Abstract
Objectives
We studied activating autoantibodies to β1-adrenergic (AAβ1AR) and M2 muscarinic receptors (AAM2R) in the genesis of atrial fibrillation (AF) in Graves’ hyperthyroidism.
Background
AF frequently complicates hyperthyroidism. AAβ1AR and AAM2R have been described in some patients with dilated cardiomyopathy and AF. We hypothesized their co-presence would facilitate AF in autoimmune Graves’ hyperthyroidism.
Methods
IgG purified from 38 patients with Graves’ hyperthyroidism with AF (n=17) or sinus rhythm (n=21) and 10 healthy controls was tested for its effects on isolated canine Purkinje fiber contractility with and without atropine and nadolol. IgG electrophysiologic effects were studied using intracellular recordings from isolated canine pulmonary veins. Potential cross-reactivity of AAβ1AR and AAM2R with stimulating thyrotropin receptor (TSHR) antibodies was evaluated before and after adsorption to CHO cells expressing human TSHRs using flow cytometry and enzyme-linked immunosorbent assays.
Results
The frequency of AAβ1AR and/or AAM2R differed significantly between patients with AF and sinus rhythm (AAβ1AR = 94% vs. 38%, p<0.001; AAM2R = 88% vs. 19%, p<0.001; and AAβ1AR+AAM2R = 82% vs. 10%, p<0.001). The co-presence of AAβ1AR and AAM2R was the strongest predictor of AF (odds ratio 33.61, 95% CI 1.17 - 964.11, p=0.04). IgG from autoantibody-positive patients induced hyperpolarization, decreased action potential duration, enhanced early afterdepolarization formation and facilitated triggered firing in pulmonary veins by local autonomic nerve stimulation. Imunoadsorption studies demonstrated that AAβ1AR and AAM2R were immunologically distinct from TSHR antibodies.
Conclusions
AAβ1AR and AAM2R when present in patients with Graves’ hyperthyroidism facilitate development of AF.
Keywords: activating autoantibodies, β-adrenergic receptors, M2 muscarinic receptor, atrial fibrillation, Graves’ hyperthyroidism
Hyperthyroidism has been associated with atrial tachyarrhythmias (1–3) and with sustained atrial fibrillation (AF) occurring in 20–30% of patients even after return to the euthyroid state (1,2). The pathogenesis of AF in these patients is postulated to result from shortening of the action potential duration in the atrial myocardium from excess thyroid hormone facilitating formation of multiple reentry circuits (4,5). Graves’ disease is one of the most common causes of hyperthyroidism (6). The prevalence of AF in patients with Graves’ disease, as in all other forms of hyperthyroidism, increases with age (1,2,6).
The autoimmune pathogenesis of Graves’ disease is accepted and attributed to autoantibodies which activate the G protein-coupled thyrotropin receptor (TSHR) (6,7). Activating autoantibodies to the β1-adrenergic (AAβ1AR) and the M2 muscarinic receptors (AAM2R) variably occur in patients with several cardiomyopathies and in a subset of patients with atrial fibrillation (8–13). AAβ1AR exhibit positive inotropic and chronotropic effects (14,15), whereas AAM2R have negative chronotropic effects (13) and decrease the action potential duration in isolated cardiomyocytes (10). The presence of AAM2R was associated with the occurrence of AF in patients with idiopathic dilated cardiomyopathy (13). Combined sympathetic and parasympathetic stimulation has been shown to generate early afterdepolarizations and rapid triggered firing in the pulmonary veins, that in turn induces AF (16,17). Given the synergistic role of sympathetic and parasympathetic activity for initiation and/or maintenance of AF (18,19), we hypothesized 1) patients with Graves’ hyperthyroidism develop significant titers of AAβ1AR and AAM2R and 2) these autoantibodies facilitate development of AF.
Methods
Study patients
Thirty-eight patients with Graves’ hyperthyroidism with AF (n=17) or sinus rhythm (n=21) were included in the study through referral and were seen by an endocrinologist and cardiologist. The diagnosis of Graves’ hyperthyroidism was based on markedly suppressed serum thyrotropin concentrations, elevated serum free thyroxine and triodothyronine concentrations and evidence of diffuse goiter with increased 24-hr radionuclide uptake (6). Measurement of TSHR antibodies was generally obtained but not required unless there was ambiguity in the diagnosis. All patients were seen during a two-year period. AF was confirmed by 12-lead electrocardiogram. Echocardiograms were performed in all but 4 patients (1 with AF and 3 with sinus rhythm). Serum was obtained from each patient and 10 voluntary healthy donors (mean age 29.5±3.2 years). This study was approved by the OUHSC Institutional Review Board and all subjects provided written informed consent.
Purification of IgG antibody
IgG was purified using the NAb Protein A/G Spin Kit (Pierce, Rockford, IL), according to the manufacturer's protocol.
