Abstract
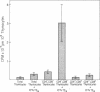
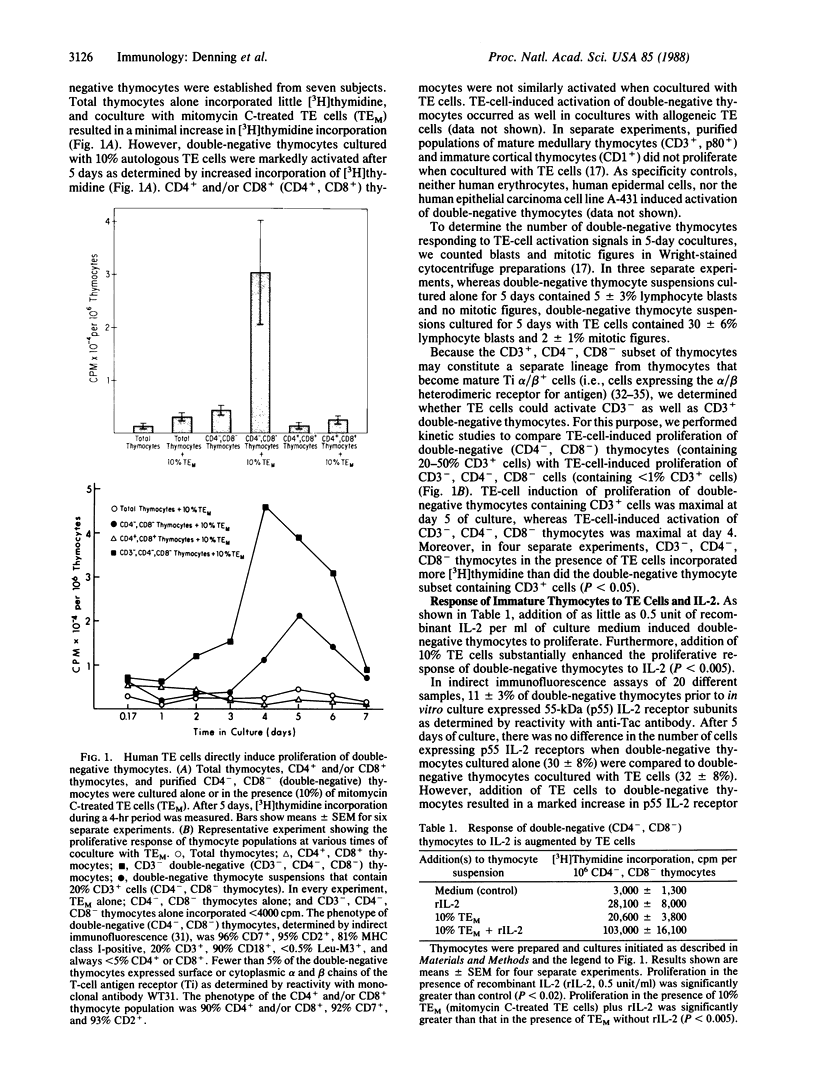
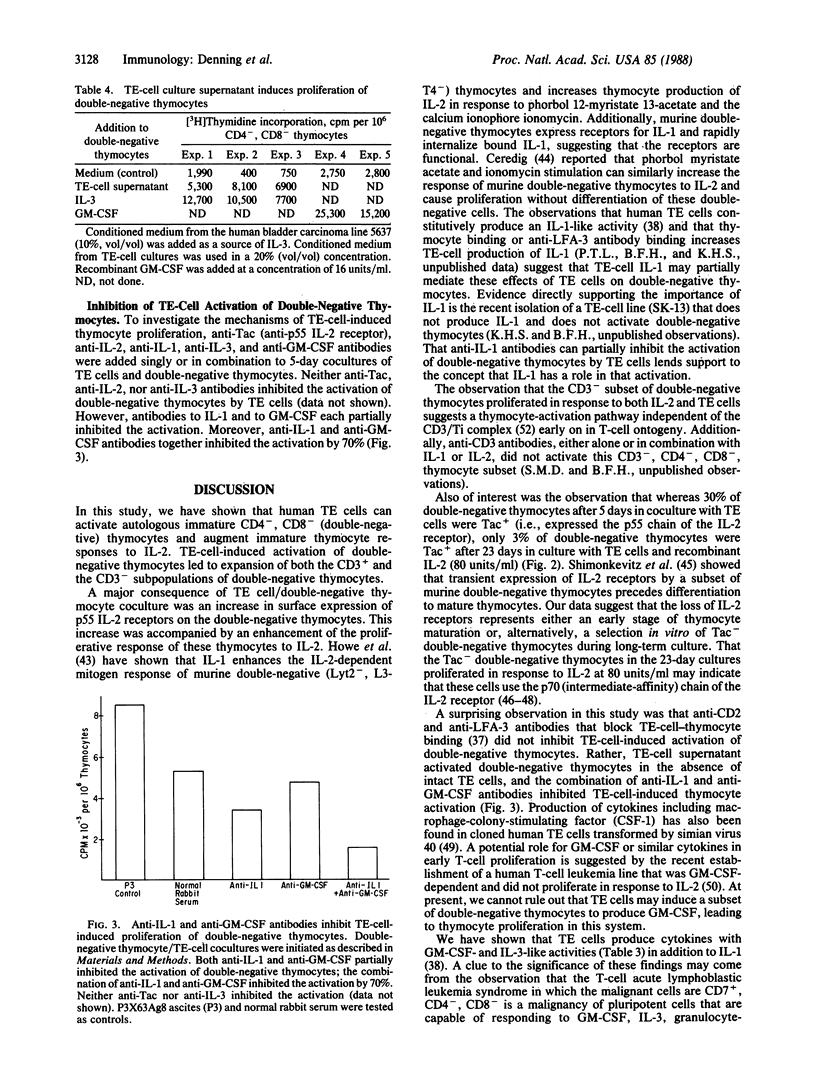
To study the role that epithelial cells of the thymic microenvironment play in promoting activation of immature CD7+, CD2+, CD4-, CD8- (double-negative) human thymocytes, we have isolated thymocyte subsets from normal postnatal thymus and have cocultured autologous double-negative thymocytes with pure populations of thymic epithelial (TE) cells. We report that TE cells directly activate double-negative thymocytes to proliferate and that TE cells enhance the ability of double-negative thymocytes to proliferate in response to stimulation with exogenous interleukin 2. Activated double-negative thymocytes that proliferated in vitro in the presence of TE cells and interleukin 2 remained double-negative after 23 days in culture. Moreover, TE-cell culture supernatants in the absence of intact TE cells contain interleukin 1, interleukin 3, and granulocyte/macrophage-colony-stimulating factor activity for human bone marrow cells and can activate double-negative thymocytes to proliferate. Antibodies against interleukin 1 and against granulocyte/macrophage-colony-stimulating factor inhibited TE-cell-induced thymocyte activation. These data indicate that one role of TE cells in vivo may be to activate double-negative thymocytes to proliferate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank I., DePinho R. A., Brenner M. B., Cassimeris J., Alt F. W., Chess L. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature. 1986 Jul 10;322(6075):179–181. doi: 10.1038/322179a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Riberdy J., Ang S. L., Seidman J. G., Devlin P., Krangel M. S. Two forms of the T-cell receptor gamma protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987 Feb 19;325(6106):689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Ceredig R. Proliferation in vitro and interleukin production by 14 day fetal and adult Lyt-2-/L3T4- mouse thymocytes. J Immunol. 1986 Oct 1;137(7):2260–2267. [PubMed] [Google Scholar]
- De la Hera A., Toribio M. L., Marquez C., Marcos M. A., Cabrero E., Martinez-A C. Differentiation of human mature thymocytes: existence of a T3+4-8- intermediate stage. Eur J Immunol. 1986 Jun;16(6):653–658. doi: 10.1002/eji.1830160611. [DOI] [PubMed] [Google Scholar]
- Denning S. M., Tuck D. T., Singer K. H., Haynes B. F. Human thymic epithelial cells function as accessory cells for autologous mature thymocyte activation. J Immunol. 1987 Feb 1;138(3):680–686. [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Fowlkes B. J., Edison L., Mathieson B. J., Chused T. M. Early T lymphocytes. Differentiation in vivo of adult intrathymic precursor cells. J Exp Med. 1985 Sep 1;162(3):802–822. