Abstract
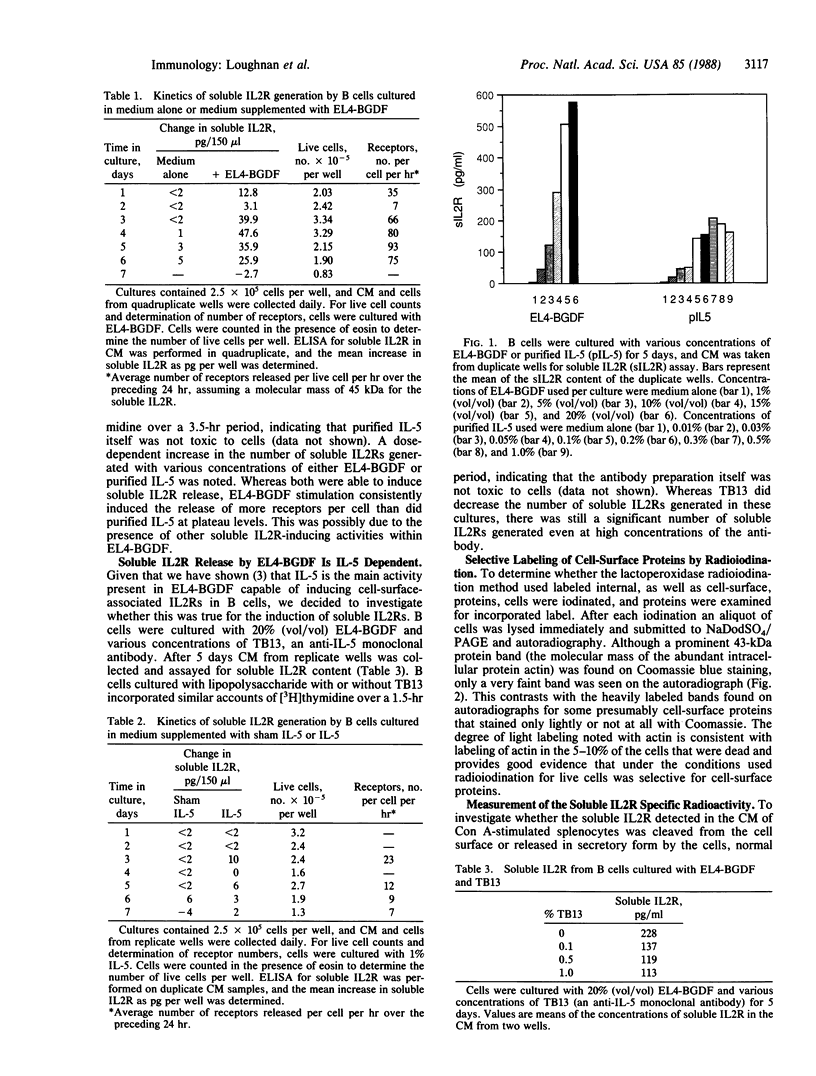
Murine T and B lymphocytes can be induced to release soluble interleukin 2 receptors (IL2Rs). This receptor is believed to be a truncated form of the 55-kDa chain of the cell-membrane-associated receptor. It has been speculated that this receptor may play an important immunoregulatory role by binding to interleukin 2 (IL-2). We report here that interleukin 5 can induce normal murine B cells to release soluble IL2Rs. This extends our finding that interleukin 5 similarly can induce murine B cells to express functional cell-surface-associated IL2Rs. Two possible mechanisms of release of soluble IL2Rs have been suggested. Soluble IL2R could be synthesized as a secretory form of the receptor lacking the transmembrane domain or by cleavage of the extracellular domain of the cell-surface-associated IL2R at the cell surface. To investigate which mechanism was operative, we radioiodinated the cell surface of normal murine splenocytes that had been cultured for 1 day with Con A to stimulate the expression of cell-surface-associated IL2Rs and the release of soluble IL2Rs. Under the conditions used, radiolabeling of internal proteins was not apparent. Labeled cells were then recultured with Con A, conditioned medium was taken from replicate cultures at various times after radioiodination, and the specific radioactivity of released soluble IL2Rs was determined by ELISA and RIA. We demonstrate that the specific radioactivity and the kinetics of change of the specific radioactivity are consistent with the hypothesis that the soluble IL2Rs are derived from the cell-surface-associated IL2Rs rather than being released in a secretory form.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berhanu P., Olefsky J. M. Photoaffinity labeling of insulin receptors in viable cultured human lymphocytes. Demonstration of receptor shedding and degradation. Diabetes. 1982 May;31(5 Pt 1):410–417. doi: 10.2337/diab.31.5.410. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Schrader J. W. Mechanism of effector-cell blockade. I. Antigen-induced suppression of Ig synthesis in a hybridoma cell line, and correlation with cell-associated antigen. J Exp Med. 1980 Jun 1;151(6):1436–1451. doi: 10.1084/jem.151.6.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell H. D., Tucker W. Q., Hort Y., Martinson M. E., Mayo G., Clutterbuck E. J., Sanderson C. J., Young I. G. Molecular cloning, nucleotide sequence, and expression of the gene encoding human eosinophil differentiation factor (interleukin 5). Proc Natl Acad Sci U S A. 1987 Oct;84(19):6629–6633. doi: 10.1073/pnas.84.19.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin R. B., Fuller T. C., MacKeen L., Kung P. C., Ip S. H., Cosimi A. B. Plasma interleukin 2 receptor levels in renal allograft recipients. Clin Immunol Immunopathol. 1987 May;43(2):273–276. doi: 10.1016/0090-1229(87)90135-8. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Harada N., Takahashi T., Matsumoto M., Kinashi T., Ohara J., Kikuchi Y., Koyama N., Severinson E., Yaoita Y., Honjo T. Production of a monoclonal antibody useful in the molecular characterization of murine T-cell-replacing factor/B-cell growth factor II. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4581–4585. doi: 10.1073/pnas.84.13.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques Y., Le Mauff B., Boeffard F., Godard A., Soulillou J. P. A soluble interleukin 2 receptor produced by a normal alloreactive human T cell clone binds interleukin 2 with low affinity. J Immunol. 1987 Oct 1;139(7):2308–2316. [PubMed] [Google Scholar]