Contractility Bioassay
Free running canine Purkinje fibers (5–7 mm) were transferred to a 36±0.1°C perfusion chamber mounted on the stage of an inverted microscope (Olympus) (20). The fibers were perfused with normal Tyrode’s solution (in mmol/L: NaCl 145, KCl 4.5, CaCl2 1.8, MgCl2 1, NaH2PO4 1, glucose 11, HEPES 10, pH 7.36) at 36±0.1°C and paced with a 4 ms duration constant current pulse at 2 Hz via extracellular platinum electrodes. Isometric contractions were recorded before, during steady state and following the washout using a video edge detector (Model VED-205, Crescent Electronics, UT). After achieving stable contractile responses over 3–5 minutes, IgG equivalent to a 1:100 serum dilution from a patient or control was administered for a 5-minute interval. With subsequent 5-minute periods, IgG plus atropine (100 nmol/L) or nadolol (100 nmol/L) was assayed to determine the effect attributable to the AAβ1AR or AAM2R components of IgG, respectively. Isoproterenol (10 nmol/L) served as a positive control. IgG from healthy donors served as negative controls. Contractility was calculated as the mean of 15 consecutive contraction cycles after a stable baseline or response was elicited and analyzed offline using pClamp 9.2 (Axon Instruments, Foster City, CA). Any response that was significantly different from the baseline with a p<0.05 was considered to be positive. Increased contractility over baseline with IgG plus atropine represented the AAβ1AR effect. The change in IgG effect on contractility with and without atropine was a surrogate marker of the AAM2R inhibitory effect. The intra-assay and inter-assay coefficient of variation was 6.6% (n=24) and 8.6% (n=38), respectively.
Electrical recordings
Isolated canine pulmonary vein preparations (16) were pinned endocardial side up and superfused with oxygenated Tyrode's solution at 36°C (20 mL/min). A bipolar electrode recording (0.10 mm diameter Teflon-coated silver wires, 1 mm apart) was obtained, filtered at 10–10,000 Hz, and recorded on a Gould WindowGraf recorder. An intracellular recording was obtained using a glass microelectrode with an intracellular resistance of 10–30 MΩ (Duo 773 electrometer, World Precision Instruments, Sarasota, FL) and was maintained for the duration of evaluation of a single IgG sample. The preparation was paced at 2–3× diastolic threshold using 4 msec duration stimuli from a Grass model S88 stimulator (Quincy, MA) at 1 Hz. Intracellular recordings were performed pre- and post-autonomic nerve stimulation from the immediate vicinity of the stimulating electrodes (within 2–3 mm) and pre- and post-superfusion of the preparation with IgG (0.15 mg/mL). Local autonomic nerve stimulation was accomplished using 300 msec duration high-frequency (100 Hz) trains of 0.05 msec duration square-wave stimuli introduced at 10–150 V in 20 V steps from a Grass stimulator. Voltage was maintained at less than 50% of the threshold voltage required to excite local myocardium when introduced as 0.05 msec duration stimuli during a 2-Hz pacing train.
Detection of TSHR antibodies by flow cytometry
Purified IgG samples were diluted (1:200) with isotonic PBS containing 4% BSA and 0.01% sodium azide and incubated with Chinese hamster ovary cells expressing full-length human thyrotropin receptor (CHO-TSHR cells) (21). Anti-human IgG (H+L) conjugated with fluorescein isothiocyanate (BD Bioscience Pharmingen, San Diego, CA) was the secondary antibody. The mean fluorescent intensity was measured by flow cytometry (BD Bioscience Pharmingen). A human monoclonal antibody (M22) to the TSHR that stimulates cAMP in CHO-TSHR cells confirmed TSHR-specific binding.
Adsorption study
CHO-TSHR cells were maintained in Ham’s F12 medium supplemented with 10% fetal bovine serum (Mediatech), 100 U/ml penicillin, and 100 U/ml streptomycin (Invitrogen, Grand Island, NY). Fully confluent cells were detached by PBS containing 1 mM EDTA and 1 mM EGTA. Counted (1×106) cells were incubated with 100 µL of diluted (1:200) purified IgG for 30 min with mild rocking at room temperature. IgG-adsorbed samples were collected by centrifugation. Flow cytometry was performed using pre- and post-adsorption samples in parallel. A reduction in mean fluorescent intensity of >20% indicated good adsorption. All experiments were performed twice. Pre- and post-adsorbed serum samples were analyzed in duplicate by enzyme-linked immunosorbent assay (ELISA) for antibody titers to the β1AR and M2R using whole receptors expressed in membranes (PerkinElmer, Waltham, MA) (20).
Statistical Analysis
Data are expressed as mean ± standard deviation. Contractility values were normalized to their baseline values. Comparison between two groups was performed by using the unpaired or paired Student’s t-test for quantitative variables, as applicable and the Fisher’s exact test for dichotomous variables. The McNemar test was used for the matched analysis. Linear correlation was performed to examine the strength of the linear relationship between the true AAM2R effect and its surrogate. Repeated measures analysis of variance was used to determine differences within a group with drug treatment. Logistic regression analysis was used to assess predictors of atrial fibrillation. Those variables with p values < 0.10 by univariate analysis were included in the multivariate logistic regression analysis model and the respective odds ratios were calculated. All analyses were two-tailed. Statistical significance was set at p<0.05.
Results
Patient characteristics
Seventeen patients had AF and 21 had sinus rhythm. The clinical, echocardiographic and biochemical characteristics of the patients are summarized in Table 1. Patients with AF were older than patients with sinus rhythm (60.9±12.7 years vs. 45.7±13.1 years, p<0.001). Otherwise, no difference was noted for the percentage of male sex, presence of hypertension, diabetes mellitus, coronary artery disease and congestive heart failure between the two groups. Echo indices, including left ventricular ejection fraction, left atrial diameter, early diastolic velocity of mitral inflow (E), deceleration time of mitral E flow velocity (DT) and the ratio between the early diastolic velocity of mitral inflow and that of mitral annulus (E/E’) did not differ significantly between the two groups. Serum thyrotropin and free thyroxine concentrations were similar in the two groups.