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding H., Munitz T. I., Singer A. Characterization of antigen-specific, Ia-restricted, L3T4+ cytolytic T lymphocytes and assessment of thymic influence on their self specificity. J Exp Med. 1985 Sep 1;162(3):943–961. doi: 10.1084/jem.162.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Eisenbarth G. S., Fauci A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5829–5833. doi: 10.1073/pnas.76.11.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Metzgar R. S., Minna J. D., Bunn P. A. Phenotypic characterization of cutaneous T-cell lymphoma. Use of monoclonal antibodies to compare with other malignant T cells. N Engl J Med. 1981 May 28;304(22):1319–1323. doi: 10.1056/NEJM198105283042202. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Reisner E. G., Hemler M. E., Strominger J. L., Eisenbarth G. S. Description of monoclonal antibody defining an HLA allotypic determinant that includes specificities within the B5 cross-reacting group. Hum Immunol. 1982 Jul;4(4):273–285. doi: 10.1016/0198-8859(82)90001-5. [DOI] [PubMed] [Google Scholar]
- Haynes B. F. The human thymic microenvironment. Adv Immunol. 1984;36:87–142. doi: 10.1016/s0065-2776(08)60900-1. [DOI] [PubMed] [Google Scholar]
- Howe R. C., Lowenthal J. W., MacDonald H. R. Role of interleukin 1 in early T cell development: Lyt-2-L3T4- thymocytes bind and respond in vitro to recombinant IL 1. J Immunol. 1986 Nov 15;137(10):3195–3200. [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Kingston R., Jenkinson E. J., Owen J. J. A single stem cell can recolonize an embryonic thymus, producing phenotypically distinct T-cell populations. 1985 Oct 31-Nov 6Nature. 317(6040):811–813. doi: 10.1038/317811a0. [DOI] [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- Kurtzberg J., Bigner S. H., Hershfield M. S. Establishment of the DU.528 human lymphohemopoietic stem cell line. J Exp Med. 1985 Nov 1;162(5):1561–1578. doi: 10.1084/jem.162.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lanier L. L., Allison J. P., Phillips J. H. Correlation of cell surface antigen expression on human thymocytes by multi-color flow cytometric analysis: implications for differentiation. J Immunol. 1986 Oct 15;137(8):2501–2507. [PubMed] [Google Scholar]
- Lanier L. L., Weiss A. Presence of Ti (WT31) negative T lymphocytes in normal blood and thymus. Nature. 1986 Nov 20;324(6094):268–270. doi: 10.1038/324268a0. [DOI] [PubMed] [Google Scholar]
- Le P. T., Tuck D. T., Dinarello C. A., Haynes B. F., Singer K. H. Human thymic epithelial cells produce interleukin 1. J Immunol. 1987 Apr 15;138(8):2520–2526. [PubMed] [Google Scholar]
- Lobach D. F., Haynes B. F. Ontogeny of the human thymus during fetal development. J Clin Immunol. 1987 Mar;7(2):81–97. doi: 10.1007/BF00916002. [DOI] [PubMed] [Google Scholar]
