Abstract
Four pigeons were trained in a procedure in which concurrent-schedule food ratios changed unpredictably across seven unsignaled components after 10 food deliveries. Additional green-key stimulus presentations also occurred on the two alternatives, sometimes in the same ratio as the component food ratio, and sometimes in the inverse ratio. In eight experimental conditions, we varied the contingencies surrounding these additional stimuli: In two conditions, stimulus onset and offset were noncontingent; in another two, stimulus onset was noncontingent, and offset was response contingent. In four conditions, both stimulus onset and offset were contingent, and in two of these conditions the stimulus was simultaneously paired with food delivery. Sensitivity to component food ratios was significantly higher when stimulus onset was response contingent compared to when it was noncontingent. Choice changes following food delivery were similar in all eight conditions. Choice changes following stimuli were smaller than those following food, and directionally were completely determined by the food-ratio:stimulus-ratio correlation, not by the stimulus contingency nor by whether the stimulus was paired with food or not. These results support the idea that conditional reinforcers may best be viewed as signals for next-food location rather than as stimuli that have acquired hedonic value, at least when the signals are differential with respect to future conditions.
Keywords: conditioned reinforcement, conditional reinforcement, conditional reinforcer, phylogenetically important event, dynamics, pecking, pigeons
The present experiment was conducted to replicate and extend some earlier results (Davison & Baum, 2006). In that research, we used a choice procedure with frequently-changing food-delivery ratios to investigate the results of presenting additional response-contingent stimuli on choice following these stimuli. One added stimulus was magazine light, which was often simultaneously paired with food delivery; the other was a 3-s keylight color change, which was not paired with food delivery. Across the seven different food-ratio components, the ratio of response-contingent keylight stimuli on the two concurrent schedules was positively correlated (+1) with food ratio in some conditions, and negatively correlated (−1) in other conditions. The results showed that paired magazine lights and unpaired keylight stimuli produced similar local changes in choice, and that the direction of these choice changes was determined by the correlation: A correlation of +1 produced preference toward the just-presented stimulus, whereas a correlation of −1 produced preference toward the alternative that had not just produced the stimulus. We argued that the stimuli were acting as discriminative stimuli, signaling the future availability of food from an alternative, and not as conditional reinforcers, even when simultaneously paired (magazine light) with food.
An early paper by Bolles (1961) foreshadowed this argument.1 Bolles trained rats in two different choice situations. In both situations, scheduling arranged that food deliveries produced by presses on two levers were distributed in time, and each food delivery was accompanied by a click. In the first situation, the schedules were arranged so that if a lever produced food, most likely next food would be again produced by that lever. In the second situation, the schedules were arranged so that if a lever produced food, most likely next food would be produced by the other lever. In the first situation, pressing tended to perseverate on a lever that produced food, whereas in the second situation, pressing tended to switch following food to the lever that had not just produced food. Following training, the rats were tested in a situation with no food (extinction) and in which only one lever produced the click that had been paired with food. Following the first training situation, preference during extinction favored the lever that produced the click. Following the second training situation, however, preference during extinction favored the lever that did not produce the click. Far from strengthening the behavior that preceded it, the click sent responding to the other lever. Bolles suggested that his experiment demonstrated “that the dominant part played by stimuli associated with reinforcement is their associative direction of behavior rather than any power they may have to reinforce it.” (p. 163.) Although put in different terms, this echoes our argument that the response-produced stimuli in our earlier experiment were functioning, not as conditional reinforcers, but as discriminative stimuli (Davison & Baum, 2006).
In a closely related experiment, Boutros, Davison, and Elliffe (2009) investigated stimulus–food correlation and pairing in conventional steady-state concurrent variable-interval (VI) schedules—that is, when the concurrent schedules were unchanging throughout sessions within conditions. Food and response-contingent stimulus presentations were arranged on a single base VI schedule, with responses producing the two events equally often. The program allocated food between the two response alternatives by assigning food to them with probabilities p and 1-p, and over conditions stimulus presentations were arranged for the alternatives either with a probabilities of p and 1-p (+1 correlation conditions), or 1-p and p (−1 correlation conditions), or .5 (zero correlation conditions). In one part of the experiment, the stimulus presentations occurred immediately prior to food delivery; in the other part, they were unpaired with food delivery. In the unpaired conditions, Boutros et al. found strong preference pulses toward the alternative that had just produced food and lesser preference pulses toward the alternative that had just produced a stimulus. The difference in the size of postfood and poststimulus preference pulses was consistent with our earlier results (Davison & Baum, 2006). But, unlike our earlier results, the direction of the correlation between food- and stimulus-frequency ratios did not control the direction of the poststimulus preference pulses. Similar results were obtained when stimuli were paired with food, but poststimulus preference pulses were larger—and more similar to postfood pulses—than when the stimuli were unpaired, and again the correlation between food and stimulus ratios did not control the direction of poststimulus pulses. Boutros et al. argued that, when food ratios change often and unpredictably, and a high positive or negative correlation links stimulus frequency to food frequency, a stimulus presentation signals where subsequent food is likely to be found, and the correlation between stimulus and food frequencies will control the direction of poststimulus choice. In contrast, when food ratios are unchanging across sessions, a stimulus presentation provides no additional information over and above the extended food ratio in effect, and so will not control the direction of poststimulus preference. In other words, either a food-related stimulus or food itself will exert local control when it signals where future food may be found (see also Krägeloh, Davison, & Elliffe, 2005). In the absence of differential local information, only more extended food ratios exert control.
