMed] [Google Scholar]
- Collatz K.G, Wilps H. The quantitative relationship between life span, food ingestion, egg production, mating, and flight-activity of protein-fed blowfly Phormia terrae novae females. Experimental Gerontology. 1985;20:347–357. doi: 10.1016/0531-5565(85)90015-4. [DOI] [PubMed] [Google Scholar]
- Davis R.L. Olfactory memory formation in Drosophila: From molecular to systems neuroscience. Annual Review of Neuroscience. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Dethier V.G. Communication by insects: Physiology of dancing. Science. 1957;125:331–336. doi: 10.1126/science.125.3243.331. [DOI] [PubMed] [Google Scholar]
- Dethier V.G. To know a fly. New York: McGraw-Hill Publishing Company; 1962. [Google Scholar]
- Dethier V.G. The hungry fly: A physiological study of the behavior associated with feeding. Cambridge, MA: Harvard University Press; 1976. [Google Scholar]
- Diegelmann S, Zars M, Zars T. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in Drosophila. Learning & Memory. 2006;13:72–83. doi: 10.1101/lm.45506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey P, Jahan-Parwar B. Cooling as reinforcing stimulus in Aplysia. American Zoologist. 1972;12:507–512. [Google Scholar]
- Dukas R. Evolutionary biology of insect learning. Annual Review of Entomology. 2008;53:145–160. doi: 10.1146/annurev.ento.53.103106.093343. [DOI] [PubMed] [Google Scholar]
- Dunlap K. The average animal. Journal of Comparative Psychology. 1935;19:1–3. [Google Scholar]
- Fresquet N, Medioni J. Effects of ageing on visual discrimination learning in Drosophila melanogaster. Quarterly Journal of Experimental Psychology B. 1993;46:399–412. [PubMed] [Google Scholar]
- Fukushi T. Classical conditioning to visual stimuli in the housefly, Musca domestica. Journal of Insect Physiology. 1976;22:361–364. [Google Scholar]
- Fukushi T. Properties of olfactory conditioning in the housefly, Musca domestica. Journal of Insect Physiology. 1979;25:155–159. [Google Scholar]
- Fukushi T. Visual learning in walking blowflies, Lucilia cuprina. Comparative Physiology. 1985;157:771–778. doi: 10.1007/BF01350074. A. [DOI] [PubMed] [Google Scholar]
- Fukushi T. Learning and discrimination of colored papers in the walking blowfly, Lucilia cuprina. Journal of Comparative Physiology A. 1989;166:57–64. doi: 10.1007/BF00190210. [DOI] [PubMed] [Google Scholar]
- Gong Z.F, Xia S.Z, Liu L, Feng C.H, Guo A.K. Operant visual learning and memory in Drosophila mutants dunce, amnesiac and radish. Journal of Insect Physiology. 1998;44:1149–1158. doi: 10.1016/s0022-1910(98)00076-6. [DOI] [PubMed] [Google Scholar]
- Grossmann K.E. Continuous, fixed-ratio and fixed-interval reinforcement in honey bees. Journal of the Experimental Analysis of Behavior. 1973;20:105–109. doi: 10.1901/jeab.1973.20-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin S.B. Energy as a constraint on the coding and processing of sensory information. Current Opinions in Neurobiology. 2001;11:475–480. doi: 10.1016/s0959-4388(00)00237-3. [DOI] [PubMed] [Google Scholar]
- Leeming F.C. Free-response escape but not avoidance learning in houseflies (Musca domestica) Psychological Record. 1985;35:513–523. [Google Scholar]
- Leeming F.C, Little G.L. Escape learning in houseflies (Musca domestica) Journal of Comparative and Physiological Psychology. 1977;91:260–269. [Google Scholar]
- Mariath H.A. Operant conditioning in Drosophila melanogaster wild-type and learning mutants with defects in the cyclic AMP metabolism. Journal of Insect Physiology. 1985;31:779–787. [Google Scholar]
- Mayr E. Behavior programs and evolutionary strategies. American Scientist. 1974;62:650–659. [PubMed] [Google Scholar]
- McGuire T.R. Learning in three species of diptera: The blow fly Phormia regina, the fruit fly Drosophila melanogaster, and the house fly Musca domestica. Behavior Genetics. 1984;14:479–526. doi: 10.1007/BF01065445. [DOI] [PubMed] [Google Scholar]
- Mery F, Kawecki T.J. An operating cost of learning in Drosophila melanogaster. Animal Behaviour. 2004;68:589–598. [Google Scholar]
- Nelson M.C. Classical conditioning in the blowfly (Phormia regina): Associative and excitatory factors. Journal of Comparative and Physiological Psychology. 1971;77:353–368. doi: 10.1037/h0031882. [DOI] [PubMed] [Google Scholar]
- Norris K.R. Bionomics of blow flies. Annual Review of Entomology. 1965;10:47–68. [Google Scholar]
- Pessotti I. Discrimination with light stimuli and a lever-pressing response in Melipona rufiventris. Journal of Apicultural Research. 1972;11:89–93. [Google Scholar]
- Putz G, Heisenberg M. Memories in Drosophila heat-box learning. Learning & Memory. 2002;9:349–359. doi: 10.1101/lm.50402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubadeau D.O, Conrad K.A. An apparatus to demonstrate and measure operant behavior of arthropoda. Journal of the Experimental Analysis of Behavior. 1963;6:429–430. doi: 10.1901/jeab.1963.6-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M. Tactics of scientific research. New York: Basic Books; 1960. [Google Scholar]
- Sigurdson J.E. Automated discrete-trials techniques of appetitive conditioning in honey bees. Behavior Research Methods & Instrumentation. 1981;13:1–10. [Google Scholar]
- Skinner B.F. A case history in scientific method. American Psychologist. 1956;11:221–233. [Google Scholar]
- Staddon J.E.R, Cerutti D.T. Operant conditioning. Annual Review of Psychology. 2003;54:115–144. doi: 10.1146/annurev.psych.54.101601.145124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Quinn W.G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. Journal of Comparative Physiology A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Waddell S, Quinn W.G. Flies, genes, and learning. Annual Review of Neuroscience. 2001;24:1283–1309. doi: 10.1146/annurev.neuro.24.1.1283. [DOI] [PubMed] [Google Scholar]
- Wall R, Shearer D. Veterinary entomology: Arthropod ectoparasites of veterinary importance. London: Chapman & Hall; 1997. [Google Scholar]
- Whitworth T. Keys to the genera and species of blow flies (Diptera: Calliphoridae) of America north of Mexico. Proceedings of the Entomological Society of Washington. 2006;108:689–725. [Google Scholar]
- Wolf R, Heisenberg M. Basic organization of operant behavior as revealed in Drosophila flight orientation. Journal of Comparative Physiology A. 1991;169:699–705. doi: 10.1007/BF00194898. [DOI] [PubMed] [Google Scholar]
- Wolf R, Voss A, Hein S, Heisenberg M. Can a fly ride a bicycle. Philosophical transactions of the Royal Society of London B. 1992;337:261–269. [Google Scholar]
- Wustmann G, Rein K, Wolf R, Heisenberg M. A new paradigm for operant conditioning of Drosophila melanogaster. Journal of Comparative Physiology A. 1996;179:429–436. doi: 10.1007/BF00194996. [DOI] [PubMed] [Google Scholar]