Table 1.
Clinical, echocardiographic and biochemical characteristics of the patients.
| Atrial fibrillation (n=17) |
Normal sinus rhythm (n=21) |
P value | |
|---|---|---|---|
| Age (years) | 60.9 ± 12.7 | 45.7 ± 13.1 | 0.001* |
| Male (%) | 47.1 | 42.9 | 1.00 |
| Hypertension (%) | 70.6 | 61.9 | 0.73 |
| Diabetes mellitus (%) | 35.3 | 23.8 | 0.49 |
| Coronary artery disease (%) | 17.6 | 9.5 | 0.64 |
| Heart failure (%) | 52.9 | 23.8 | 0.09 |
| Ejection fraction (%) | 51.6 ± 16.1 | 52.4 ± 16.2 | 0.88 |
| Left atrium (mm) | 42.3 ± 7.8 | 38.3 ± 7.6 | 0.15 |
| E (m/s) | 0.83 ± 0.25 | 0.98 ± 0.37 | 0.17 |
| DT (ms) | 245.2 ± 72.2 | 242.4 ± 77.9 | 0.90 |
| E/E' | 5.9 ± 2.5 | 6.6 ± 2.5 | 0.50 |
| Serum thyrotropin (mU/L) | 0.075 ± 0.12 | 0.071 ± 0.18 | 0.94 |
| Serum free thyroxine (ng/dL) | 2.35 ± 1.3 | 3.08 ± 2.3 | 0.22 |
E, early diastolic velocity of mitral inflow; DT, deceleration time of mitral E flow velocity; E/E', ratio between the early diastolic velocity of mitral inflow (E) and that of mitral anulus (E').
Contractility Bioassay
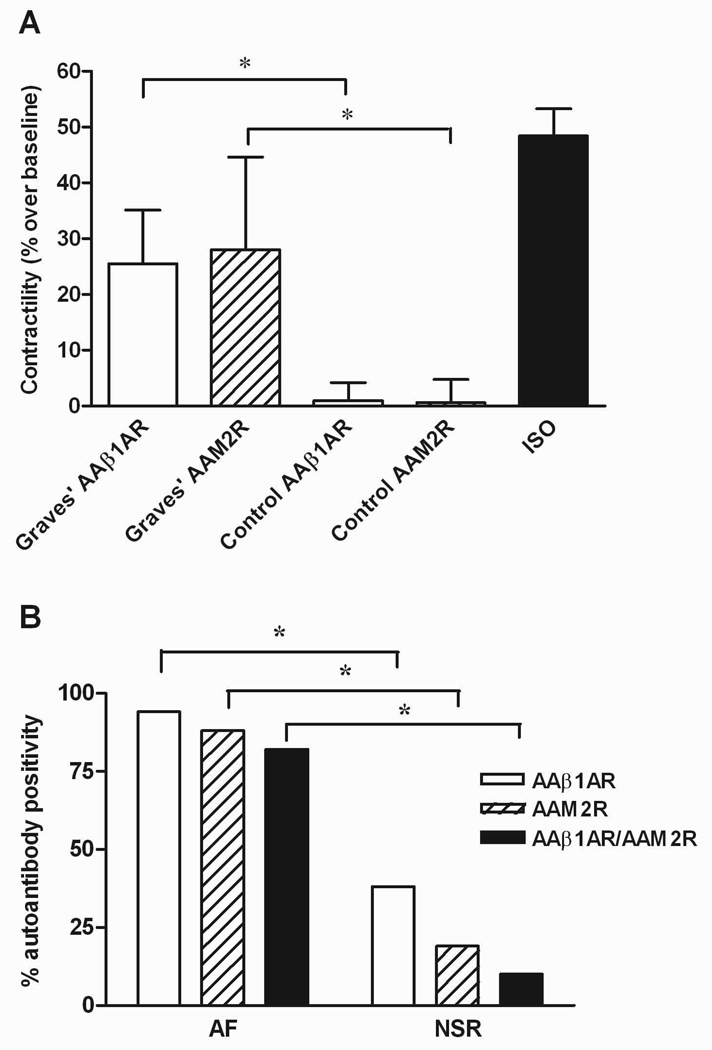
Twenty-four (63%) and 19 (50%) of the 38 IgG samples demonstrated AAβ1AR and AAM2R, respectively. In 16 (42%) IgG samples, AAβ1AR and AAM2R coexisted. None of 10 control IgG samples demonstrated either autoantibody group. The β-adrenergic receptor agonist isoproterenol (10 nmol/L) increased contractility 48.4±4.9% over baseline (p<0.001). The mean IgG agonist effect (% over baseline) from the 24 AAβ1AR-positive patients in the presence of M2R blockade was 25.6±9.6% (p<0.001 vs. control subjects). The absolute mean inhibitory AAM2R effect (% change over baseline) in the 19 AAM2R-positive patients was 28.1±16.6% (p<0.001 vs. control subjects) (Figure 1A). The change in IgG effect on contractility with and without atropine correlated strongly with the IgG effect in the presence of nadolol (R2=0.67, P=0.001, n=12), supporting the use of the atropine-induced change as a surrogate for the AAM2R effect. During each assay, the effect of combined βAR and M2R blockade with nadolol and atropine led to a return of the IgG response to baseline. These data, not shown, provide additional evidence against the co-presence of additional autoantibodies causing Purkinje contractile response.