The strong postfood preference pulses following food-paired stimuli might suggest that the stimuli were acting as conditional reinforcers. However, as Boutros et al. (2009) argued, some part of poststimulus and postfood preference pulses may have resulted from the inclusion of a 2-s changeover delay, which effectively penalized changing over after a stimulus or food presentation (Krageloh et al., 2005). This effect may account for the preference pulses following unpaired stimuli. A difference between preference pulses from unpaired and paired conditions might indicate some conditional reinforcement from the food-paired stimuli. However, we found no such difference between unpaired and paired stimuli (Davison & Baum, 2006). Thus, while conditional reinforcement effects may occur in steady-state preparations, they may be absent in situations in which food availability changes frequently—which, arguably, are the more common conditions of animal life and foraging.
The present experiment aimed to investigate in detail the effects of contingencies surrounding the keylight stimuli in the frequently changing food-ratio procedure (Davison & Baum, 2006) because, if the stimuli were acting as conditional reinforcers, the presence versus absence of a response–stimulus contingency should affect whether they would produce a poststimulus increment in choice. Thus, we investigated procedures in which the stimuli were (1) presented noncontingently; or (2) presented noncontingently with a response requirement to turn them off (thus making the stimuli more salient as predictors of future food, but keeping them non-response contingent); or (3) contingent on responding with a further contingency to turn them off (perhaps a conditional reinforcer effect with saliency); or (4) as in (3), but also with the stimuli simultaneously paired with food delivery. For each of these procedures, we arranged both positive and negative correlations between stimulus ratio and food ratio, as in the earlier study (Davison & Baum, 2006).
METHOD
Subjects
Four homing pigeons numbered 91, 92, 93 and 96 were maintained at 85% ± 15 g of their free-feeding body weights. These pigeons participated in the earlier study (Davison & Baum, 2006). Two other pigeons (Pigeons 94 and 95) commenced the experiment but died during the present experiment and were excluded.
Apparatus and Procedure
The pigeons were individually housed in 375-mm high by 370-mm deep by 370-mm wide cages, and these cages also acted as the experimental chambers. On one wall of the cage were three 20-mm diameter plastic pecking keys set 100 mm apart center to center and 220 mm from a wooden perch situated 100 mm from the wall and 20 mm from the floor. Each key could be transilluminated by yellow, green, or red LEDs, and responses to illuminated keys exceeding about 0.1 N were counted as effective responses. Beneath the center key, and 60 mm from the perch, was a 40 by 40-mm magazine aperture. During food delivery, the keylights were extinguished, the aperture was illuminated, and the hopper, containing wheat, was raised for 2.5 s. The subjects could see and hear pigeons in other experiments, but no personnel entered the experimental room while the experiments were running.
The pigeons lived and worked in the same home cages under shifted day–night conditions. The room lights turned on at midnight, and experimentation started at 0100 h. The room lights were extinguished at 1600 h. Sessions were conducted pigeon by pigeon in numerical order. The sessions started with the lighting of keylights and ended in blackout after all seven components had been completed or 1 hr, whichever came first. As the pigeons had previously worked on a similar procedure, no training was required, and they commenced this experiment on Condition 1 (see Table 1).
Table 1.
Sequence of experimental conditions.
| Stimulus contingency | ||||
| Correlation | ON Contingency | OFF Contingency | Paired/Unpaired | |
| 1 | +1 | NC | C | UNP |
| 2 | −1 | NC | C | UNP |
| 3 | −1 | NC | NC | UNP |
| 4 | +1 | NC | NC | UNP |
| 5 | −1 | C | - | P |
| 6 | −1 | C | C | UNP |
| 7 | +1 | C | C | UNP |
| 8 | +1 | C | - | P |
Note. Correlation is the correlation between the arranged food ratios and stimulus ratios across the seven components. Stimulus contingency is either C (response contingent) or NC (response noncontingent) for both the onset and offset of the green stimulus. Paired refers to whether food deliveries were preceded by the green stimulus (P) or not (UNP). 100 sessions were conducted for each experimental condition.
Sessions commenced with either the left or right key lit yellow (randomly selected with p = .5), and the center (switching) key lit red. The component in effect was randomly chosen without replacement from the set of seven components. The procedure was a switching-key arrangement, with the center red key as the switching key. Responding on the two yellow side keys intermittently produced food (2.5-s access to wheat in a lit hopper) or a green keylight for 2.5 s (in some conditions, the green keylight was presented noncontingently). When the red center key was pecked, the side key on which the pigeon had previously been pecking was extinguished, and a further red-key peck turned on the other side key, turned off the red center key, and made it inoperative. A peck on the newly presented side key turned the center-keylight on again, and switches again became available. Technically, then, the procedure was a changeover ratio (COR: Stubbs, Pliskoff & Reid, 1977) of two responses. Responses to the changeover key were not used in any analyses. The schedules timed during changing over. The experimental contingencies were controlled by MED-PC IV programs arranged on a remote PC-compatible computer, and this computer also recorded the time of every experimental and behavioral event with a resolution of 0.01 s.