- Lugo J. P., Krishnan S. N., Sailor R. D., Koen P., Malek T., Rothenberg E. Proliferation of thymic stem cells with and without receptors for interleukin 2. Implications for intrathymic antigen recognition. J Exp Med. 1985 May 1;161(5):1048–1062. doi: 10.1084/jem.161.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage M., Mathieson B., Sharrow S., McHugh L., Hämmerling U., Kanellopoulos-Langevin C., Brideau D., Jr, Thomas C. A., 3rd Preparative nonlytic separation of Lyt2+ and Lyt2- T lymphocytes, functional analyses of the separated cells and demonstration of synergy in graft-vs.-host reaction of Lyt2+ and Lyt2- cells. Eur J Immunol. 1981 Mar;11(3):228–235. doi: 10.1002/eji.1830110312. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Longton G., Ledbetter J. A., Newman W., Braun M. P., Beatty P. G., Hansen J. A. Identification and functional characterization of two distinct epitopes on the human T cell surface protein Tp50. J Immunol. 1983 Jul;131(1):180–185. [PubMed] [Google Scholar]
- Mathieson B. J., Fowlkes B. J. Cell surface antigen expression on thymocytes: development and phenotypic differentiation of intrathymic subsets. Immunol Rev. 1984 Dec;82:141–173. doi: 10.1111/j.1600-065x.1984.tb01121.x. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Watt S. M., Robertson D., Hussein S., Healy L. E., Furley A. J., Greaves M. F. Cloning of human thymic subcapsular cortex epithelial cells with T-lymphocyte binding sites and hemopoietic growth factor activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4999–5003. doi: 10.1073/pnas.84.14.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Hemopoietic colony-forming cells in umbilical cord blood with extensive capability to generate mono- and multipotential hemopoietic progenitors. J Clin Invest. 1982 Dec;70(6):1324–1328. doi: 10.1172/JCI110734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Ritter M. A., Sauvage C. A., Cotmore S. F. The human thymus microenvironment: in vivo identification of thymic nurse cells and other antigenically-distinct subpopulations of epithelial cells. Immunology. 1981 Nov;44(3):439–446. [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A. Ability of human leukocytic pyrogen to enhance phytohemagglutinin induced murine thymocyte proliferation. Cell Immunol. 1981 Sep 1;63(1):134–142. doi: 10.1016/0008-8749(81)90034-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlunk T., Schleyer M. The influence of culture conditions on the production of colony-stimulating activity by human placenta. Exp Hematol. 1980 Feb;8(2):179–184. [PubMed] [Google Scholar]
- Scollay R., Shortman K. Identification of early stages of T lymphocyte development in the thymus cortex and medulla. J Immunol. 1985 Jun;134(6):3632–3642. [PubMed] [Google Scholar]
- Shimonkevitz R. P., Husmann L. A., Bevan M. J., Crispe I. N. Transient expression of IL-2 receptor precedes the differentiation of immature thymocytes. Nature. 1987 Sep 10;329(6135):157–159. doi: 10.1038/329157a0. [DOI] [PubMed] [Google Scholar]