Figure 1. Functional effects of IgG from patients with Graves’ disease and controls on Purkinje fiber contractility.
A. The mean effect (% over baseline) of AAβ1AR from the 24 AAβ1AR-positive patients (Graves’ AAβ1AR) and the absolute value of the mean inhibitory effect (% change over baseline) of AAM2R in the 19 AAM2R-positive patients (Graves’ AAM2R) are compared with control IgG. The "antibody-negative" Graves’ values were not significantly different from the controls and therefore are not shown. Isoproterenol (ISO, 10 nmol/L) served as a positive control. Both AAβ1AR and AAM2R were significantly greater in patients than controls. *p<0.001. B. The percentages of autoantibody-positive patients for AAβ1AR and AAM2R and their combination are shown. Autoantibodies were significantly more frequent in patients with atrial fibrillation (AF), compared to patients with normal sinus rhythm (NSR). *p<0.001.
The frequency of autoantibody-positivity differed significantly between the two groups (Figure 1B). Sixteen of 17 (94%) patients with AF were positive for AAβ1AR, compared to only 8 of 21 (38%) patients with sinus rhythm (p<0.001). Likewise, 15 (88%) patients with AF were positive for AAM2R compared to 4 (19%) patients with sinus rhythm (p<0.001). Both autoantibodies co-existed in 14 (82%) patients with AF, compared to only 2 (10%) patients with sinus rhythm (p<0.001).
Electrophysiologic effects of IgG
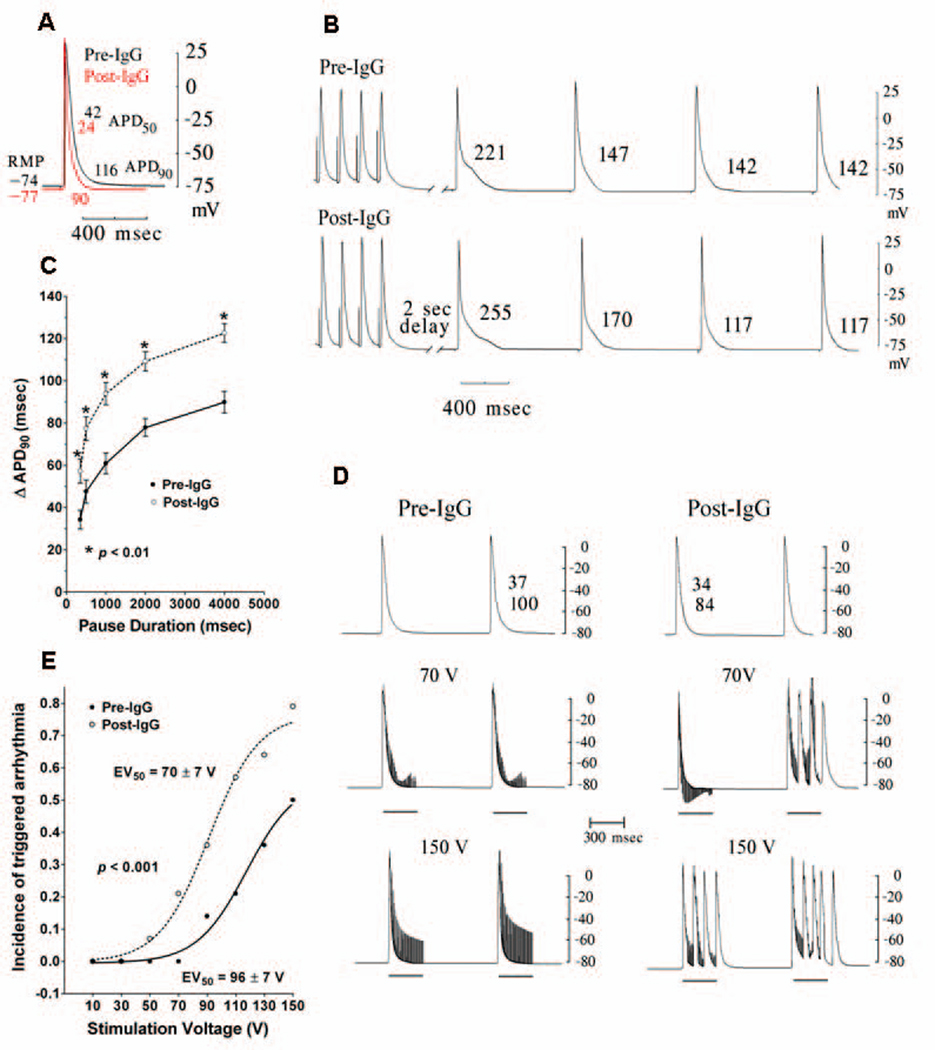
The electrophysiologic effects of IgG from 14 autoantibody-positive patients on canine pulmonary vein sleeves are summarized in Table 2. IgG equivalent to a 1:100 serum dilution (0.15 mg/mL) reduced the resting membrane potential compared to pre-IgG values, increased the action potential amplitude, and decreased the action potential duration at 50% and 90% of repolarization (Fig 2A). Pause-duration-dependent prolongation of the terminal action potential duration (action potential duration at 90% of repolarization) was enhanced after a 20-beat pacing train at 6 Hz for pause durations of 250, 500, 1,000, 2,000, and 4,000 msec, respectively, in the presence of IgG compared to control (p<0.01 for each pause duration). With rapid pacing followed by a prolonged pause, prolongation of the terminal phase of the action potential clearly assumes the form of an early afterdepolarization (Fig 2B and 2C). Triggered firing with local autonomic nerve stimulation was observed in 50% and 79% of the pulmonary sleeve preparations pre- and post-IgG, respectively (p=NS). IgG decreased the voltage of the stimulus train needed to induce triggered firing, significantly moving the stimulus voltage-response curve to the left (EV50=70±2 vs. 96±2 V post- and pre-IgG, respectively, p<0.001) (Fig 2D and 2E). Early afterdepolarization formation and local autonomic nerve stimulation-induced triggered firing were blocked by atenolol (32 nmol/L). Hyperpolarization, action potential shortening and local autonomic nerve stimulation-induced triggered firing were blocked by atropine (32 nmol/L).