Events, which might be food delivery or green keylight presentation, were scheduled on a base VI 15-s schedule arranged by querying a probability gate set at 0.037 every 1 s (technically, a random-interval schedule). The overall probability of a food versus a green keylight event was .5 in all seven components of all conditions. Components, which were not differentially signaled, ended after 10 food deliveries and were separated by 60-s blackouts. The left:right food ratios in the seven components were 1:27, 1:9, 1:3, 1:1, 3:1, 9:1 and 27:1 as in earlier studies (e.g., Davison & Baum, 2000, 2006), and occurred in random order without replacement in each session. Food deliveries and green keylights were scheduled dependently—that is, when one event had been arranged on the left or right key, schedule timing stopped until that event had been produced, except when green keylights were arranged noncontingently. The onset of the green keylight was contingent on a key peck in some conditions (C in Table 1), and was presented noncontingently (NC, Table 1) in other conditions, and could not occur during changeover. In all four noncontingent stimulus-onset conditions, stimulus onset could only occur, if it was arranged, following 2 s since the last peck on an alternative. The offset of the green key was also contingent on a side-key peck in some conditions, and noncontingent in other conditions (see Table 1). The changeover key was inoperative during food and green keylight presentations—following these, two red-center key pecks again switched to the other alternative.
In Conditions 1 and 2, green keys occurred noncontingently, and their offset was contingent on responding to the green key. In Conditions 3 and 4, both the onset and offset of the green key occurred noncontingently, and thus the duration always 2.5 s. In Conditions 5, 6, 7, and 8, both the onset and the offset of the green keys were contingent on responding. Additionally, in Conditions 5 and 8 the green key was also simultaneously paired with food delivery—the peck that produced food also produced the immediate onset of a green keylight on the pecked key, which was extinguished when food delivery ended. In these paired conditions, the stimulus ratio was the ratio of stimulus presentations that were not paired with food. Thus, in Condition 5 (r = −1, paired), in the 9:1 food-ratio component, the ratio of stimulus presentations that were presented alone was 1:9, but the ratio of stimulus presentations that were paired with food was the same as the 9:1 food ratio. In Conditions 1, 2, and 5 to 8, the green keylight had neither minimum nor maximum duration, but was turned off by the next peck. Each pair of conditions differed according to the relation between Left:Right key component food ratios and component green-key stimulus-presentation ratios. In one of each pair, the ratio of food deliveries on the left and right keys across the components was the same as the ratio of green keylight presentations across components (r = +1 in Table 1); in the other of the pair the ratio of food deliveries and green keylight presentations were inversely related across the components (r = −1).
The sequence of experimental conditions is shown in Table 1. Each condition lasted 100 daily sessions, and the data from the last 85 sessions were used in analyses.
The present experiment focuses on the effects of the contingencies surrounding presentations of the green keylight stimulus on postevent preference pulses—whether or not a response was required to produce it or to remove it, and whether the stimulus itself was simultaneously paired with food or not. General considerations might suggest that noncontingent presentation of a stimulus unpaired with food would leave subsequent responding unaffected, because the absence of a response–stimulus and a stimulus–food contingency might make the stimulus irrelevant. Adding a contingency, such as requiring a response to start and/or end the stimulus, should make it more salient. Under the pairing hypothesis of conditional reinforcement, pairing the stimulus with food should enhance poststimulus responding on whichever alternative produced the stimulus. However, we found no such effect in the earlier study (Davison & Baum, 2006), when we paired a stimulus (magazine light) with food and found the general direction of poststimulus choice was determined by the sign of the correlation between stimulus and food ratios.
RESULTS
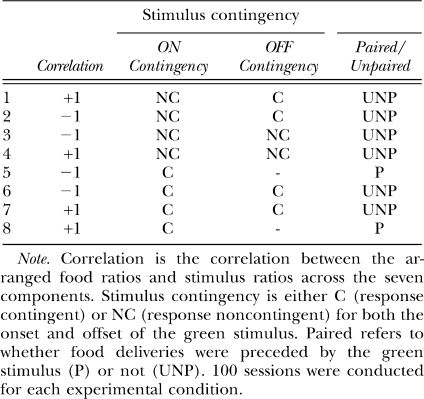
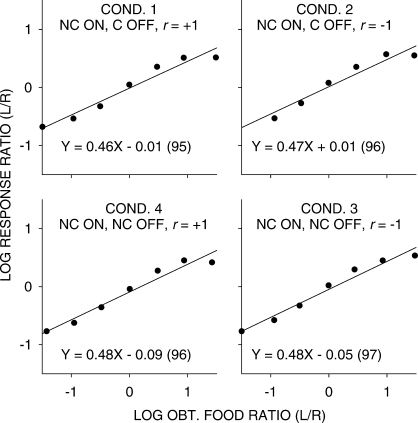
Figures 1 and 2 show log response ratios as a function of log obtained food ratios in all conditions of the experiment. Straight lines have been fitted to these data by the method of least squares in order to obtain estimates of sensitivity and bias according to the generalized matching law (Baum, 1974). A comparison of Figures 1 and 2 suggests that sensitivity to food ratio was less in conditions in which the stimulus onset was noncontingent (Figure 1; mean 0.47) than when it was contingent (Figure 2; mean 0.56). A Friedman 2-way analysis of variance using individual pigeon data was significant (χr2 = 20.6, N = 4, k = 8, p < .05), and a post-hoc test showed that noncontingent-onset sensitivity estimates as a group were smaller than contingent-onset sensitivity estimates at p < .05.
Fig 1.
Pooled data. Log response ratios as a function of log food ratios in the seven components in conditions in which stimulus onset was noncontingent (NC ON). The straight lines were fitted by least-squares linear regression, and the equations of these lines (with percent variance accounted for in parentheses) are shown for each condition.
Fig 2.