- Lotze M. T., Custer M. C., Sharrow S. O., Rubin L. A., Nelson D. L., Rosenberg S. A. In vivo administration of purified human interleukin-2 to patients with cancer: development of interleukin-2 receptor positive cells and circulating soluble interleukin-2 receptors following interleukin-2 administration. Cancer Res. 1987 Apr 15;47(8):2188–2195. [PubMed] [Google Scholar]
- Loughnan M. S., Takatsu K., Harada N., Nossal G. J. T-cell-replacing factor (interleukin 5) induces expression of interleukin 2 receptors on murine splenic B cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5399–5403. doi: 10.1073/pnas.84.15.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Moreau J. L., Nabholz M., Diamantstein T., Malek T., Shevach E., Thèze J. Monoclonal antibodies identify three epitope clusters on the mouse p55 subunit of the interleukin 2 receptor: relationship to the interleukin 2-binding site. Eur J Immunol. 1987 Jul;17(7):929–935. doi: 10.1002/eji.1830170706. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Rubin L. A., Kurman C. C., Fritz M. E., Boutin B. An analysis of the cellular requirements for the production of soluble interleukin-2 receptors in vitro. J Clin Immunol. 1986 Mar;6(2):114–120. doi: 10.1007/BF00918743. [DOI] [PubMed] [Google Scholar]
- Osawa H., Josimovic-Alasevic O., Diamantstein T. Enzyme-linked immunosorbent assay of mouse interleukin-2 receptors. J Immunol Methods. 1986 Aug 21;92(1):109–115. doi: 10.1016/0022-1759(86)90510-7. [DOI] [PubMed] [Google Scholar]
- Osawa H., Josimovic-Alasevic O., Diamantstein T. Interleukin 2 receptors are released by cells in vitro and in vivo. I. Detection of soluble IL 2 receptors in cell culture supernatants and in the serum of mice by an immunoradiometric assay. Eur J Immunol. 1986 Apr;16(4):467–469. doi: 10.1002/eji.1830160426. [DOI] [PubMed] [Google Scholar]
- Pike B. L., Vaux D. L., Clark-Lewis I., Schrader J. W., Nossal G. J. Proliferation and differentiation of single hapten-specific B lymphocytes is promoted by T-cell factor(s) distinct from T-cell growth factor. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6350–6354. doi: 10.1073/pnas.79.20.6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M. High and low affinity receptors for interleukin 2: implications of pronase, phorbol ester, and cell membrane studies upon the basis for differential ligand affinities. J Immunol. 1986 Jul 1;137(1):142–149. [PubMed] [Google Scholar]
- Robinson P. J. Two different biosynthetic pathways for the secretion of Qa region-associated class I antigens by mouse lymphocytes. Proc Natl Acad Sci U S A. 1987 Jan;84(2):527–531. doi: 10.1073/pnas.84.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Sethi K. K., Näher H. Elevated titers of cell-free interleukin-2 receptor in serum and cerebrospinal fluid specimens of patients with acquired immunodeficiency syndrome. Immunol Lett. 1986 Oct;13(4):179–184. doi: 10.1016/0165-2478(86)90052-0. [DOI] [PubMed] [Google Scholar]
- Tsudo M., Uchiyama T., Uchino H. Expression of Tac antigen on activated normal human B cells. J Exp Med. 1984 Aug 1;160(2):612–617. doi: 10.1084/jem.160.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. K., York-Jolley J., Malek T. R., Berzofsky J. A., Nelson D. L. Antigen-specific murine T cell clones produce soluble interleukin 2 receptor on stimulation with specific antigens. J Immunol. 1986 Jul 15;137(2):592–596. [PubMed] [Google Scholar]
- Zubler R. H., Lowenthal J. W., Erard F., Hashimoto N., Devos R., MacDonald H. R. Activated B cells express receptors for, and proliferate in response to, pure interleukin 2. J Exp Med. 1984 Oct 1;160(4):1170–1183. doi: 10.1084/jem.160.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]