Table 2.
Electrophysiologic effects of IgG (n=14).
| Pre-IgG | IgG (0.15 mg/mL) | |
|---|---|---|
| Resting membrane potential (mV) | −74.6 ± 1.8 | −76.9 ± 2.0 * |
| Action potential amplitude (mV) | 101.6 ± 7.6 | 105.7 ± 7.8 * |
| Action potential duration at 50% of repolarization (ms) | 44.3 ± 9.3 | 39.6 ± 7.0 * |
| Action potential duration at 90% of repolarization (ms) | 134.5 ± 17.8 | 118.0 ± 15.6 * |
| Triggered firing (incidence) | 50 % | 79 % |
| Stim EV50 (V) | 96 ± 7 | 72 ± 7 ** |
Stim EV50, the stimulation voltage that results in 50% of the total incidence of triggered firing.
p < 0.01,
p < 0.001 vs. pre-IgG
Figure 2. Electrophysiologic effects of IgG on canine pulmonary vein sleeves.
A. Action potentials are shown before (black) and after (red) IgG administration demonstrating hyperpolarization, increased action potential amplitude and decreased action potential duration. RMP, resting membrane potential, APD50 and APD90, action potential duration at 50% and 90% of repolarization, respectively. B. Enhanced early afterdepolarization formation produced by tachycardia (6 Hz)-pause (2 sec) pacing is shown post-IgG in electrical recordings from pulmonary vein sleeves. Numbers represent action potential duration at 90% of repolarization. C. Pause-duration-dependent prolongation of the terminal action potential duration (change in action potential duration at 90% of repolarization, ΔAPD90) with tachycardia (20 beat train at 6 Hz)-pause pacing is shown with IgG compared to control (n=14). *p<0.01 for each pause duration. D. Electrical recordings from pulmonary vein sleeves during local autonomic nerve stimulation (marked by the dark bars) demonstrates enhanced triggered firing post-IgG vs. pre-IgG administration. The numbers in the upper panel represent the action potential duration at 50% and 90% of repolarization. E. Stimulus response curves (local autonomic nerve stimulation) are shown before and after IgG administration demonstrating enhancement of triggered firing by IgG in canine pulmonary vein sleeves (significant movement of the stimulus voltage-response curve to the left) (n=14).
Determinants of atrial fibrillation
Univariate analyses were performed for 14 variables listed in Table 3. The co-presence of AAβ1AR and AAM2R, old age, heart failure, and increased AAβ1AR (% over baseline) and AAM2R effects (% change over baseline) significantly were related to the presence of AF. Multivariate analysis demonstrated the co-presence of AAβ1AR and AAM2R was the strongest independent predictor of AF (odds ratio 33.61, 95% CI 1.17 - 964.11, p=0.04). Older age also independently predicted the presence of AF (odds ratio 1.15, 95% CI 1.02 - 1.31, p=0.03) (Table 3).
Table 3.
Univariate and multivariate logistic regression analyses for 14 variables.
| Variable | Odds ratio | 95% CI (univariate) |
P value | Odds ratio | 95% CI multivariate |
P value |
|---|---|---|---|---|---|---|
| Co-presence of AAβ1AR/AAM2R |
44.33 | 6.51 – 301.90 | <0.001* | 33.61 | 1.17 – 964.11 | 0.04* |
| Age (years) | 1.10 | 1.03 – 1.18 | 0.006* | 1.15 | 1.02 – 1.31 | 0.03* |
| Male sex | 1.19 | 0.33 – 4.29 | 1.00 | |||
| Hypertension | 1.48 | 0.38 – 5.79 | 0.73 | |||
| Diabetes mellitus | 1.75 | 0.43 – 7.18 | 0.49 | |||
| Coronary artery disease |
2.01 | 0.30 – 13.86 | 0.64 | |||
| Congestive heart failure |
3.60 | 0.90 – 14.37 | 0.09** | 0.84 | 0.05 – 15.40 | 0.91 |
| Ejection fraction (%) | 1.00 | 0.96 – 1.04 | 0.92 | |||
| Left atrium diameter (mm) |
1.09 | 0.98 – 1.21 | 0.13 | |||
| E/E’ | 0.73 | 0.32 – 1.69 | 0.49 | |||
| Serum thyrotropin concentration (mU/L) |
1.21 | 0.01 – 118.42 | 0.94 | |||
| Serum free thyroxine concentration (ng/dL) |
0.77 | 0.50 – 1.18 | 0.23 | |||
| AAβ1AR effect | 1.13 | 1.04 – 1.21 | 0.002* | 1.06 | 0.93 – 1.20 | 0.37 |
| AAM2R effect | 1.13 | 1.04 – 1.22 | 0.003* | 1.07 | 0.94 – 1.22 | 0.31 |
AAβ1AR, activating autoantibodies to the β1-adrenergic receptor; AAM2R, activating autoantibodies to the M2 muscarinic receptor; E/E', ratio between the early diastolic velocity of mitral inflow (E) and that of mitral anulus (E').
p < 0.05,
p < 0.10.