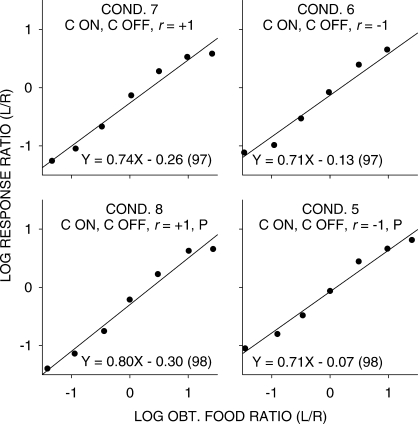
Pooled data. Log response ratios as a function of log food ratios in the seven components in conditions in which stimulus onset was response contingent (C ON). The straight lines were fitted by least-squares linear regression, and the equations of these lines (with percent variance accounted for in parentheses) are shown for each condition.
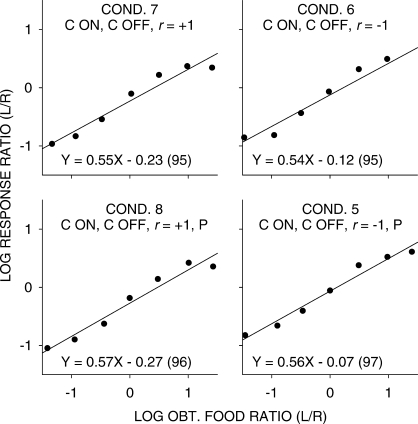
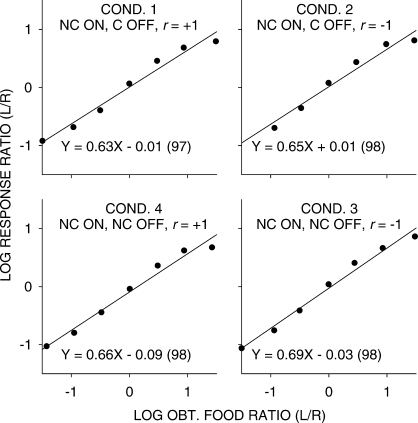
While the straight lines fitted well (a minimum of 96% of the data variance accounted for), it is evident that there were consistent deviations from the fitted lines, with extreme values being underestimated. One reason for this underestimation could be carryover between components: Because of the selection of components without replacement, extreme components would always have been preceded by less extreme components. Thus, we carried out a multiple linear regression using current choice as the dependent variable, and the current and previous component log food ratios as independent variables. Sensitivity to the previous-component food ratio equaled 0.19 (significantly greater than 0 in each condition), and was unchanging across all experimental conditions. Figures 3 and 4 are similar to Figures 1 and 2, except the effects of the previous-component food ratio have been removed. The relations between log response and log food ratios were steeper with the effect of the prior component removed, but the nonlinearities shown in Figures 1 and 2 remained. Thus, the nonlinearities evident in Figures 1 and 2 did not arise from systematically different samples of prior-component food ratios for each of the data points. The significant difference in sensitivity between conditions in which the stimuli were noncontingent (mean 0.66) and those in which stimuli were response contingent (0.74) was also found in the multiple-linear regression analysis.
Fig 3.
Pooled data with carryover from previous component removed. Log response ratios as a function of log food ratios in the seven components in conditions in which stimulus onset was noncontingent (NC ON). The straight lines were fitted by least-squares linear regression, and the equations of these lines (with percent variance accounted for in parentheses) are shown for each condition.
Fig 4.
Pooled data with carryover from previous component removed. Log response ratios as a function of log food ratios in the seven components in conditions in which stimulus onset was response contingent (C ON). The straight lines were fitted by least-squares linear regression, and the equations of these lines (with percent variance accounted for in parentheses) are shown for each condition.
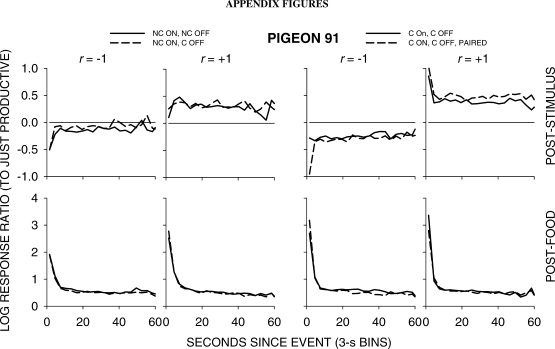
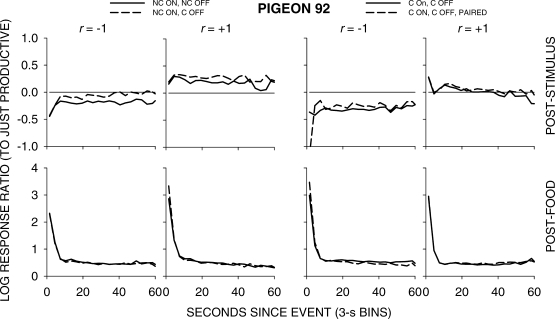
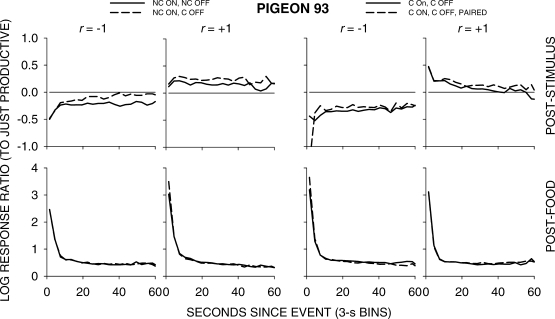
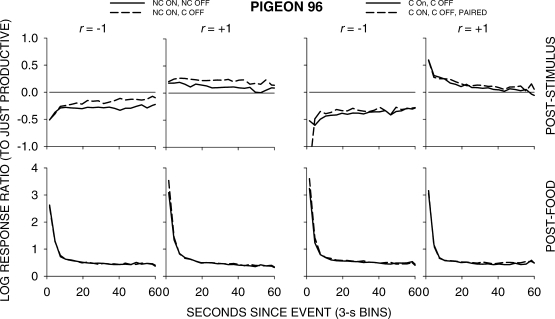
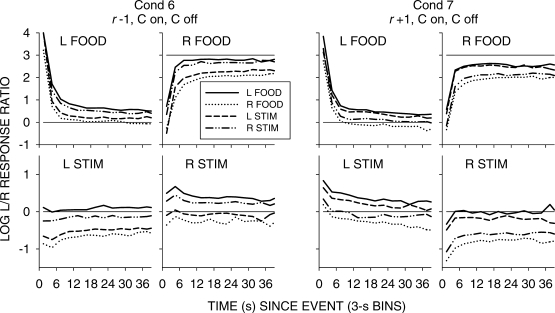
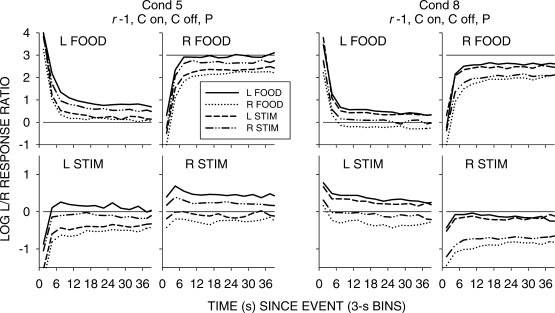