- Singer K. H., Harden E. A., Robertson A. L., Lobach D. F., Haynes B. F. In vitro growth and phenotypic characterization of mesodermal-derived and epithelial components of normal and abnormal human thymus. Hum Immunol. 1985 Jul;13(3):161–176. doi: 10.1016/0198-8859(85)90009-6. [DOI] [PubMed] [Google Scholar]
- Spits H., Borst J., Tax W., Capel P. J., Terhorst C., de Vries J. E. Characteristics of a monoclonal antibody (WT-31) that recognizes a common epitope on the human T cell receptor for antigen. J Immunol. 1985 Sep;135(3):1922–1928. [PubMed] [Google Scholar]
- Stutman O. Intrathymic and extrathymic T cell maturation. Immunol Rev. 1978;42:138–184. doi: 10.1111/j.1600-065x.1978.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Nelson D. L., Fleisher T. A., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. II. Expression of Tac antigen on activated cytotoxic killer T cells, suppressor cells, and on one of two types of helper T cells. J Immunol. 1981 Apr;126(4):1398–1403. [PubMed] [Google Scholar]
- Valtieri M., Santoli D., Caracciolo D., Kreider B. L., Altmann S. W., Tweardy D. J., Gemperlein I., Mavilio F., Lange B., Rovera G. Establishment and characterization of an undifferentiated human T leukemia cell line which requires granulocyte-macrophage colony stimulatory factor for growth. J Immunol. 1987 Jun 1;138(11):4042–4050. [PubMed] [Google Scholar]
- Vollger L. W., Tuck D. T., Springer T. A., Haynes B. F., Singer K. H. Thymocyte binding to human thymic epithelial cells is inhibited by monoclonal antibodies to CD-2 and LFA-3 antigens. J Immunol. 1987 Jan 15;138(2):358–363. [PubMed] [Google Scholar]
- Weissman I. L. Thymus cell maturation. Studies on the origin of cortisone-resistant thymic lymphocytes. J Exp Med. 1973 Feb 1;137(2):504–510. doi: 10.1084/jem.137.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Platzer E., Lu L., Gabrilove J. L., Levi E., Mertelsmann R., Moore M. A. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiranowska M., Kaido T., Caspritz G., Cook J., Hadden J. Interleukin-2 and coculture with thymic epithelial cells synergistically induce prothymocyte differentiation and proliferation. Thymus. 1987;10(3-4):231–245. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Hera A., Toribio M. L., Marquez C., Martinez C. Interleukin 2 promotes growth and cytolytic activity in human T3+4-8- thymocytes. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6268–6271. doi: 10.1073/pnas.82.18.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Crisanti A., Kisielow P., Haas W. Absence of growth by most receptor-expressing fetal thymocytes in the presence of interleukin-2. Nature. 1985 Apr 11;314(6011):539–540. doi: 10.1038/314539a0. [DOI] [PubMed] [Google Scholar]