To minimize the impact of age, we examined a (within 5 year) matched subgroup in our patient population. Ten patients with AF and 10 patients with sinus rhythm (mean age = 54.3±11.7 vs. 53.0±12.6 years, p=0.81) could be compared. The co-presence of AAβ1AR and AAM2R was significantly more prevalent in patients with AF (90% vs. 0%, p=0.008).
AAβ1AR and AAM2R are distinct from TSHR antibodies
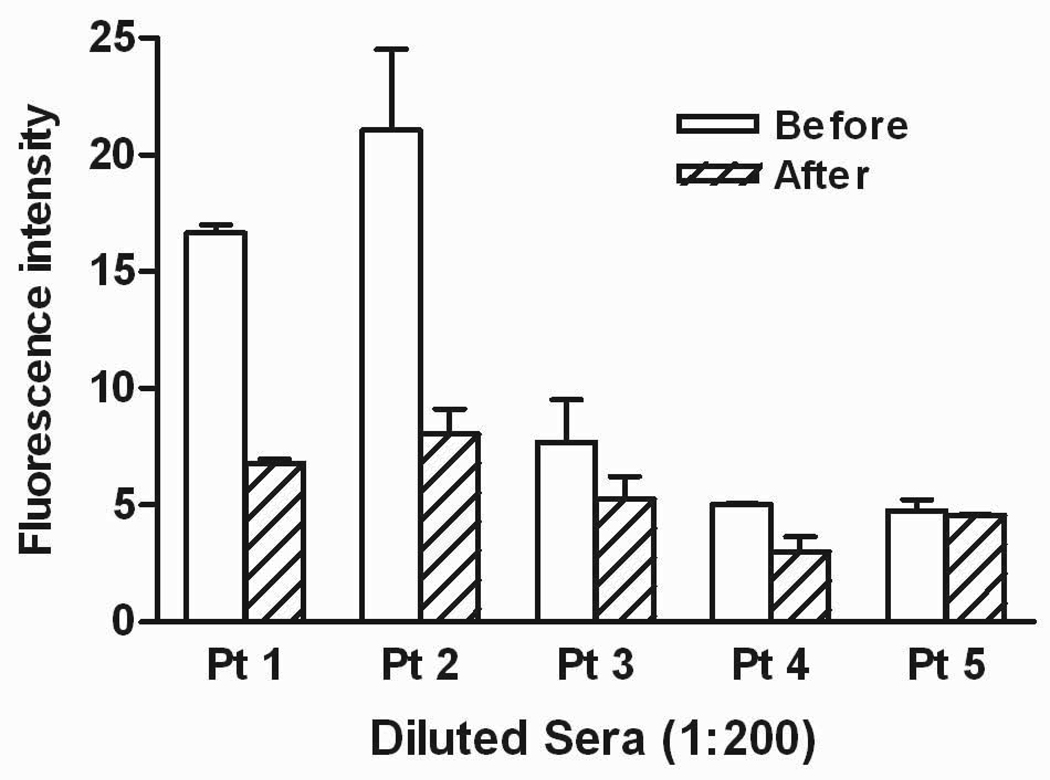
We examined the potential binding of AAβ1AR and AAM2R to TSHRs expressed in CHO cells. Diluted TSHR-preadsorbed sera from 5 subjects, 4 with elevated AAβ1AR and AAM2R and one ELISA-positive but non-active normal control were incubated with fresh CHO-TSHR cells in triplicates. These cells were double-labeled with anti-IgG antibodies and subjected to flow cytometry. Non-adsorbed sera from the same patients were used for controls. There was little binding and less than 25% decrease in binding after adsorption in the non-active normal control subject and in the 2 non-Graves’ subjects that were negative for TSHR antibodies. By contrast, the two subjects with concurrent TSHR antibodies and activating autonomic receptor antibodies had significantly higher baseline binding and a greater than 50% decrease in TSHR binding after adsorption (Figure 3). Serial dilutions of the adsorbed and non-adsorbed sera were examined by ELISA using β1AR and M2R. There was no significant loss in the adsorbed IgG reactivity to the autonomic receptor targets (data not shown).
Figure 3. Binding of patient sera to CHO-TSHR cells by flow cytometry.
Fluorescence was measured after exposing these cells to TSHR pre- and post-adsorbed purified IgG (1:200 dilution) and fluorescein isothiocyanate-labeled anti-human IgG. A 50% decrease supports a significant loss of binding activity. Patients 1 and 2 had documented stimulating thyrotropin receptor antibodies and coexisting activating autoantibodies to AAβ1AR and AAM2R. Patients 3 and 4 were non-Graves’ patients harboring AAβ1AR and AAM2R. Patient 5 was a control subject with non-activating autoantibodies to β1AR and M2R by ELISA. There were significant adsorbable antibodies to the TSHR in the two Graves’ patients. The other three had either no or small amounts of adsorbable activity.