Figure 5 shows group choice across the 4 pigeons over the first 60 s following all food and stimulus events in 3-s bins; Appendix Figures A1 to A4 show individual-pigeon preference in a similar way. The group results shown in Figure 5 were obtained by pooling the raw data of the 4 pigeons and calculating choice as if the peck counts came from 1 pigeon. A comparison of Figure 5 with Figures A1 to A4 shows that the individual results were directionally and quantitatively very similar to the group results. Thus, we shall concentrate on the analysis of the group choice data, noting any individual-pigeon differences.
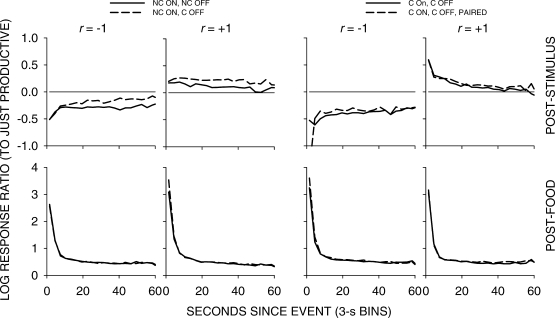
Fig 5.
Pooled data. Log response ratios showing choice of the just-productive alternative in 3-s bins across the first 60 seconds following stimulus and food events in each condition of the experiment. For green-key stimulus events, C means response contingent on and/or off, and NC means noncontingent on and/or off. P denotes that the stimulus was also paired with food delivery.
Fig A1.
Pigeon 91: Log response ratios showing choice of the just-productive alternative in 3-s bins across the first 60 seconds following stimulus and food events in each condition of the experiment. For green-key stimulus events, C means response contingent on and/or off, and NC means noncontingent on and/or off. P denotes that the stimulus was also paired with food delivery.
Fig A2.
Pigeon 92. Please see legend to Figure A1.
Fig A3.
Pigeon 93. Please see legend to Figure A1.
Fig A4.
Pigeon 96. Please see legend to Figure A1.
The top four graphs in Figure 5 show that, as in the earlier study, stimulus-ratio:food-ratio correlation determined the direction of preference following a stimulus presentation. When the correlation was +1, choice favored the just-productive key, but when the correlation was -1, choice favored the other key. The left two graphs indicate that when a peck was required to turn the stimulus off, preference shifted slightly in favor of the just-productive key. The right two graphs show that pairing the green keylight with food had no effect. The bottom row of graphs shows that preference pulses following food were unaffected by any of the variations.
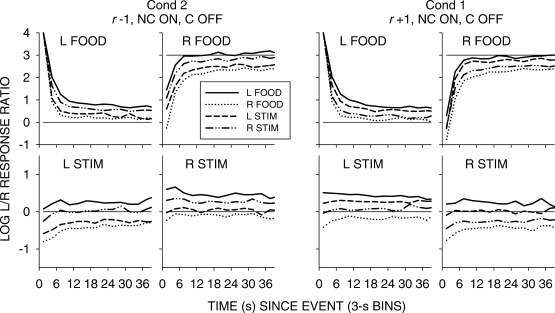
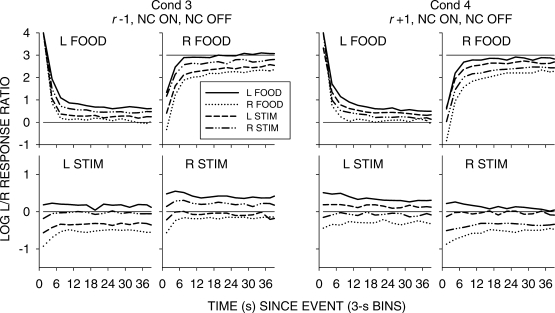
In previous reports, (e.g., Baum & Davison, 2004) we have presented graphs showing how interfood choice was affected by different sequences of food deliveries. However, in the present experiment, with two different events on each alternative, sequences become complex. As an alternative, Figures 6 (NC on, C off), 7 (NC on, NC off), 8 (C on, C off) and 9 (C on, C off, paired) show how choice changed following events according to the previous event—for example, a left food delivery when this had been preceded by another left food, a right food, a left stimulus, and a right stimulus. The analyses were pooled across all seven components. Each of these figures shows the results from a pair of conditions with the same stimulus-on and stimulus-off contingencies, but with opposing stimulus-ratio:food-ratio correlations.
Fig 6.
Pooled data from Conditions 1 and 2. Log left/right response ratios as a function of time since events in 3-s bins for conditions in which stimulus onset was noncontingent and stimulus offset was response contingent. Each separate graph shows choice following a single event according to the four prior events.
Postfood Preference Pulses
A comparison of Figures 6 to 9 again shows that postfood preferences were unaffected by stimulus contingency, stimulus-ratio:food-ratio correlation, and pairing. The figures show similar food-sequence effects: Prior food deliveries on the same alternative produced postfood preference pulses that were more extreme and lasted longer than prior food deliveries on the other alternative, as we have previously reported (Baum & Davison, 2004; Davison & Baum, 2003). Prior stimulus presentations had effects that were intermediate between the effects of prior food deliveries, and depended on the stimulus-ratio:food-ratio correlation. When the correlation was −1, postfood preference was more extreme when the prior event had been a stimulus on the other key compared with postfood preference when it had been on the same key. Conversely, when the correlation was +1, postfood preference was more extreme when the prior event had been a stimulus on the same key compared with postfood preference when it had been on the other key. These sequence effects were manifest for postfood preferences for all stimulus contingencies, whether or not the stimuli were paired with food (Figures 6 to 9).
Fig 7.
Pooled data from Conditions 3 and 4. Log left/right response ratios as a function of time since events in 3-s bins for conditions in which both stimulus onset and stimulus offset were response noncontingent. Each separate graph shows choice following a single event according to the four prior events.
Fig 8.
Pooled data from Conditions 6 and 7. Log left/right response ratios as a function of time since events in 3-s bins for conditions in which both stimulus onset and offset were response contingent. Each separate graph shows choice following a single event according to the four prior events.
Fig 9.
Pooled data from Conditions 5 and 8. Log left/right response ratios as a function of time since events in 3-s bins for conditions in which stimulus onset and offset were response contingent and stimuli were paired with food delivery. Each separate graph shows choice following a single event according to the four prior events.
Poststimulus Preference Pulses
Poststimulus preferences were ordered in the same way as postfood preferences, with stimulus-ratio:food-ratio correlation having the same effect. Stimuli were followed by smaller choice changes than those produced by food delivery, and transient strong preferences immediately following stimulus presentations were often absent when stimulus onset was noncontingent (Figures 6 and 7). In all conditions, when the stimulus–food correlation was −1, two successive stimuli on the same key moved choice further toward the other alternative than did alternations of stimuli. When the correlation was +1, two successive stimuli on the same key moved choice further toward the same alternative than did stimulus alternations. Two successive stimuli on opposite keys approximately canceled each other's effects, producing near indifference, regardless of correlation. Thus, in all conditions, stimuli and food deliveries had the same sequential effect, though stimulus effects were smaller and their direction depended completely on the stimulus-ratio:food-ratio correlation.
However, when the stimuli were both turned on and turned off by responses (Figures 8 and 9), stronger transient poststimulus choices did occur in some conditions. In Condition 7, when the stimulus–food correlation was +1, stimuli produced transient preferences to the left key following left-key stimuli, and to the right key following right-key stimuli. In Condition 5, the paired condition with negative stimulus–food correlation, left-key stimuli were followed by a strong pulse toward right-key choice, but no transient was found for right-key stimuli. In Condition 8 (r = +1, paired), left-key stimuli were followed by a small pulse toward left-key choice, and right-key stimuli were followed by a small pulse toward right-key choice. Thus, the comparison of Conditions 5 and 8 shows that the effects of paired stimuli depend on the stimulus-ratio:food-ratio correlation: In Condition 5, preference was generally more to the left key following right stimuli, and to the right key (with a strong pulse) following left-key stimuli. Although the pulses in Condition 8 (Figure 9) appear to support some conditional reinforcement effect of the stimuli, the comparison of Conditions 5 and 8 argues against conditional reinforcement and strongly for a signaling effect based on stimulus-ratio:food-ratio correlation, as we (Davison & Baum, 2006) previously suggested.
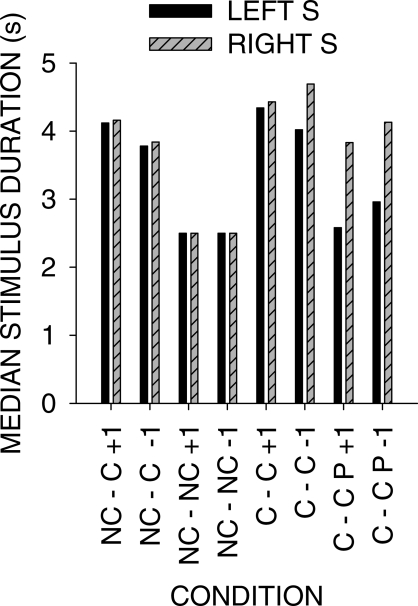
Figure 10 shows the duration of the stimuli across the various conditions. When a peck was required to turn the light off, the light remained on between 2.5 and 4.5 s across conditions before the next response turned it off. No systematic difference in duration was evident between the +1 and −1 stimulus-ratio:food-ratio correlations.