Discussion
Graves’ disease and autoantibodies
A significant percentage of patients with Graves’ disease have activating autoantibodies against the β1AR and M2R. This increased frequency was observed mainly in patients with AF. Autoantibodies were present in some patients with sinus rhythm with a frequency greater than the 10% of a normal population reported in a previous study (22). This correlates with the fact that Graves’ disease is an autoimmune disease (6,7). Thyroid-specific autoantibodies, such as thyroid peroxidase antibodies and TSHR antibodies are present in 75% and 90–95% of patients with Graves’ disease, respectively (6,23). The genetic, environmental and endogenous factors responsible for the pathogenesis of Graves’ disease increase the propensity of these patients to develop other autoantibodies (6).
Graves’ hyperthyroidism and AF
In our study, traditional risk factors including hypertension, heart failure, increased left atrial diameter and increased left ventricular filling pressures (as predicted by E/E’ ratio) did not identify AF risk in patients with Graves’ disease. Stimulation of atrial M2Rs facilitates initiation and maintenance of AF (18) and AAM2R facilitate AF in patients with dilated cardiomyopathy (13). Our results likewise indicate AAβ1AR and AAM2R facilitate AF formation in Graves’ hyperthyroidism. Recent experimental findings describing rapid triggered firing from the canine pulmonary veins (16–19,24) provide important insights into a possible mechanism for these observed arrhythmogenic effects of AAM2R and AAβ1AR in patients with Graves’ disease. Simultaneous activation of sympathetic and parasympathetic outflow from ganglionated plexi located on the epicardial surface of the atrium (18,19), local stimulation of both parasympathetic and sympathetic nerve endings (17), and simultaneous administration of acetylcholine plus norepinephrine (or isoproterenol) (16) all initiate rapid triggered firing from canine pulmonary veins. M2R activation (action potential shortening) and β1AR activation (enhancement of the calcium transient) are important and necessary components for such triggered firing. Herein, we provide evidence suggesting that both AAM2R and AAβ1AR exert sufficient electrophysiologic effects on pulmonary vein sleeve myocardium to facilitate triggered firing. Shortening of the action potential (AAM2R effect) and enhancement of tachycardia-pause early afterdepolarization formation (AAβ1AR effect) can generate an increased sodium-calcium exchange inward current and early afterdepolarization formation (24). In the concentrations used within the isolated pulmonary vein sleeve, the antibodies induced early afterdepolarizations, but were not sufficient alone to provoke triggering. However, the antibodies facilitated the generation of triggered firing elicited by local autonomic nerve stimulation. Although excess thyroid hormone per se can cause shortening of the action potential duration in atrial and pulmonary vein myocytes (4,5), the effects of AAβ1AR and AAM2R resulted from activation of their respective receptors, since their effects could be blocked with the β-blocker atenolol and M2R blocker atropine, respectively. Elimination of the observed electrophysiologic effects with β-adrenergic and M2 muscarinic blockade suggests that it is unlikely that the TSHR autoantibodies directly caused these effects. Evidence from 4 subjects suggests the autoantibody effects did not result from cross-reactivity of TSHR autoantibodies with the β1AR and M2R. These data from our ex vivo experiments are consistent with the concept that autoantibody activation of both β1AR and M2R facilitates initiation and maintenance of AF in patients and is responsible in part for the high incidence of AF in Graves’ hyperthyroidism.
Age was an independent predictor of AF in our patient population as in other studies (2,6). Autoantibody prevalence also increases with age in the normal population (22). It is likely that age, activating autoantibodies and thyroid hormone act synergistically in this population.
Limitations
It is possible that age differences in the AF and non-AF groups in this observational cross-sectional study might confound our data. Although not a case-control study, in an age-matched subgroup of our patient population the association between the co-presence of AAβ1AR and AAM2R and AF remained highly statistically significant. We did not use long-term monitoring to identify patients in the non-AF group with unrecognized episodes of AF. However, the high rates of AF in patients with Graves’ hyperthyroidism make the identification of a significant number of such episodes unlikely. The results of the multivariate analysis, although of interest, are limited by the relatively small number of observations included in the model and should be interpreted with caution. Finally, although other autoantibodies directed toward other receptors might exist, it is unlikely they exert a significant electrophysiologic action since the observed electrophysiologic effects were blocked completely with atenolol and atropine.
Conclusions
In patients with Graves’ hyperthyroidism, the co-presence of AAβ1AR and AAM2R facilitates autonomic-induced rapid triggered firing in pulmonary veins and is the strongest independent predictor of AF. These unique activating autoantibodies may play a role in the initiation and maintenance of AF in this patient population.
Acknowledgments
Supported by the American Heart Association, Presbyterian Health Foundation (X.Y.), Heart Rhythm Institute, University of Oklahoma Health Sciences Center and the Oklahoma City Veterans Administration Research Foundation (E.P., D.C.K), NIDDKD (DK06973) and the VA Merit Award program (T.F.D.). Private grants from Will and Helen Webster, Britani T. and Paul E. Bowman, Jr., and Stan and Gayle Ward were gratefully received.