Fig 10.
Median stimulus durations from data pooled across the 4 pigeons in all conditions of the experiment. In the NC-ON and NC-OFF conditions, the stimulus duration was fixed at 2.5 s.
DISCUSSION
The generalized-matching relation did not fit the obtained across-component data without systematic deviations, although this relation did account for a large proportion of the data variance. That allows us to take the slopes obtained as indicative of the general relation between choice and food ratios. In these analyses, whether we removed carryover from the prior component or not (Figures 1 to 4), we found that sensitivity was significantly greater in conditions in which stimulus onset was response contingent compared to conditions in which this was noncontingent. However, sensitivity values were unaffected by varying the correlation between food ratio and stimulus ratio from +1 to −1. If the stimuli had any hedonic value, especially when they were response contingent and positively correlated with the food ratio (Figures 3 and 4), the additional stimuli should have acted as if there were more “reinforcers” on the higher food-rate component than on the lower food-rate component, thus increasing apparent sensitivity to the food ratio. Conversely, when the correlation was −1, adding stimuli with hedonic value more frequently to the lower food-rate component should have decreased sensitivity. Because no such differences occurred, the stimuli, even when paired with food, and even when predictive of the likely location of future food, had no hedonic value.
The present results fully replicated the effect of stimulus-ratio:food-ratio correlation on choice following stimulus presentation reported earlier (Davison & Baum, 2006). This correlation determined the effect of stimulus presentation on subsequent choice: A positive correlation increased both shorter- and longer-term poststimulus choice toward the alternative that just provided the stimulus; and a negative correlation produced poststimulus choice favoring the alternative that had not just provided the stimulus. The direction of this change in choice was independent of whether the stimulus onset was contingent or noncontingent, whether stimulus offset was contingent or noncontingent, and whether or not the stimulus was paired with food. Since we found the same directional change following magazine-light presentation when magazine light was paired with food (Davison & Baum, 2006), the effect was independent of whether the paired stimulus occurred in the food magazine or on the key. The poststimulus direction of preference, then, indicates no conditional reinforcement of preceding responses, neither in any of the present conditions nor in the earlier study. The direction of the poststimulus behavior change is a discriminative effect, with the stimulus signaling the location (one or the other alternative) of the likely next food, as we (Davison & Baum) concluded earlier. Krägeloh, Davison & Elliffe (2005) reported a similar result for choice following food itself: Rather than increasing responses that occurred prior to food delivery, food delivery increased responding on the other alternative if food delivery signaled that the other alternative was more likely to produce the next food delivery.
Noncontingent stimulus onset produced smaller changes, both in the shorter- and longer-term, than did response-contingent stimulus onset. Perhaps this is some evidence that the added stimuli acted like conditional reinforcers when they were response contingent. Noncontingent stimulus onset would provide a longer and more variable delay between responding and stimulus presentation, which would be expected to decrease the reinforcing effect of a subsequent conditional reinforcer, leading to a smaller increment in responding following noncontingent stimulus onset. Thus, the difference in poststimulus preference pulses following contingent and noncontingent stimulus onset was in the expected direction if the stimuli had become conditional reinforcers. However, the directional changes argue implacably against a conditional reinforcement explanation of this difference. A parsimonious interpretation is that noncontingent stimuli are less salient than contingent stimuli—some presentations may be completely missed when onset and offset are noncontingent because the pigeon is doing something else—and hence were poorer predictors of future food.
Still, the absence of any difference between contingent and noncontingent stimulus offset seems inconsistent with this salience notion—shouldn't the stimulus be sufficiently salient when the pigeon has to peck to remove it? The difference between onset and offset contingency may be compared to results with observing responses. The peck that produces stimulus onset, like an observing response, produces a relatively informative event, whereas the peck that removes the stimulus produces the white key, a stimulus that was on most of the time and was relatively uninformative about the availability of food. This line of reasoning is supported by the finding that observing is maintained only by stimuli that predict food (e.g., Mueller & Dinsmoor, 1986). Although that result is often taken to support the theory of conditional reinforcement, another view might be that the stimuli predicting food are relatively more informative. In keeping with this view, Shahan and Podlesnik (2005) found that stimuli produced by observing responses fail to enhance resistance of responding to disruption, arguing against the theory that observing is maintained by conditional reinforcement. The idea that the response-produced stimuli are only discriminative stimuli would predict no effect on resistance to disruption. If, as we are suggesting, all events predictive of food, including food itself, serve as discriminative stimuli, relative predictiveness of an event should determine its value, at least in part. These ideas are consistent with Rescorla's (1967) idea of contingency, rather than pairing, as the important operation in Pavlovian conditioning—with the caveat that stimulus–food contingencies do not make a stimulus into a conditional reinforcer. Figure 1 illustrates that food itself is more salient than lights that are also predictive of food, so predictiveness is not the only factor, but the ability of both food and lights that predict likelihood of food elsewhere to drive responding away from a just-productive response alternative remains obstinately opposed to any stimulus–food pairing theory of conditional reinforcement (Davison & Baum, 2006; Krägeloh, Davison, & Elliffe, 2005).
When an activity like key pecking produces food or a stimulus related to food, and the food or stimulus predicts the likelihood of further food for continuing in that activity or for pursuing a different activity, the food or stimulus will induce more of the same activity or more of the different activity, depending on the prediction made. Activity-contingent events induce behavior change when they signal future phylogenetically important events (PIEs; Baum, 2005), like food, water, or a mate, but the direction of the behavior change depends on their signaling the activity likely to produce future events. The vector of behavior change is jointly determined by both contingency and predictive direction. Contingent events do not simply increase or decrease the activity that preceded them according to the valence of the event. Contingency plays a role, but contingency acts only by way of the correlation it creates among events.
In the present experiment and the earlier one (Davison & Baum, 2006), food delivery always produced a subsequent increase in both short-term and longer-term preference toward the alternative that had just produced food. This might seem surprising, because food on a lower food-rate alternative could signal subsequent food on the other, higher food-rate alternative (e.g., Krägeloh et al., 2005). However, because such signaling was inconsistent across the differing food-ratio components in Davison and Baum's experiment, and because food deliveries across sessions were always approximately equal for the two alternatives in the present experiment, a food delivery from either alternative did not on that basis predict the location of the next food. Food signaled only that, if food is gained from an alternative, more food may be likely. In other words, stimuli and food deliveries in the present procedure signal local food availability, rather than global or extended food rates. The situation differs, for example, from that investigated by Boutros et al. (2009). They arranged simple concurrent VI VI schedules with added stimuli that were either positively correlated or negatively correlated with the global food ratio across extended conditions and found no differential effect of stimulus-ratio and food-ratio correlation on poststimulus choice—poststimulus preferences were generally in the same direction as, but smaller than, postfood preferences. As they suggested, the stimuli in such a procedure add nothing informative to the prediction of the next food delivery over and above the signal provided by the food deliveries themselves (they are redundant relevant cues, whether positively or negatively correlated with food). In contrast, stimuli paired with food by presenting them prior to food deliveries—thus, not entirely redundant—resulted in poststimulus preferences that resembled postfood preferences.
In summary, then, environmental events, whether they be PIEs or PIE-related stimuli, affect behavior by signaling the likelihood (or unlikelihood) of future PIEs (Baum, 2005). Such signals change choice locally if differential local contingencies exist and are effective. If local contingencies are ineffective, or if no differential local contingencies exist—only global contingencies (as in Boutros et al., 2009)—local choice changes will be muted, and global choice will come under the control of global differential contingencies. We doubt control can ever be complete at either of these time scales—behavior must remain at least partially under the control of both global and local contingencies for behavior to change when contingencies change.
Acknowledgments
We thank the staff and students in the Experimental Analysis of Behaviour Research Unit who helped run this experiment, and to Mick Sibley who looked after the pigeons.
Footnotes
We are indebted to Timothy Shahan for drawing this paper to our attention.
REFERENCES
- Baum W.M. On two types of deviation from the matching law: Bias and undermatching. Journal of the Experimental Analysis of Behavior. 1974;22:231–242. doi: 10.1901/jeab.1974.22-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum W.M. Understanding behaviorism: Behavior, culture, and evolution (2nd Ed.) Medford, MA: Blackwell Publishing; 2005. [Google Scholar]
- Baum W.M, Davison M. Choice in a variable environment: Visit patterns in the dynamics of choice. Journal of the Experimental Analysis of Behavior. 2004;81:85–127. doi: 10.1901/jeab.2004.81-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles R.C. Is the “click” a token reward. The Psychological Record. 1961;11:163–168. [Google Scholar]
- Boutros N, Davison M, Elliffe D. Conditional reinforcers and informative stimuli in a constant environment. Journal of the Experimental Analysis of Behavior. 2009;91:41–60. doi: 10.1901/jeab.2009.91-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M, Baum W.M. Choice in a variable environment: Every reinforcer counts. Journal of the Experimental Analysis of Behavior. 2000;74:1–24. doi: 10.1901/jeab.2000.74-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M, Baum W.M. Every reinforcer counts: Reinforcer magnitude and local preference. Journal of the Experimental Analysis of Behavior. 2003;80:95–129. doi: 10.1901/jeab.2003.80-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M, Baum W.M. Do conditional reinforcers count. Journal of the Experimental Analysis of Behavior. 2006;86:269–283. doi: 10.1901/jeab.2006.56-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krägeloh C.U, Davison M, Elliffe D.M. Local preference in concurrent schedules: The effects of reinforcer sequences. Journal of the Experimental Analysis of Behavior. 2005;84:37–64. doi: 10.1901/jeab.2005.114-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K.L, Dinsmoor J.A. The effect of negative stimulus presentations on observing-response rates. Journal of the Experimental Analysis of Behavior. 1986;46:281–291. doi: 10.1901/jeab.1986.46-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla R.A. Pavlovian conditioning and its proper control procedures. Psychological Review. 1967;74:71–80. doi: 10.1037/h0024109. [DOI] [PubMed] [Google Scholar]
- Shahan T.A, Podlesnik C.A. Rate of conditioned reinforcement affects observing rate but not resistance to change. Journal of the Experimental Analysis of Behavior. 2005;84:1–17. doi: 10.1901/jeab.2005.83-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs D.A, Pliskoff S.S, Reid H.M. Concurrent schedules: A quantitative relation between changeover behavior and its consequences. Journal of the Experimental Analysis of Behavior. 1977;27:85–96. doi: 10.1901/jeab.1977.27-85. [DOI] [PMC free article] [PubMed] [Google Scholar]