Abbreviations
- AF
atrial fibrillation
- AAβ1AR
activating autoantibodies to β1-adrenergic receptor
- AAM2R
activating autoantibodies to M2 muscarinic receptor
- ELISA
enzyme-linked immunosorbent assay
- β1AR
β1-adrenergic receptor
- M2R
M2 muscarinic receptor
- CHO-TSHR
Chinese hamster ovary cells expressing full-length thyrotropin receptor
- TSHR
thyrotropin receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Cunningham has been a consultant for Wyeth Vaccines, Aventis-Pasteur, ID Biomedical, Shire Biologics, Novartis, and Talecris. Dr. Davies has served as a board member for Kronus Corp. Other authors: No Disclosures.
Presented in part at the Annual Meeting of the American College of Cardiology, March 2008, Chicago, IL; and the Annual Meeting of the Endocrine Society, June 2008, San Francisco, CA.
References
- 1.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 2.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 3.Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Jones SV, Dillmann WH. Effects of hyperthyroidism on delayed rectifier K+ currents in left and right murine atria. Am J Physiol Heart Circ Physiol. 2005;289:H1448–H1455. doi: 10.1152/ajpheart.00828.2004. [DOI] [PubMed] [Google Scholar]
- 5.Chen YC, Chen SA, Chen YJ, Chang MS, Chan P, Lin CI. Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. J Am Coll Cardiol. 2002;39:366–372. doi: 10.1016/s0735-1097(01)01731-4. [DOI] [PubMed] [Google Scholar]
- 6.Weetman AP. Graves' disease. N Engl J Med. 2000;343:1236–1248. doi: 10.1056/NEJM200010263431707. [DOI] [PubMed] [Google Scholar]
- 7.Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest. 2005;115:1972–1983. doi: 10.1172/JCI26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnusson Y, Marullo S, Hoyer S, et al. Mapping of a functional autoimmune epitope on the beta 1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1990;86:1658–1663. doi: 10.1172/JCI114888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu LX, Magnusson Y, Bergh CH, et al. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;91:1964–1968. doi: 10.1172/JCI116416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Corsso C, de Carvalho AC, Martino HF, Varanda WA. Sera from patients with idiopathic dilated cardiomyopathy decrease ICa in cardiomyocytes isolated from rabbits. Am J Physiol Heart Circ Physiol. 2004;287:H1928–H1936. doi: 10.1152/ajpheart.00044.2004. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Hu D, Li J, Wu Y, Liu X, Yang X. Autoantibodies against the myocardial beta1-adrenergic and M2-muscarinic receptors in patients with congestive heart failure. Chin Med J (Engl) 2002;115:1127–1131. [PubMed] [Google Scholar]
- 12.Hernandez CC, Barcellos LC, Gimenez LE, et al. Human chagasic IgGs bind to cardiac muscarinic receptors and impair L-type Ca2+ currents. Cardiovasc Res. 2003;58:55–65. doi: 10.1016/s0008-6363(02)00811-8. [DOI] [PubMed] [Google Scholar]
- 13.Baba A, Yoshikawa T, Fukuda Y, et al. Autoantibodies against M2-muscarinic acetylcholine receptors: new upstream targets in atrial fibrillation in patients with dilated cardiomyopathy. Eur Heart J. 2004;25:1108–1115. doi: 10.1016/j.ehj.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Christ T, Wettwer E, Dobrev D, et al. Autoantibodies against the beta1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J Mol Cell Cardiol. 2001;33:1515–1525. doi: 10.1006/jmcc.2001.1414. [DOI] [PubMed] [Google Scholar]
- 15.Chiale PA, Ferrari I, Mahler E, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103:1765–1771. doi: 10.1161/01.cir.103.13.1765. [DOI] [PubMed] [Google Scholar]
- 16.Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Scherlag BJ, Patterson E, Po SS. The neural basis of atrial fibrillation. J Electrocardiol. 2006;39:S180–S183. doi: 10.1016/j.jelectrocard.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Po SS, Scherlag BJ, Yamanashi WS, et al. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm. 2006;3:201–208. doi: 10.1016/j.hrthm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Kem DC, Yu X, Patterson E, et al. Autoimmune hypertensive syndrome. Hypertension. 2007;50:829–834. doi: 10.1161/HYPERTENSIONAHA.107.096750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ando T, Latif R, Pritsker A, Moran T, Nagayama Y, Davies TF. A monoclonal thyroid-stimulating antibody. J Clin Invest. 2002;110:1667–1674. doi: 10.1172/JCI16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu HR, Zhao RR, Zhi JM, Wu BW, Fu ML. Screening of serum autoantibodies to cardiac beta1-adrenoceptors and M2-muscarinic acetylcholine receptors in 408 healthy subjects of varying ages. Autoimmunity. 1999;29:43–51. doi: 10.3109/08916939908995971. [DOI] [PubMed] [Google Scholar]
- 23.Costagliola S, Morgenthaler NG, Hoermann R, et al. Second generation assay for thyrotropin receptor antibodies has superior diagnostic sensitivity for Graves' disease. J Clin Endocrinol Metab. 1999;84:90–97. doi: 10.1210/jcem.84.1.5415. [DOI] [PubMed] [Google Scholar]
- 24.Patterson E, Jackman WM, Beckman KJ, et al. Spontaneous pulmonary vein firing in man: relationship to tachycardia-pause early afterdepolarizations and triggered arrhythmia in canine pulmonary veins in vitro. J Cardiovasc Electrophysiol. 2007;18:1067–1075. doi: 10.1111/j.1540-8167.2007.00909.x. [DOI] [PubMed] [Google Scholar]